Published online Mar 21, 2008. doi: 10.3748/wjg.14.1790
Revised: January 14, 2008
Published online: March 21, 2008
AIM: To identify the role of herbal compound 861 (Cpd 861) in the regulation of mRNA expression of collagen synthesis- and degradation-related genes in human hepatic stellate cells (HSCs).
METHODS: mRNA levels of collagen typesIand III, matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), membrane type-1 matrix metalloproteinase (MT1-MMP), tissue inhibitor of metalloproteinase 1 (TIMP-1), and transforming growth factor β1 (TGF-β1) in cultured-activated HSCs treated with Cpd 861 or interferon-γ (IFN-γ) were determined by real-time PCR.
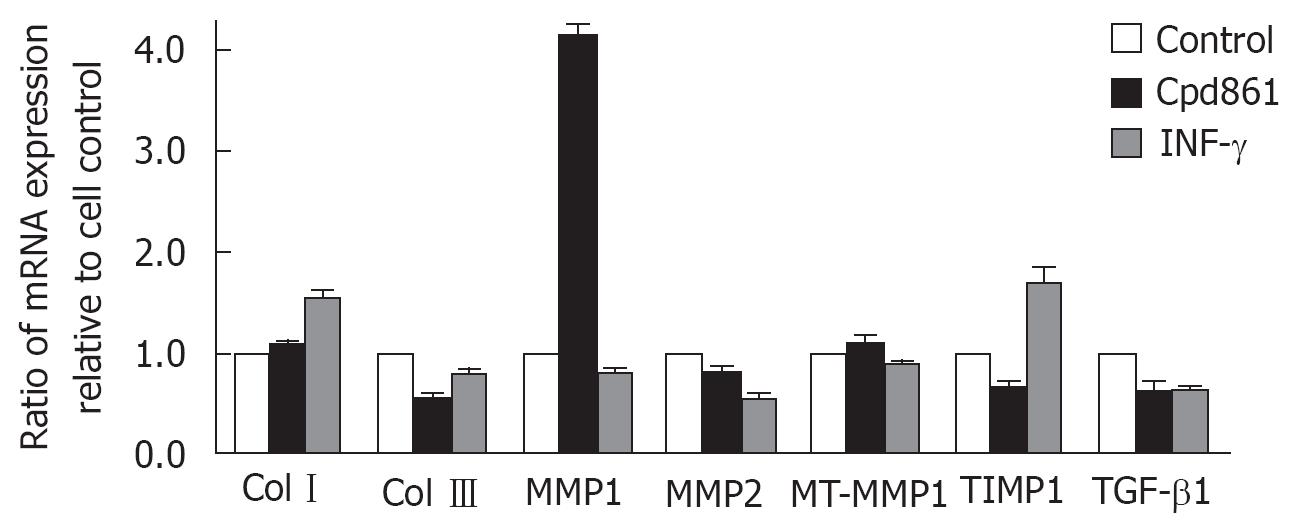
RESULTS: Both Cpd 861 and IFN-γ reduced the mRNA levels of collagen type III, MMP-2 and TGF-β1. Moreover, Cpd 861 significantly enhanced the MMP-1 mRNA levels while down-regulated the TIMP-1 mRNA expression, increasing the ratio of MMP-1 to TIMP-1 to (6.3 + 0.3)- fold compared to the control group.
CONCLUSION: The anti-fibrosis function of Cpd 861 may be mediated by both decreased interstitial collagen synthesis by inhibiting the transcription of collagen type III and TGF-β1 and increased degradation of these collagens by up-regulating MMP-1 and down-regulating TIMP-1 mRNA levels.
- Citation: Wang L, Wang BE, Wang J, Xiao PG, Tan XH. Herbal compound 861 regulates mRNA expression of collagen synthesis- and degradation-related genes in human hepatic stellate cells. World J Gastroenterol 2008; 14(11): 1790-1794
- URL: https://www.wjgnet.com/1007-9327/full/v14/i11/1790.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1790
Hepatic fibrosis, a wound-healing response to a variety of chronic liver injuries, is characterized by the increased deposition of remodeled extracellular matrix (ECM). Hepatic stellate cells (HSCs), the major source of ECM in liver, play a central role in the progress of fibrogenesis[1–4]. After acute or chronic injury, HSCs are activated by autocrine and paracrine mediators and have greater fibrogenic, contractile and migration activities than “resting hepatic stellate cells” accompanied with expressing activation markers (e.g., α-smooth muscle actin, α-SMA). In addition to the synthesis of greater amounts of ECM components (predominantly collagen typesIand III), activated HSCs also show altered expression/activity of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), thereby leading to increased deposition of extracellular matrix and formation of scar tissue in the fibrotic liver[6–8]. Thus, the activated HSCs are considered a therapeutic target for antifibrotic drugs[2–5].
