Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4979
Revised: July 23, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
Alcoholic liver injury comprises of interactions of various intracellular signaling events in the liver. Innate immune responses in the resident Kupffer cells of the liver, oxidative stress-induced activation of hepatocytes, fibrotic events in liver stellate cells and activation of liver sinusoidal endothelial cells all contribute to alcoholic liver injury. The signaling mechanisms associated with alcoholic liver injury vary based on the cell type involved and the extent of alcohol consumption. In this review we will elucidate the oxidative stress and signaling pathways affected by alcohol in hepatocytes and Kupffer cells in the liver by alcohol. The toll-like receptors and their down-stream signaling events that play an important role in alcohol-induced inflammation will be discussed. Alcohol-induced alterations of various intracellular transcription factors such as NFκB, PPARs and AP-1, as well as MAPK kinases in hepatocytes and macrophages leading to induction of target genes that contribute to liver injury will be reviewed. Finally, we will discuss the significance of heat shock proteins as chaperones and their functional regulation in the liver that could provide new mechanistic insights into the contributions of stress-induced signaling mechanisms in alcoholic liver injury.
- Citation: Mandrekar P. Signaling mechanisms in alcoholic liver injury: Role of transcription factors, kinases and heat shock proteins. World J Gastroenterol 2007; 13(37): 4979-4985
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4979.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4979
Alcohol induced liver injury is marked by pathological changes in the liver ranging from steatosis, steatohepatitis to cirrhosis and sometimes hepatocellular carcinoma. The complex pathogenesis of acute and chronic alcohol consumption is multifactorial with diverse consequences in different tissues and cell types. Alcohol consumption leads to elevated endotoxin in the blood and liver leading to activation of immune cells that produce inflammatory mediators (i.e. cytokines). Abnormal cytokine production is a major feature of alcoholic liver disease. Elevated serum concentrations of TNFα, IL-6 and IL-8 have been reported in alcoholic patients and correlated with liver injury and clinical outcome[1]. Among inflammatory cytokines, TNFα is a critical factor in alcoholic liver injury, a hypothesis that has been confirmed in animal models and human studies[2,3]. Resident macrophages/Kupffer cells in the liver increase their production of cytokines in patients with alcoholic liver disease. Cultured monocyte/macrophages from alcoholic hepatitis patients produce TNFα spontaneously that is enhanced further in response to lipopolysaccharide (LPS)[1]. In alcoholic liver injury, studies have shown that it is TNFα that is responsible for hepatocyte killing resulting from increased sensitivity of otherwise resistant cells to TNF-induced killing/apoptosis[4]. It appears that early alcoholic liver injury involves interactions of cytokine over-production due to induction of the “hyper-inflammatory” state in monocytes/macrophages and sensitization of hepatocytes to cell death. The intracellular molecular mechanisms in response to alcohol exposure leading to inflammatory gene expression in the innate immune cell compartment and its down-stream effect on parenchymal cells of the liver are of considerable current interest.
In this article we will review the key components involved in alcohol-induced sensitization to TLR-signaling pathways and the pivotal role of transcription factors and mitogen-activated protein kinases (MAPK) contributing to alcohol-induced inflammation and hepatocyte injury. Furthermore, we will highlight the possible role of stress-induced heat shock proteins as chaperones in alcoholic liver injury.
The currently accepted model of alcoholic liver injury is characterized by increased gut permeability due to prolonged alcohol consumption resulting in increased endotoxin levels in portal circulation[5]. Endotoxin is recognized by the resident macrophages/Kupffer cells in the liver via the toll-like receptor-4 (TLR4) leading to activation of intracellular signaling pathways in the macrophages and production of pro-inflammatory cytokines (TNFα, IL-1), chemokines (IL-8, MCP-1) and TGFβ[6,7]. Kupffer cell-derived mediators then activate the other cell types in the liver resulting in damage. Chemokines recruit polymorphonuclear neutrophils and other inflammatory cells such as macrophages and T cells which contribute to amplification of the inflammatory response. Hepatocytes undergoing oxidative stress due to ROS generation and CYP2E1 induction are sensitized to TNFα induced apoptosis and necrosis[3,8]. Furthermore, mediators such as TGFβ and LPS activate stellate cells to proliferate and produce collagen leading to fibrosis and progression of liver injury[9]. Alcohol-related injury of liver sinusoidal endothelial cells and their role in progression of disease has not been well studied. Some studies suggest that liver sinusoidal endothelial cells can be activated to produce cytokines and chemokines by malondialdehyde-acetaldehyde adduct proteins, generated by alcohol metabolism and thus could further contribute to amplification of alcohol-induced inflammation[10]. Collectively, alcoholic liver injury involves various liver cell types during progression of disease.