Herbal compound 861 (Cpd 861) is an extract of mixed Chinese herbs that have been used for liver disease treatment in traditional Chinese midicine (TCM). Randomized double-blinded clinical studies have verified that Cpd 861 could significantly improve clinical manifestations and biochemical parameters of chronic HBV-related liver fibrosis patients, as well as regression of hepatic fibrotic change[9–11]. Our preliminary data show that Cpd 861 could inhibit HSC proliferation and reduce the expression of α-SMA in culture-activated HSCs[12]. The aim of the present study was to identify the role of Cpd 861 in the regulation of collagen synthesis and degration in culture-activated HSCs and to define its antifibrosis molecular mechanisms of action on the expression of collagens, MMPs, and TIMP-1 in human hepatic stellate cells.
Human hepatic stellate cell line LX-2 used in this study was kindly provided by Dr. Friedman SL of the Mount Sinai School of Medicine. The cells express α-SMA under all culture conditions and therefore are regarded as at least partially activated even after immediate replating[13]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, Carlsbad, CA), supplemented with 5% heat-inactivated fetal bovine serum (FBS; Hyclone, USA), 200 mmol/L L-glutamine(Gibco, Invitrogen, Carlsbad, CA), 100 000 U/L penicillin(Sigma, USA) and 0.1 g/L streptomycin (Gibco, Invitrogen, Carlsbad, CA) in an atmosphere containing 50 mL/L CO2.
Cells were cultured in DMEM containing 5% FBS in an atmosphere containing 50 mL/L CO2. The medium was carefully removed before compound treatment. The fresh medium containing Cpd 861 (0.01 g/L, in this concentration Cpd 861 had no effect on the proliferation of LX-2 cells)[12] and IFN-γ (1 000 000 U/L, Fosun Pharma, Shanghai, China) was added. IFN-γ, which can reduce HSC activation both in vivo and in vitro associated with reduced extracellular matrix deposition in animal hepatic fibrosis progression[14–17], was used as the positive control. The changes in mRNA expression of collagen typesIand III, matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), membrane type-1 matrix metalloproteinase (MT1-MMP), tissue inhibitor of metalloproteinase 1 (TIMP-1), and transforming growth factor β1 (TGF-β1) were detected by quantitative real-time PCR.
Total RNA was extracted from LX-2 cells as previously described[12]. The concentration of total RNA was determined spectrophotometrically with ND-1000 spectrophotometer (NanoDrop, USA) and the integrity of samples was confirmed by visualizing 28 S and 18 S ribosomal RNA bands under ultraviolet light after agarose gel electrophoresis. One &mgr;g of RNA was added to each reaction tube and converted to complementary DNA (cDNA) using oligo (dT)15 primers (Promega, USA) and SuperScript™ II reverse transcriptase(Invitrogen, CA).
Real-time PCR reaction was performed (10 min at 95°C for activation, 15 s at 95°C and 60 s at 60°C for 40 cycles of amplification) on the Applied Biosystems 7300 Real Time PCR System using SYBR® Green PCR Master Mix (Applied Biosystems, USA). Standard curve method and/or comparative CT method were used to quantify the mRNA expression levels[1819]. Standard curves were generated using 103-109 copies of plasmids containing cDNA of each target gene, and results were normalized for RNA input using glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Samples and standards were analyzed in triplicate for each set of primers (Table 1).
| Oligonucleotide | Oligonucleotide primer | Fragment sequence size (bp) |
| Col I | L: GTCGAGGGCCAAGACGAAG | 143 |
| R: CAGATCACGTCATCGCACAAC | ||
| Col III | L: TGGTCCCCAAGGTGTCAAAG | 117 |
| R: GGGGGTCCTGGGTTACCATTA | ||
| MMP-1 | L: TCTGGGGTGTGGTGTCTCA | 114 |
| R: GCCTCCCATCATTCTTCAGGTT | ||
| MMP-2 | L: ACATCAAGGGCATTCAGGAG | 268 |
| R: GCCTCCGTATACCGCATCAAT | ||
| MT1-MMP | R: GAAGCCTGGCTACAGCAATATG | 119 |
| L: TGCAAGCCGTAAAACTTCTGC | ||
| TIMP-1 | L: CTTCTGCAATTCCGACCTCGT | 127 |
| R: CCCTAAGGCTTGGAACCCTTT | ||
| TGF-β1 | L: GGCCAGATCCTGTCCAAGC | 201 |
| R: GTGGGTTTCCACCATTAGCAC | ||
| GAPDH | L: ATGGGGAAGGTGAAGGTCG | 108 |
| R: GGGGTCATTGATGGCAACAATA |
After PCR, the melting curves of all final PCR products were analyzed as previously described[12]. To ensure that the correct products were amplified in the reaction, all samples were also separated on 2% agarose gel electrophoresis. All PCR conditions and primers were optimized to produce a single product of the correct basepair size.
Data were expressed as mean ± SE. Statistical analysis was performed using GraphPad Prism software (version 3.0). t-test was used for comparison between the groups. P < 0.05 was considered statistically significant.
Cpd 861 regulated the mRNA expression of hepatic fiborosis related genes.
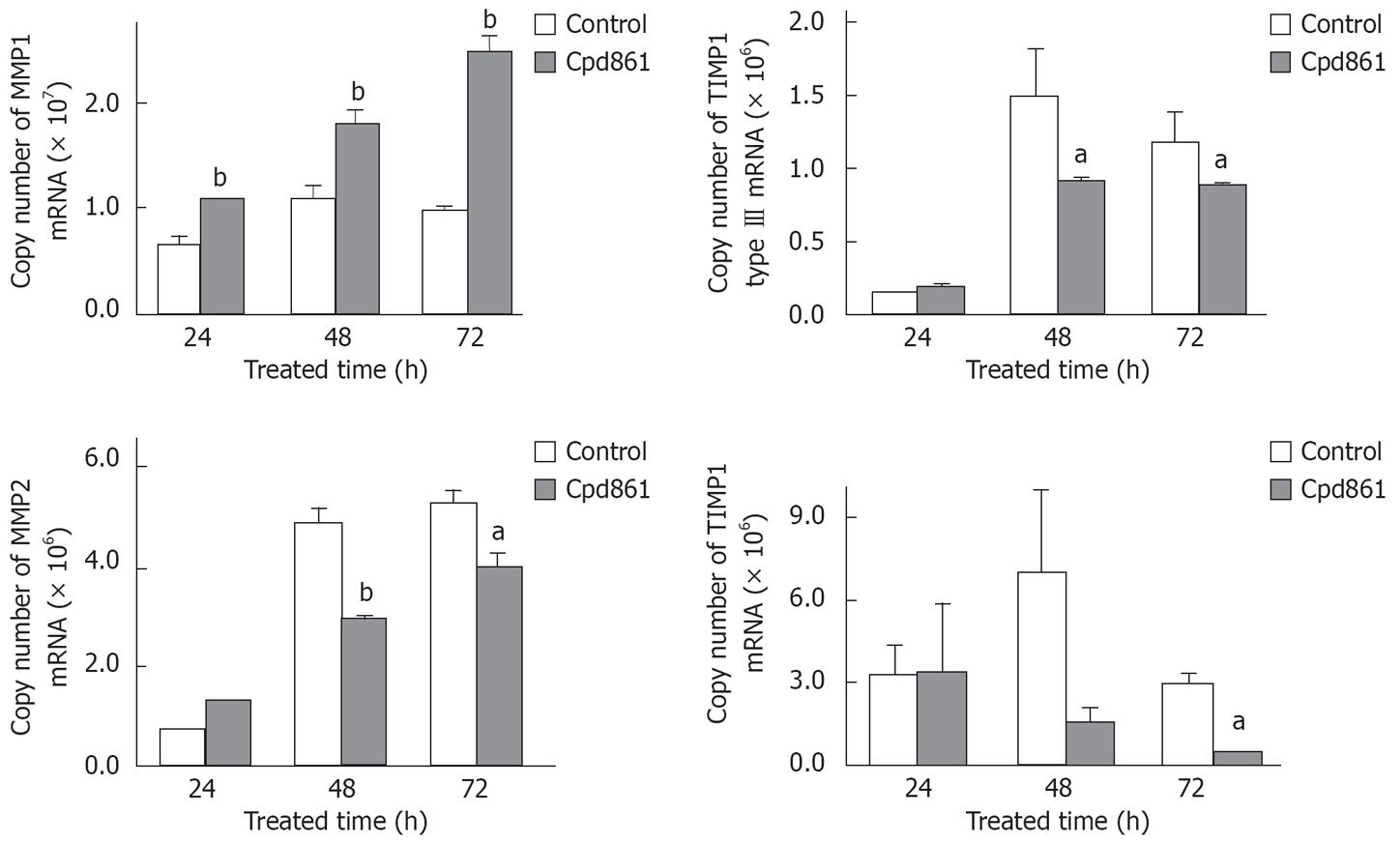
To detect the effects of Cpd 861 on collagen synthesis and degradation in LX-2 cells, the mRNA levels of collagen typesIand III, MMP-1, MMP-2, MT1-MMP, TIMP-1, and TGF-β1 in cultured-activated HSCs after 48 h of treatment with Cpd 861 (0.01 g/L) or interferon-γ (1 000 000 U/L) were determined by real-time PCR. The data were normalized by GAPDH. As shown in Figure 1, both Cpd 861 and interferon-γ reduced the mRNA levels of collagen type III, MMP-2 and TGF-β1. Moreover, Cpd 861, but not interferon-γ, significantly enhanced the MMP-1 mRNA levels, but at the same time inhibited the TIMP-1 mRNA expression which increased the ratio of MMP-1 to TIMP-1 to (6.3 ± 0.3)-fold compared to the control group. In our study, the mRNA levels of collagen typeIand MT1-MMP in LX-2 cells did not change much after 48 h of treatment with Cpd 861. To further understand the mechanisms of Cpd 861, the change over time was also detected. As shown in Figure 2, after 24 h of treatment with Cpd 861, the MMP-1 mRNA expression progressively increased significantly, while the expression levels of collagen type III, MMP-2, and TIMP-1 decreased significantly after exposed to Cpd 861 for 48 h or 72 h.