Chronic alcohol induced inflammatory responses in the liver are thought to be central to alcoholic liver injury. Excessive generation of reactive oxygen species (ROS) called free radicals plays an important role in alcohol-induced cellular damage[8]. A number of studies have shown that alcohol increases generation of ROS in vitro[11-13]. However, to determine the in vivo generation of ROS by alcohol has been rather challenging. Nevertheless, ROS-induced cellular responses are critical in alcohol-induced inflammation as well as TNF-induced hepatocyte killing[2,13]. The ROS-related intracellular mechanisms leading to the sensitization of cellular injury by alcohol are underway in various laboratories. In the liver, Kupffer cells produce ROS in response to chronic alcohol exposure as well as endotoxin[13]. Recent evidence shows that direct interaction of NADPH oxidase isozyme 4 with TLR4 is involved in LPS-mediated ROS generation and NFκB activation[14]. In alcohol fed rats, pretreatment with diphenyliodonium (DPI), which inhibits NADPH oxidase and normalizes ROS production, decreased LPS-induced ERK1/2 phosphorylation and inhibited increased TNFα production in Kupffer cells[13]. Thurman and his group have shown that p47 phox-/-mice are resistant to alcohol-induced liver injury, indicating an important role for NADPH oxidase in not only inflammatory responses but also liver injury[15]. Furthermore, dilinoleoylphosphotidylcholine (DPC) also prevented LPS induced NFκB and ERK1/2 activation and TNFα production in Kupffer cells of chronic alcohol fed rats[16]. It is now widely accepted that ROS not only plays a critical role in direct hepatocyte injury but also contributes to increased inflammatory responses further enhancing liver injury. Hence, the mechanisms affecting interaction of ROS and inflammatory responses as well as alcohol-induced sensitization mechanisms leading to hepatocyte death by alcohol need further elucidation.
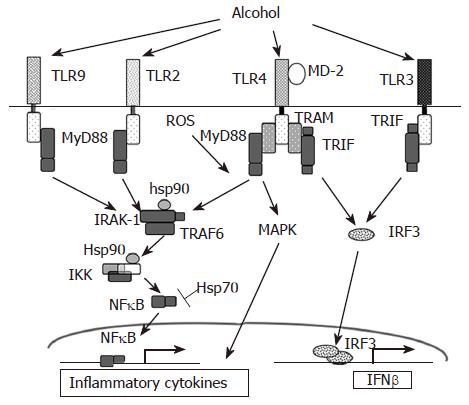
A major role for TLR mediated signaling, via endotoxin, in alcoholic liver disease (ALD) (Figure 1) was established by studies of Thurman and colleagues[7,17]. Innate immune responses activated via the Kupffer cells, the primary effector cell in the liver, play a key role in the early pathogenesis of alcohol-induced liver injury[18]. Increased levels of circulating lipopolysaccharide (LPS) in alcoholic patients have been shown[19]. The currently accepted model of alcoholic liver injury elucidates that LPS promotes hepatic injury via induction of Kupffer cell activation resulting in production of TNFα and other inflammatory mediators. The Kupffer cells respond to stimulation by gut-derived endotoxins and apoptotic dead cells in the tissue resulting in increased inflammatory responses. Studies in knock out mouse models have shown that chronic alcohol feeding in CD14, TLR4 and LPS-binding protein (LBP) deficient mice results in alleviation of alcohol-induced liver injury indicating an important role for the TLR4 pathway[7,20]. Furthermore, LPS recognition by TLR4 expressed on hepatic stellate cells and sinusoidal epithelial cells may also contribute to the progression of ALD[21,22].
Circulating TNFα is increased in chronic alcoholics as well as in mouse chronic alcohol feeding models[1,23]. Alcohol sensitizes Kupffer cells and monocytes/macrophages to produce increased TNFα in response to endotoxin[24]. Although studies on effects of alcohol on membrane proximal events using mutant and knock out mice have shown an important role for CD14[20] and TLR4[7], recent studies show hepatic expression of TLR2 or TLR4 mRNA was not changed by chronic alcohol feeding or by acute alcohol administration[25]. Upon activation of TLR4, IRAK is recruited to the TLR4 complex via interaction with MyD88 (Figure 1). Acute and chronic alcohol exposure affects activation and recruitment of the IRAK-1 and IKK kinase activation[28] Mandrekar et al[26-28] unpublished. In contrast to chronic alcohol consumption, acute alcohol exposure inhibits TLR4 signaling in monocytes and macrophages after in vitro as well as in vivo alcohol treatment in mice leading to decreased LPS-induced TNFα production. Acute alcohol administration also suppressed TLR3 downstream signaling[29]. In vitro acute alcohol exposure of human monocytes or macrophages suppresses LPS-induced IRAK-1 phosphorylation[28] and inhibits poly I:C induced IRAK-1 degradation[29] (Figure 1). Furthermore, acute alcohol exposure of murine macrophages inhibits TLR2, TLR4 and TLR9 ligand-induced IL-6 and TNFα production[30]. Thus, it is evident that TLR-associated molecules such as CD14, TLR4 and LPS-binding protein (LBP) as well as their intra-cytoplasmic mediators IL-1 receptor associated kinases (IRAKs), IκB-kinase complex (IKK) and NFκB are altered by alcohol and can contribute to alcoholic liver injury.
In response to alcohol exposure, multiple signal transduction pathways are activated by different receptors such as TLRs, TNFα, etc. in various liver cell types culminating in nuclear events involving binding of transcription factors to the promoter elements of target genes. The progression of alcoholic liver disease is characterized by initial appearance of fatty liver and inflammation, necrosis and apoptosis followed by fibrosis. It is generally accepted that the molecular mechanisms regulating the different stages of alcoholic liver disease from fatty liver and inflammation to fibrosis and cirrhosis are diverse. Since early alcoholic liver injury, a reversible condition, which comprises of development of fatty liver and inflammation, research on intracellular mechanisms has been focused primarily on these early stages. Chronic alcohol exposure increases expression of genes regulating fatty acid synthesis and suppresses genes involved in fatty acid oxidation resulting in increased fatty acid accumulation or steatosis. Transcription factors regulating fatty acid metabolism including sterol regulatory element binding protein (SREBP) and peroxisomal proliferating factor α (PPARα) that is involved in fatty acid oxidation play a pivotal role in early alcoholic liver injury. Liver specific overexpression of SREBP1α or SREBP1c results in fatty liver with significantly increased hepatic triglyceride content[31]. In hepatoma cultures and ethanol-consuming mice, SREBP mRNA and active SREBP1 protein levels were significantly increased[32,33] and accompanied by hepatic triglyceride accumulation. PPARα, an essential regulatory factor up-regulating fatty acid oxidation also plays an important role in alcoholic fatty liver induction. French and colleagues reported that chronic alcohol feeding decreased PPARα mRNA[34]. Further, exposure of primary hepatocytes and hepatoma cells to chronic alcohol resulted in impaired PPARα DNA binding activity that was prevented by 4-methylpyrazole, an inhibitor of alcohol dehydrogenase[35]. Although chronic alcohol decreased PPARα activity, treatment of mice with WY14643, a PPARα agonist restored DNA binding activity without affecting quantities of PPARα, indicating additional mechanisms affected by alcohol to regulate fatty acid oxidation.
Similar to the effects of chronic alcohol on transcription factors involved in lipid homeostasis, alcohol-induced inflammatory mediators that play an important role in disease progression, are induced by key transcription factors. The most well studied example is the activation of NFκB in monocytes and macrophages controlling pro-inflammatory cytokine induction. Studies have shown increased LPS-induced NFκB DNA binding activity in monocytes of patients with alcoholic hepatitis compared to controls[36]. While chronic alcohol exposure increases LPS-induced NFκB binding in monocytes and macrophages, acute alcohol exposure decreases LPS-induced NFκB binding resulting in different regulation of pro-inflammatory cytokine genes based on duration of alcohol exposure. While several animal models have shown increased hepatic LPS-induced NFκB DNA binding activity, some studies have failed to observe any effect of chronic alcohol feeding on NFκB activity[37-39]. Many studies have described a positive effect of ROS on NFκB regulation[38,40,41]. Recent studies have shown that ROS production due to activation of the NADPH oxidase system and interaction with adapters of the TLR4 signaling pathway influence NFκB DNA binding and promote pro-inflammatory cytokine production[14]. It is likely that chronic alcohol exposure may activate NFκB via ROS-dependent mechanisms since treatment of liver macrophages with dilinoleoylphosphotidylcholine (DPC) protects liver injury and prevents NFκB activation[16].