The altered balance between the synthesis and degradation of matrix proteins is the major pathogenic feature in the hepatic fibrosis process, which leads to the quantitative and qualitative change of composition in hepatic ECM. In advanced liver fibrosis, the total components of ECM increase accompanying a decrease in the normal low density basement membrane-like matrix (collagen type IV) being replaced by interstitial type matrix (mainly collagen typesIand type III), leading eventually to the net deposition of fibrillar matrix[20].
The extracellular matrix synthesis is mediated at both transcriptional and post-transcriptional levels, while its degradation is predominantly regulated by MMPs, which can degrade almost all ECM components. For example, MMP-2 (gelatinase A), one of the major MMPs during liver fibrogenesis, degrades type IV collagen, and this function can enhance the normal basement replacement by fibril-forming collagen which contributes to the pathogenesis of liver fibrosis. MMP-2 is secreted as a pro-enzyme, and its activation involves a membrane type MMP (MT1-MMP) expressed on the cell surface[2122]. In contrast to MMP-2, interstitial collagenases (MMP-1 in humans, MMP-13 in rats) are involved in the degradation of interstitial type matrix by cleaving such substrates at a specific Gly-Ile/Leu site[6]. The activities of MMPs are inhibited by TIMPs (a family of tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2 especially in the liver)[6]. The combination of MMPs and TIMPs plays a critical role in the remodeling of hepatic ECM during liver fibrogenesis. When liver fibrosis progresses, in addition to increased matrix synthesis, the enhanced expression and release of MMP-2 and MT1-MMP as well as TIMP-1 are also the striking features, while the expression of MMP-1 decreases or remains unchanged, which serves to promote progression of liver fibrosis by preventing degradation of interstitial collagens[623].
In the liver, HSCs are the key cell type involved both in the synthesis and in the degration of matrix proteins. These cells are the major fibrogenic cell type that contributes to collagen accumulation in the liver. In a normal liver, HSCs in the space of Disse are maintained in a quiescent, nonfibrogenic phenotype. When the liver is injured, these cells are activated and transformed to myofibroblast-like cells characterized by fibrogenesis, contractility, and migration, production of much larger amounts of the majority components of extracellular matrix, particularly collagen types II and III. Moreover, satellite cells also express almost all the key components required for matrix degradation such as MMP-1, MMP-2 and TIMP-1[124]. Changes in the activity of MMPs and TIMPs lead to the remodeling of hepatic ECM during injury to the liver, which in turn directly and indirectly accelerates stellate cell activation.