Activator protein-1 (AP-1) another transcription factor is also regulated by acute and chronic alcohol exposure in monocyte/macrophages and hepatocytes. Acute and chronic alcohol exposure increases AP-1 DNA binding activity[28,42,43] in monocytes/macrophages whereas isolated Kupffer cells do not show any effect on AP-1 activity after chronic ethanol feeding[44]. Furthermore, chronic alcohol increases AP-1 expression and induces activation in livers of chronic alcohol fed mice and in isolated primary hepatocytes[42,45]. Increased activation of AP-1 could influence pro-inflammatory and anti-inflammatory cytokine gene induction and hence could contribute to the amplification of the inflammatory response after chronic alcohol exposure. Since AP-1 regulates collagen synthesis, increased AP-1 activation could also be implicated in alcohol-induced fibrotic changes in the liver[46].
PPARγ, another transcription factor known to inhibit inflammatory responses is also regulated by chronic alcohol exposure in macrophages. PPARγ expression was increased in Kupffer cells and hepatocytes during chronic alcohol exposure[47]. Treatment with PPARγ agonists prevented development of chronic alcohol induced steatosis and inflammation[48]. The exact mechanism by which PPARγ exerts its effect to resolve alcohol-induced liver injury remains to be studied.
Early growth response factor-1 (Egr-1), a zinc finger transcription factor induced in response to environ-mental stress and shown to regulate cellular growth and proliferation is up-regulated during chronic alcohol exposure in Kupffer cells[44,49]. Increased LPS-stimulated Egr-1 expression is dependent on ERK1/2 activation in Kupffer cells of chronic alcohol fed mice compared to pair-fed controls[44]. Furthermore, recent data show that chronic alcohol feeding induced liver injury is blocked in Egr-1 knock out mice, indicating a role for the ERK1/2-Egr-1 pathway in the pathogenesis of alcoholic liver injury[50]. These studies illustrate that based on the cell type involved and duration of alcohol exposure, regulation of transcription factors is highly complex and requires further evaluation. Future investigations on regulation of transcription factors during the different stages of alcoholic liver injury will aid in designing effective therapeutic strategies.
LPS recognition also activates MAPK family members including extracellular receptor activated kinases 1/2 (ERK1/2), p38 and c-jun-N-terminal kinase (JNK) resulting in TNFα production[51]. Chronic alcohol increases LPS-induced ERK1/2 activation which contributes to TNFα expression in macrophages[44]. Similarly, LPS stimulation of Kupffer cells exposed to chronic alcohol showed increased p38 activity whereas decreased JNK activity was observed in livers after chronic alcohol feeding[39]. Activation of p38 MAPK by LPS has been shown to contribute to TNFα mRNA stability via interaction with tristetraprolin (TTP)[52]. Inhibition of p38 activation completely abrogated alcohol-mediated stabilization of TNFα mRNA[39]. On the other hand, ERK1/2 inhibition did not affect TNFα mRNA stability but affected its transcription[44]. LPS stimulation of JNK leads to phosphorylation of c-jun and subsequent binding of c-jun to the CRE/AP-1 site in the TNFα promoter[51]. Although chronic alcohol feeding decreased JNK activity without any effect on TNFα mRNA, acute alcohol exposure increased JNK phosphorylation as well as AP-1 binding in the presence of combined TLR4 plus TLR2 stimulation[28] in human monocytes. Furthermore, LPS-induced ERK1/2 phosphorylation was decreased in acute alcohol exposed monocytes[28], whereas p38 MAPK activity was increased contributing to anti-inflammatory mediators such as IL-10 after acute alcohol exposure in monocytes[43]. Increased oxidative stress in chronic alcohol exposed rats promotes hepatocyte apoptosis and necrosis and is implicated in the alcohol-induced sensitization to the pro-apoptotic action of TNFα[2]. Besides modulation of MAPK activity in macrophages, potentiation of alcohol induced hepatocyte death has been attributed to increased mitochondrial permeability transition and caspase-3 activation in hepatocytes and depends on p38 MAPK activation but is independent of caspase-8[4,53].
Mammalian heat shock proteins (hsps) induced in response to cellular oxidative stress serves as chaperones in refolding, disaggregation and degradation of damaged polypeptides[54,55]. Amongst the family of heat shock proteins, Hsp70, Hsp60, Hsp90 and Hsp32 (also termed HO-1) have been implicated in protective mechanisms against increased oxidative stress in liver injuries. Upregulation of hsps in liver cells in culture has been shown to diminish the toxicity of a number of hepatotoxicants. Immunohistochemical detection revealed elevated Hsp70 in livers of alcoholic patients[56]. Male Wistar rats fed with acute as well as chronic ethanol for 12 wk showed induction of Hsp70 in various regions of the brain and to a small extent in the liver[57,58]. However, the intensity of induction of Hsp70 in the liver, the principal organ of ethanol oxidation was much less pronounced than the hippocampus or striatal areas of the brain[57,58]. Recent studies also reveal that acutely and chronically ethanol-treated primary astrocyte cultures showed increased Hsp70 expression at 50 mmol/L, but not at 200 mmol/L ethanol concentration suggesting that severe toxicity of a 200 mmol/L ethanol concentration seems to exceed the power of inducible protective mechanisms elicited by heat shock proteins in astrocytes[59]. The mechanism by which Hsp70 exerts its protective role is not clear. Oxidative stress due to depletion of glutathione (GSH) and induction of Hsp70 has been closely linked[60]. Antisense-Hsp70 experiments in rat astrocyte cultures resulted in moderate oxidative damage in control astrocytes and a consequent drastic decrease in the viability of ethanol-treated cells, with mitochondrial functionality being affected[59]. Thus, heat shock proteins confer a survival advantage to the cells preventing oxidative damage. Hsp70 induction using geranylgeranylacetone showed subsequent inhibition of alcohol-induced apoptosis of hepatocytes[61]. Induction of hsp72 by hyperthermia pre-conditioning increased hsp70 and reduced TNFα responses in CCl4-induced cirrhotic rats[62]. Use of drugs that elevate intracellular hsp70 may serve to exert a cytoprotective effect in alcoholic liver disease. In addition to its role in cytoprotection, hsp70 also inhibits inflammatory responses in immune cells[63]. Hsp70 interacts with various components of the NFκB signaling pathway (Figure 1). Overexpression of Hsp70 leads to repression of NFκB mediated gene expression[63]. Further, nuclear translocation of NFκB is also affected in cells transfected with the Hsp70 gene[64]. Hsp70-induced NFκB inhibition is attributed to both increased IκBα expression and attenuated IκBα degradation[65]. In addition, Hsp70 can directly interact with NFκB p65 and NFκB p50 to influence NFκB mediated responses[66].
Hsp90 also plays an important role in regulating the TLR signaling pathway and can thus influence inflammatory responses (Figure 1) by chaperoning key signaling molecules. Inhibition of Hsp90 impairs function of its client signaling proteins and thus alters cell function[67]. Treatment of cells with inhibitors of Hsp90 such as the benzoquinone ansamycin, geldanamycin activates a heat shock response without the stress[68]. Geldanamycin also inhibits LPS-induced NFκB activity and TNFα production in macrophages[69,70]. Hsp90 is crucial for biogenesis and activity of IKKα and IKKβ, kinases responsible in IκBα phosphorylation and activation of NFκB, and inhibition of Hsp90 results in IKKα and IKKβ depletion[71]. Thus Hsp90 can play a crucial role in maintaining the activity of various components of the NFκB signaling pathway. In alcoholic liver disease, ethanol induced oxidative stress using the intragastric feeding model induces thiol modification of Hsp90 in the liver[72]. Whether the thiol modification of Hsp90 contributes to progression of alcoholic liver disease needs further evaluation. Studies have shown that chronic alcohol exposure modulates endothelial cell function by increasing NO production via PI3 K-dependent up-regulation of eNOS and its interaction with Hsp90[73]. Furthermore, Hsp90 and Hsp70 have been shown to form insoluble aggregates with cytokeratins to form Mallory bodies[74] in alcoholic liver disease. Selective enhancement of cytochrome P450 activity in rat hepatocytes can be achieved by heat shock treatment[75]. Alcohol inducible cytochrome P450 2E1 (CYP2E1) is also shown to be a Hsp90 “client” protein and regulation of Hsp90 can profoundly affect enzyme turnover[76]. Thus, taken together, it is tempting to speculate that chaperoning activity of Hsp90 and Hsp70 could be modulated using pharmacological inhibitors to alleviate alcohol-induced oxidative stress and inflammation and reversal of liver injury.