LX-2 cells, a human hepatic stellate cell line, were used in our study. LX-2 cells retain features of HSCs and express key proteins involved in matrix remodelling[13]. The cells express α-SMA under all culture conditions and are regarded as, at least, partially activated even after immediate replating. Furthermore, during growth on a plastic surface, the cells undergo further activation as defined by the increase in α-SMA[25]. In our study, the collagen type III and MMP-2 mRNA expression increased, while MMP-1 mRNA expression was down-regulated when these cells were further activated on a plastic surface (data not shown), consistent with those seen in clinical or animal studies[623]. Thus, LX-2 cells provide a valuable tool in our anti-fibrotic drug research.
Cpd 861 formulated by one of the authors (Bao’en Wang)[10] according to Chinese medical theory, is comprised of Salvia miltiorrhiza, Astragalus membranaceus and Spatholobus suberectus as its chief components. This herbal compound has been proven to be effective for the treatment of patients with hepatic fibrosis and its therapeutic effectiveness in making hepatic fibrosis regress has been confirmed by several random clinical tests with paired liver biopsies and animal model studies[10112627]. Our previous study demonstrated that Cpd 861 can significantly inhibit cell proliferation in a dose-dependent manner and reduce the mRNA expression level of α-SMA in LX-2 cells[12]. In this study, we used real-time PCR to identify mRNA expressions of collagens, MMPs, and TIMPs in culture-activated HSCs. The data show both Cpd 861 and positive control IFN-γ inhibited the mRNA expressions of TGF-β1, MMP-2, and collagen type III, while there was no difference in the mRNA expression of MT1-MMP and collegen typeIobserved in LX-2 cells treated with either Cpd 861 or IFN-γ alone.
As the dominant stimulus of ECM production by HSCs, the increasing expression of TGF-β1 has been demonstrated in both culture-activated HSCs and in vivo studies, and the autocrine expression in activated HSCs is its most important resource[28–30]. Besides direct transcriptional down-regulation of collagen type III, the restraining of mRNA expression of TGF-β1 may also play an important role in both Cpd 861 and IFN-γ anti-collagen-synthesis functions.
As discussed above, the degradation of basement membrane by HSC-derived MMP-2 is critical to further activation of HSCs and scar formation during liver wound remodeling. The transcriptional down-regulation of MMP-2 by both Cpd 861 and IFN-γ may protect the normal basement membrane from being replaced by fibrillar matrix and indirectly inhibit the activation of HSCs.
It is particularly interesting that, in contrast to IFN-γ, Cpd 861 also can significantly enhance the mRNA level of MMP-1 and decrease the TIMP-1 mRNA expression, indicating that Cpd 861 can directly (enhancing the expression of MMP-1) and indirectly (inhibiting the expression of TIMP-1) enhance the degration of collagens. The data from the time course study indicate that the up-regulation of MMP-1 expression preceded the changes in MMP-2, TIMP-1, and collagen type III mRNA levels, suggesting that the fibrillar matrix degration has already enhanced before its synthesis is inhibited by Cpd 861. In addition to their role in inhibiting extracellular matrix degradation, there is increasingly recognized that TIMPs play a significant role in regulating apoptosis of some cell types[6]. Thus, the down-regulation of TIMP-1 mRNA levels by Cpd 861 may also contribute to pro-apoptosis function in the HSCs due to its mechanism underlying anti-fibrosis.
In conclusion, the anti-fibrotic function of Cpd 861 may be attributed to both the decreased interstitial collagen synthesis by down-regulating the mRNA levels of collagen type III and TGF-β1 as well as increased degradation of these collagens by up-regulating MMP-1 and down-regulating TIMP-1, accompanying protection of the normal basement membrane from destruction by MMP-2, and perhaps at some points associated with the apoptosis of activated HSCs. Further work is required to accurately analyze the relative roles of these different functions in detail, and this will be important in the development of novel antifibrotic therapies.
The altered balance between the synthesis and degradation of matrix proteins is the major pathogenic feature in the hepatic fibrosis process. The purpose of this study was to identify the role of herbal compound 861 (Cpd 861) in the regulation of mRNA expression of collagen synthesis- and degradation-related genes in human hepatic stellate cells (HSCs).