In conclusion, the biological effects of acute and chronic alcohol exposure and the net result on intracellular signaling pathways in liver cell types are complex. It is evident that the innate immune response plays a significant role in the development of alcoholic liver injury. A wealth of information is available on the contribution of pro-inflammatory responses in alcohol-induced liver damage. Studies on interactions of various signaling mechanisms in the different cell types of the liver and their outcomes in the liver microenvironment will provide a better understanding of the pathogenesis of alcoholic liver disease. Future studies performed to delineate novel molecular pathways are necessary and will provide a better understanding of liver injury induced by chronic alcohol consumption.
S- Editor Ma N L-Editor Alpini GD E- Editor Yin DH
| 1. | McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 389] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S-171S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [PubMed] [Cited in This Article: ] |
| 7. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Zima T, Kalousová M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:110S-115S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21 Suppl 3:S84-S87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Dicker E, Cederbaum AI. Increased NADH-dependent production of reactive oxygen intermediates by microsomes after chronic ethanol consumption: comparisons with NADPH. Arch Biochem Biophys. 1992;293:274-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589-3593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 403] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases acetaldehyde-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats. Biochem Biophys Res Commun. 2002;299:459-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, Thurman RG. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310-G314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S-54S. [PubMed] [Cited in This Article: ] |
| 20. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 22. | Deaciuc IV, Spitzer JJ. Hepatic sinusoidal endothelial cell in alcoholemia and endotoxemia. Alcohol Clin Exp Res. 1996;20:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 317] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood). 2003;228:882-890. [PubMed] [Cited in This Article: ] |
| 25. | Romics L, Mandrekar P, Kodys K, Velayudham A, Drechsler Y, Dolganiuc A, Szabo G. Increased lipopolysaccharide sensitivity in alcoholic fatty livers is independent of leptin deficiency and toll-like receptor 4 (TLR4) or TLR2 mRNA expression. Alcohol Clin Exp Res. 2005;29:1018-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Romics L, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, Szabo G. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. Hepatology. 2004;40:376-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26:1609-1614. [PubMed] [Cited in This Article: ] |
| 28. | Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628-7635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004;173:2715-2724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3203] [Cited by in F6Publishing: 3192] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 32. | You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem. 2002;277:29342-29347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 393] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 33. | Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 424] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 34. | Wan YJ, Morimoto M, Thurman RG, Bojes HK, French SW. Expression of the peroxisome proliferator-activated receptor gene is decreased in experimental alcoholic liver disease. Life Sci. 1995;56:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Uesugi T, Froh M, Arteel GE, Bradford BU, Gäbele E, Wheeler MD, Thurman RG. Delivery of IkappaB superrepressor gene with adenovirus reduces early alcohol-induced liver injury in rats. Hepatology. 2001;34:1149-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Jokelainen K, Reinke LA, Nanji AA. Nf-kappab activation is associated with free radical generation and endotoxemia and precedes pathological liver injury in experimental alcoholic liver disease. Cytokine. 2001;16:36-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930-41937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Hill DB, Devalaraja R, Joshi-Barve S, Barve S, McClain CJ. Antioxidants attenuate nuclear factor-kappa B activation and tumor necrosis factor-alpha production in alcoholic hepatitis patient monocytes and rat Kupffer cells, in vitro. Clin Biochem. 1999;32:563-570. [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Wang XD, Liu C, Chung J, Stickel F, Seitz HK, Russell RM. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol. 2006;177:2592-2600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6-G15. [PubMed] [Cited in This Article: ] |
| 45. | Román J, Colell A, Blasco C, Caballeria J, Parés A, Rodés J, Fernández-Checa JC. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kappaB. Hepatology. 1999;30:1473-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Armendariz-Borunda J, Simkevich CP, Roy N, Raghow R, Kang AH, Seyer JM. Activation of Ito cells involves regulation of AP-1 binding proteins and induction of type I collagen gene expression. Biochem J. 1994;304:817-824. [PubMed] [Cited in This Article: ] |