Although remarkable progress has been made in understanding the mechanisms underlying hepatic fibrosis and plenty of agents have been studied, few effective “anti-fibrogenic” drugs have been approved for use in humans.
The study not only verified the clinical anti-fibrotic effect of the herbs, but also explored the multiple action sites of the herbal compound which is evidently different from that of the purified ingredients in Western drugs.
The results of this study could allow for developing the effective anti-fibrogenic drugs from the Chinese herbs.
This is an interesting paper, describing the effect of plant extract Cpd 861 on the levels of mRNAs encoding collagens (typesIand III, metalloproteinases (MMP-1 and MMP-2), and various other factors (TIMP-1 and TGF-beta 1) in LX-2 cells of stellate cell origin. The plant extract appeared to regulate the mRNA levels of collagen synthesis- and degradation-related genes, thus demonstrating an anti-fibrotic effect.
| 1. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [Cited in This Article: ] |
| 2. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [Cited in This Article: ] |
| 3. | Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728-1734. [Cited in This Article: ] |
| 4. | Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S73-S78. [Cited in This Article: ] |
| 5. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82-S88. [Cited in This Article: ] |
| 6. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [Cited in This Article: ] |
| 7. | Wang JC. Importance of plasma matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinase (TIMP) in development of fibrosis in agnogenic myeloid metaplasia. Leuk Lymphoma. 2005;46:1261-1268. [Cited in This Article: ] |
| 8. | Manoury B, Nenan S, Guenon I, Lagente V, Boichot E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharmacol. 2007;7:900-911. [Cited in This Article: ] |
| 9. | Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol. 2005;42 Suppl:S22-S36. [Cited in This Article: ] |
| 10. | Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67-E70. [Cited in This Article: ] |
| 11. | Yin SS, Wang BE, Wang TL, Jia JD, Qian LX. The effect of Cpd 861 on chronic hepatitis B related fibrosis and early cirrhosis: a randomized, double blind, placebo controlled clinical trial. Zhonghua Ganzangbing Zazhi. 2004;12:467-470. [Cited in This Article: ] |
| 12. | Wang L, Wang J, Wang BE, Xiao PG, Qiao YJ, Tan XH. Effects of herbal compound 861 on human hepatic stellate cell proliferation and activation. World J Gastroenterol. 2004;10:2831-2835. [Cited in This Article: ] |
| 13. | Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142-151. [Cited in This Article: ] |
| 14. | Baroni GS, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [Cited in This Article: ] |
| 15. | Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248-258. [Cited in This Article: ] |
| 16. | Rockey DC, Chung JJ. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 42(4):660-670. [Cited in This Article: ] |
| 17. | Henri S, Chevillard C, Mergani A, Paris P, Gaudart J, Camilla C, Dessein H, Montero F, Elwali NE, Saeed OK. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169:929-936. [Cited in This Article: ] |
| 18. | Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503-512. [Cited in This Article: ] |
| 19. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [Cited in This Article: ] |
| 20. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [Cited in This Article: ] |
| 21. | Benyon RC, Hovell CJ, Da Gaca M, Jones EH, Iredale JP, Arthur MJ. Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology. 1999;30:977-986. [Cited in This Article: ] |
| 22. | Theret N, Lehti K, Musso O, Clement B. MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology. 1999;30:462-468. [Cited in This Article: ] |
| 23. | Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176-184. [Cited in This Article: ] |
| 24. | Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647-1659. [Cited in This Article: ] |
| 25. | Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87-95. [Cited in This Article: ] |
| 26. | Wang BE, Zhao HT. Histopathological evaluation of the therapeutic effect of herbal Cpd 861 on liver fibrosis. Zhonghua Ganzangbing Zazhi. 1997;5:77-78. [Cited in This Article: ] |
| 27. | Wang BE, Wang TL, Jia JD, Ma H, Duan ZP, Li ZM, Li J, Wang AM, Qian LX. Experimental and Clinical Study on Inhibition and Reversion of Liver Fibrosis with Integrated Chinese and Western Medicine. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;5:6-11. [Cited in This Article: ] |
| 28. | Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28-36. [Cited in This Article: ] |
| 29. | Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77-87. [Cited in This Article: ] |
| 30. | Herrmann J, Haas U, Gressner AM, Weiskirchen R. TGF-beta up-regulates serum response factor in activated hepatic stellate cells. Biochim Biophys Acta. 2007;1772:1250-1257. [Cited in This Article: ] |