| 47. | Boelsterli UA, Bedoucha M. Toxicological consequences of altered peroxisome proliferator-activated receptor gamma (PPARgamma) expression in the liver: insights from models of obesity and type 2 diabetes. Biochem Pharmacol. 2002;63:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KI, Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003;306:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002;277:14777-14785. [PubMed] [Cited in This Article: ] |
| 50. | McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8-26. [PubMed] [Cited in This Article: ] |
| 52. | Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461-6469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 53. | Pastorino JG, Shulga N, Hoek JB. TNF-alpha-induced cell death in ethanol-exposed cells depends on p38 MAPK signaling but is independent of Bid and caspase-8. Am J Physiol Gastrointest Liver Physiol. 2003;285:G503-G516. [PubMed] [Cited in This Article: ] |
| 54. | Jäättelä M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 352] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2714] [Cited by in F6Publishing: 2543] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 56. | Omar R, Pappolla M, Saran B. Immunocytochemical detection of the 70-kd heat shock protein in alcoholic liver disease. Arch Pathol Lab Med. 1990;114:589-592. [PubMed] [Cited in This Article: ] |
| 57. | Calabrese V, Renis M, Calderone A, Russo A, Barcellona ML, Rizza V. Stress proteins and SH-groups in oxidant-induced cell damage after acute ethanol administration in rat. Free Radic Biol Med. 1996;20:391-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Calabrese V, Renis M, Calderone A, Russo A, Reale S, Barcellona ML, Rizza V. Stress proteins and SH-groups in oxidant-induced cellular injury after chronic ethanol administration in rat. Free Radic Biol Med. 1998;24:1159-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Russo A, Palumbo M, Scifo C, Cardile V, Barcellona ML, Renis M. Ethanol-induced oxidative stress in rat astrocytes: role of HSP70. Cell Biol Toxicol. 2001;17:153-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Antiapoptotic response to induced GSH depletion: involvement of heat shock proteins and NF-kappaB activation. Antioxid Redox Signal. 2005;7:446-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Ikeyama S, Kusumoto K, Miyake H, Rokutan K, Tashiro S. A non-toxic heat shock protein 70 inducer, geranylgeranylacetone, suppresses apoptosis of cultured rat hepatocytes caused by hydrogen peroxide and ethanol. J Hepatol. 2001;35:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Mikami K, Otaka M, Goto T, Miura K, Ohshima S, Yoneyama K, Lin JG, Watanabe D, Segawa D, Kataoka E. Induction of a 72-kDa heat shock protein and protection against lipopolysaccharide-induced liver injury in cirrhotic rats. J Gastroenterol Hepatol. 2004;19:884-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Calderwood SK. Regulatory interfaces between the stress protein response and other gene expression programs in the cell. Methods. 2005;35:139-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996;271:17724-17732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 221] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 65. | Wong HR, Ryan M, Wispé JR. Stress response decreases NF-kappaB nuclear translocation and increases I-kappaBalpha expression in A549 cells. J Clin Invest. 1997;99:2423-2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 67. | Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Duncan RF. Inhibition of Hsp90 function delays and impairs recovery from heat shock. FEBS J. 2005;272:5244-5256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Byrd CA, Bornmann W, Erdjument-Bromage H, Tempst P, Pavletich N, Rosen N, Nathan CF, Ding A. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1999;96:5645-5650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Chakravortty D, Kato Y, Sugiyama T, Koide N, Mu MM, Yoshida T, Yokochi T. The inhibitory action of sodium arsenite on lipopolysaccharide-induced nitric oxide production in RAW 267.4 macrophage cells: a role of Raf-1 in lipopolysaccharide signaling. J Immunol. 2001;166:2011-2017. [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23:5378-5386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Polikandriotis JA, Rupnow HL, Hart CM. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase-and hsp90-dependent mechanisms. Alcohol Clin Exp Res. 2005;29:1932-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Riley NE, Li J, McPhaul LW, Bardag-Gorce F, Lue YH, French SW. Heat shock proteins are present in mallory bodies (cytokeratin aggresomes) in human liver biopsy specimens. Exp Mol Pathol. 2003;74:168-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Rajagopalan P, Berthiaume F, Tilles AW, Toner M, Yarmush ML. Selective enhancement of cytochrome p-450 activity in rat hepatocytes by in vitro heat shock. Tissue Eng. 2005;11:1527-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Morishima Y, Peng HM, Lin HL, Hollenberg PF, Sunahara RK, Osawa Y, Pratt WB. Regulation of cytochrome P450 2E1 by heat shock protein 90-dependent stabilization and CHIP-dependent proteasomal degradation. Biochemistry. 2005;44:16333-16340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |