Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4699
Revised: April 23, 2007
Accepted: April 25, 2007
Published online: September 21, 2007
There are several cofactors which affect body iron metabolism and accelerate iron overload. Alcohol and hepatic viral infections are the most typical examples for clarifying the role of cofactors in iron overload. In these conditions, iron is deposited in hepatocytes and Kupffer cells and reactive oxygen species (ROS) produced through Fenton reaction have key role to facilitate cellular uptake of transferrin-bound iron. Furthermore, hepcidin, antimicrobial peptide produced mainly in the liver is also responsible for intestinal iron absorption and reticuloendothelial iron release. In patients with ceruloplasmin deficiency, anemia and secondary iron overload in liver and neurodegeneration are reported. Furthermore, there is accumulating evidence that fatty acid accumulation without alcohol and obesity itself modifies iron overload states. Ineffective erythropoiesis is also an important factor to accelerate iron overload, which is associated with diseases such as thalassemia and myelodysplastic syndrome. When this condition persists, the dietary iron absorption is increased due to the increment of bone marrow erythropoiesis and tissue iron overload will thereafter occurs. In porphyria cutanea tarda, iron is secondarily accumulated in the liver.
- Citation: Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Iron overload and cofactors with special reference to alcohol, hepatitis C virus infection and steatosis/insulin resistance. World J Gastroenterol 2007; 13(35): 4699-4706
- URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4699
In hereditary hemochromatosis, patients having HFE trait are more susceptible to iron overload when cofactors such as alcohol, hepatitis viruses, and abnormal porphyrin metabolism are present. Even in the absence of hereditary hemochromatosis, there are several conditions associated with secondary iron overload in which iron deposition is rather mild[1]. For example, in alcoholics and patients with chronic hepatitis C, intrahepatic iron is increased and liver injury is accelerated, followed by development of fibrosis, cirrhosis and hepatocellular carcinoma (HCC). In addition, abnormal copper metabolism and several causes for iron-loaded anemia are also important cofactors which influence the background iron overload. Furthermore, there is accumulating evidences that fatty acid accumulation without alcohol and obesity itself modifies insulin resistance through iron[2] and fibrogenesis of the liver[3]. In this review, the role of cofactors on iron overload will be discussed in three categories such as alcohol, hepatitis C virus infection and steatosis with obesity, the most common cofactors in liver iron overload.
There are several factors which affect body iron metabolism and accelerates iron overload. Table 1 lists cofactors and disease conditions which are known to accelerate hepatic iron accumulation independent from responsible genes for hereditary hemochromatosis. Alcoholic and hepatic viral infections are the most typical examples for clarifying the role of cofactors in iron overload. In addition, abnormal copper metabolism and several causes for iron-loaded anemia such as thalassemia and myelodysplastic syndrome are also important factors which influence the background iron overload. When this condition persists, the dietary iron absorption is increased due to the increment of bone marrow erythropoiesis[4] and tissue iron overload will occur thereafter. These patients are usually anemic in spite of increased body iron stores (iron-loaded anemia), and require frequent blood transfusions, which further exaggerate secondary iron overload, in which conditions of new oral iron chelators are effective[5]. In patients with ceruloplasmin deficiency, anemia and secondary iron overload in liver and neurodegeneration are reported[6]. Furthermore, there are accumulating evidences that fatty acid accumulation without alcohol and obesity itself modifies iron overload states. Ineffective erythropoiesis is also an important factor to accelerate iron overload. This condition is associated with diseases such as thalassemia, aplastic anemia, and myelodysplastic syndrome. In porphyria cutanea tarda, iron is secondarily accumulated in the liver and phlebotomy and oral iron chelators are effective as well as in hemochromatosis.
| 1 Alcohol (Alcoholic liver disease) |
| 2 Infection (Hepatitis C virus infection, etc) |
| 3 Obesity and insulin resistance (Nonalcoholic steatohepatitis) |
| 4 Copper (Ceruloplasmin deficiency) |
| 5 Porphyrin (Porphyria) |
| 6 Ineffective erythropoiesis (Thalassemia, myelodysplastic syndrome) |
| 7 Others |
Alcohol is one of the most important cofactors to modify or enhance iron accumulation in the liver. Excess intake of alcohol induces alcoholic liver diseases (ALD) such as fatty liver, fibrosis, hepatitis, and cirrhosis, in which iron overload is frequently associated[7]. By Perls’ iron stain, excess iron accumulation was found in hepatic tissues with ALD, but not in any normal hepatic tissues[8]. In ALD, iron is deposited in both hepatocytes and reticuloendothelial (Kupffer) cells. In advanced cases of ALD, which is also called as “alcoholic siderosis”, the reticuloendothelial iron deposition is dominant. In earlier stages of ALD such as fatty liver and fibrosis, iron deposition is mild and is preferentially present in hepatocytes rather than in Kupffer cells, which finding is more frequently observed in Japanese patients who have mild clinical phenotype comparing with those in US[9].
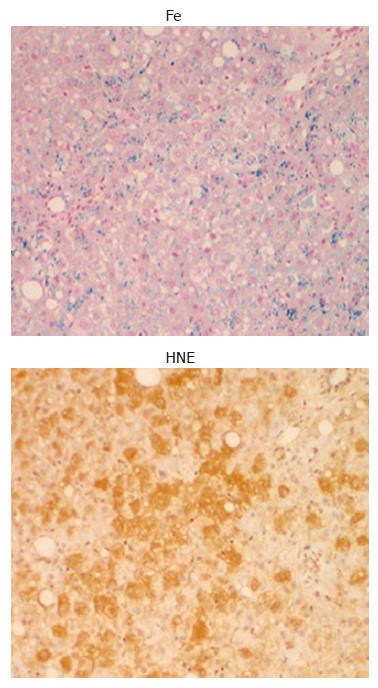
The reactive oxygen species (ROS) produced play an important role in the development of ALD[10]. The expression of 4-hydroxy-2-nonenal (HNE)-protein adducts, which is a lipid peroxidative product is increased in oxidized hepatocytes[11]. Chronic alcohol ingestion in experimental animals is associated with oxidative stress as reflected by increased hepatic levels of lipid peroxidation products such as malondialdehyde and HNE, both of which have been implicated in hepatic fibrogenesis in the intragastric ethanol infusion model[12]. Furthermore, lipid peroxidation products induce gene expression of procollagen α-1 (I) and increase collagen production by several folds in cultured hepatic stellate cell[13]. In human ALD, there is a positive correlation between iron deposition and histological intensity of HNE-protein adduct[14]. As shown in Figure 1, the distribution of HNE-protein adducts and iron granules appeared to be identical, suggesting that iron may be associated with the production of HNE-protein adduct. As hepatic iron is visualized by Perls’ reaction as an insoluble protein-bound iron such as hemosiderin, this form of iron may be inactive for the production of ROS. But, the free iron responsible for Fenton reaction should be present close to the protein-bound iron, and may be involved in the production of HNE-protein adducts. There are two pathways to generate ROS through ethanol metabolism. Oxidation of ethanol by alcohol dehydrogenase to form acetaldehyde, which is subsequently oxidized to acetate and ultimately carbon dioxide and water. During the oxidation process of acetaldehyde involving aldehyde oxidase and xanthine oxidase, superoxide (O2-) is produced[15]. In addition, cytochrome P450 is involved in the metabolism of ethanol, in which ROS are also generated in microsomes[16]. Among ROS, hydroxy radical (OH-) is most potent, which is produced via Fenton reaction in the presence of free iron and the resulted OH- can easily cause cell damage by oxidizing lipid, proteins, and nucleic acids. In an intragastric infusion mouse model of ALD, supplementation of carbonyl iron advanced peri-venular fibrosis to bridging fibrosis and cirrhosis[17]. Oxidative stress arising from hepatocytes and macrophage activates hepatic stellate cells by increasing the production of cytokines such as transforming growth factor-β (TGFβ), directly or indirectly. The dietary iron supplementation was associated with increased NF-κB activation[18], and the up regulation of NF-κB responsive proinflammatory genes such as IL-1β, TNFα, and MIP-1[19].
In advanced cases of ALD, iron is accumulated more prominently in Kupffer cells than in hepatocytes, mainly due to repeated endotoxemia and hyper-cytokinemia of TNFα and IL-1β[20]. These cytokines induced hepatic uptake of transferrin iron in vitro[21] and in vivo[22]. In mild cases of ALD, iron is preferentially stained in hepatocytes, rather than in Kupffer cells, suggesting that hepatocyte is the main site of early iron storage in the liver. However, it is not clear why iron is accumulated in liver parenchymal cells of alcoholics in such conditions. Two possibilities can be drawn: one is the increased uptake of iron in hepatocytes, and another is the increased iron absorption through hepcidin, which is a newly found antimicrobial peptide, and is a negative regulator of iron absorption and reticuloendothelial iron releases[23]. Hepatocytes have several pathways for iron uptake. Iron in serum is usually bound to transferrin and iron-bound transferrin is taken up via transferrin receptor (TfR) with high affinity or via other unknown mechanism with greater capacity, but low affinity independent of high affinity receptor[24]. There are two molecules of transferrin receptor: transferrin receptor 1 (TfR1) and transferrin receptor 2 (TfR2). TfR1 has a high affinity to serum transferrin and considered to be functional, while the function of TfR2 is not clear yet, even though the TfR2 gene is responsible for genetic hemochromatosis[25]. In normal hepatocytes, TfR2 is constitutively expressed. But, TfR1 is down-regulated, suggesting that TfR1 does not contribute to the steady state hepatic iron uptake. Recently, Wallace et al[26] reported that homozygous TfR2 knockout mice had no TfR2 associated with typical iron overload, and there was no upregulation of hepcidin mRNA, suggesting that TfR2 is required to iron regulated expression and is involved in a pathway to HFE and hemojuvelin. In addition, DMT1 may be involved when serum iron concentration exceeds transferrin iron binding capacity[27]. It is noteworthy that TfR1 is regulated by cellular iron levels or oxidative stresses post-transcriptionally and it is possible that ethanol may augment TfR1 expression by producing oxidative stresses. According to immunohistochemical investigation, TfR1 expression was increased in hepatocytes in 80% of hepatic tissues with ALD, but was not detected in any normal hepatic tissues[28]. It is noteworthy that the mean duration of abstinence of patients who demonstrated positive TfR1 expression in hepatocytes was significantly shorter than that of patients who demonstrated negative TfR1 expression.
Ethanol exposure in the presence of iron to the primary cultured-hepatocytes demonstrated an increase of TfR expression, and this augmentation was suppressed by the inhibitor of alcohol dehydrogenase, 4-methyo pyrazole, but enhanced by a inhibitor of acetaldehyde dehydrogenase, cyanamide, suggesting that ethanol metabolite acetaldehyde itself is involved for the induction of TfR1 by ethanol[29]. By functional uptake assay using 59Fe-transferrin, the additional ethanol exposure increased transferrin-iron uptake into hepatocytes, while non-transferrin-bound iron (NTBI) uptake[30] was not increased. It has been reported that TfR1 expression was up-regulated both transcriptionally[31] and posttranscriptionally[32]. This regulation is induced either by iron deficiency state or oxidative stress such as H2O2 and nitric oxide via iron regulatory protein, IRP[33]. In addition to the direct cell toxicity, acetaldehyde produces free radicals[34] and free radicals modify IRP activity[35,36].
Body iron homeostasis is strictly regulated by a balance between the processes such as dietary iron absorption in enterocytes, iron transport by transferrin in circulation, iron utilization and storage in bone marrow and liver. The increase of intestinal iron absorption was one of the mechanisms of the hepatic iron deposition in alcoholics[37]. In patients with hereditary hemochromatosis, serum pro-hepcidin is lower than that of normal controls, suggesting that iron absorption is increased in spite of high iron storage[38]. It is speculated that down-regulation of hepcidin might be one of important factors for pathogenesis of iron overload in ALD[39]. Serum pro-hepcidin concentration in ALD was significantly lower than that in healthy subjects, and pro-hepcidin/ferritin ratios in ALD were lower than healthy subjects[40]. In the ethanol-loaded mouse model which has a mild steatotic change, the hepcidin mRNA and protein expression were significantly lower than that of control. In addition, alcohol-loading might disrupt the sensing signal of inflammatory cytokines, and then down-regulate hepcidin expression, following the increased iron absorption from small intestine. Recently, the mechanism of hepcidin downregulation by alcohol has been elucidated: a decreased hepcidin expression in mouse liver is accompanied with an increase of DMT1 and ferroportin1, and a decrease of hepcidin promoter activity and DNA-binding activity of CCAAT/enhancer-binding, protein α (C/EBPα)[41].
Hepatitis C virus infection is one of the most common disorders in liver diseases involving chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). Table 2 summarizes the effect of iron on hepatitis C virus infection. In the Third National Health and Nutrition Examination Survey, HCV infection is significantly associated with higher serum levels of ferritin and iron in the US population[42]. The mean serum levels of ferritin and iron were significantly higher among subjects with HCV infection than among subjects without liver disease[43]. In addition, serum ferritin levels were directly and significantly correlated with serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase, whereas platelet counts were inversely correlated with serum ferritin. It is also found that lipid peroxidative products such as malondialdehyde are increased in hepatic tissues with CH-C[44]. In 1994, an initial report was published that phlebotomy was effective in improving the serum ALT level in patients with CH-C[45] and a national prospective study confirmed the results[46]. Since then, it was reported that hepatic iron accumulation in CH-C predict a response to interferon (IFN) therapy[47], and phlebotomy before and during IFN therapy improved virological and histological response to short-term IFN therapy evaluated at the end-of-treatment[48]. This observation is reasonable considering the finding that oxidative stress impairs interferon alpha signal by blocking JAK-STAT pathway[49]. The standard therapy for hepatitis C is now a combined therapy of interferon-α and ribavirin, in which patients with viral response to treatment seemed to develop higher soluble transferrin receptor levels[50] with decline in serum iron and ferritin than non-responders, revealing intracellular reduction of iron store depending on the result of treatment including hemolytic reaction by ribavirin[51]. This is an interesting observation that decrease of iron status may be an additional effect of the combination therapy with interferon and ribavirin. Moreover, HFE mutations are also associated with increased sustained virologic responses by antiviral long-term treatment, while it is well known that HFE mutations are associated with increased iron loading[52]. However, some reports suggest that iron depletion was unable to trigger interferon response, so that there are conflicting data. It should be further investigated whether hepatic iron content modify the response to interferon[53,54]
| 1 Immunological modification (Immunological escape of HCV) |
| Decrease of Th1 activity |
| Impaired function of macrophage and Kupffer cells |
| Decrease of innate immunity (Natural resistance macrophage protein 2) |
| 2 Increase of liver toxicity by iron-mediated radical formation |
| Reactive oxygen production through fenton reaction |
| Induction of apoptosis |
| Acceleration of fibrinogenesis |
| DNA damage and carcinogenesis |
| 3 Effect on cell signalling |
| Decrease of interferon responsiveness by NFκB activation |
| 4 HCV proliferation |
| Activation of translation initiation factor 3 (eIF3) |
| Suppression of HCV RNA polymerase (NS5B) activity |
From these observations, iron and related molecules seem to be key factors in the hepatocytes to influence the disease condition of CH-C, and also development of cirrhosis and maybe hepatocellular carcinoma. Clinical data on phlebotomy on CH-C generally indicates that phlebotomy does not influence the viral load in vivo. On the other hand, in vitro study on HCV replication is controversial: iron promotes HCV translation by up-regulating expression of the translation initiation factor eIF3 by reporter assay[55], whereas iron suppresses HCV replication by inactivating the RNA polymerase NS5B[56].
As previously described, hepatocytes have two iron uptake systems, transferrin-mediated and nontransferrin-bound iron-mediated pathway. Transferrin and TfR1 are molecules involved in the classical pathway of cellular iron uptake, but are faintly expressed in normal hepatocytes, and is down-regulated in iron-loaded hepatic tissues with hemochromatosis. Concerning the post-transcriptional regulation of TfR1, two mechanisms are postulated through the activity change of IRP which is already mentioned. In CH-C, TfR1 expression was up-regulated and DMT1 expression was down-regulated in the condition of hepatic excess iron accumulation, suggesting that regulation of DMT1 expression is iron-dependent, but that of TfR1 expression is iron-independent in CH-C[57]. In patients with CH-C, serum values of inflammatory cytokines such as IL-1β, IL-6, and TNFα have been reported to be high in comparison with those in normal controls. In addition, TfR1 was up-regulated by IL-1β, IL-6, and TNFα in HepG2. Administration of IL-6 augments hepatic uptake of transferrin-bound iron (59Fe), and this is mainly mediated through hepatocytes, but not through Kupffer cells. These results suggest that the up-regulation of TfR1 expression in CH-C might be caused by increase of inflammatory cytokines that proceeded from HCV infection, although there is a possibility that the components of HCV themselves may induce TfR1 expression directly or indirectly.
Like wise, the up-regulated TfR1 might act as a key molecule for hepatic excess iron accumulation in CH-C; however, there are several candidate molecules which cause this condition. For instance, each mutant of HFE, TfR2, hepcidin, hemojuvelin and ferroportin1 (also known as Ireg1 or MTP1) with substitution of amino acid causes the similar phenotype of hemochromatosis. That is, these facts indicate that at least 5 molecules are involved in the familiar hemochromatosis[58]. In hepatocytes, TfR2 predominantly expresses in the normal condition[59] and the disruption of TfR2 gene caused the hepatic iron overload, a phenotype of hemochromatosis, suggesting that TfR2 should also have important role in hepatic iron metabolism[60]. This receptor might act as a sensor of iron status because hepatic TfR2 protein level was increased in iron loaded rats and was decreased in iron deficient rats. Recently, Takeo et al[61] reported that in CH-C TfR2 protein expression is increased parallel with ferroportin1, although the meaning of this TfR2 elevation is still to be elucidated[62].
In addition, there was a significant correlation of hepcidin mRNA expression in the liver with hepatic iron concentration and serum ferritin, but did not correlate with ALT, AST, HAI, or viral load. In inflammatory conditions, hepcidin is regulated transcriptionally by IL-6[63] and IL-1β[64] independent of liver iron content. It is noteworthy that, in contrast to other inflammatory states, hepcidin mRNA expression in the liver was independent of markers of inflammation in hepatitis C, suggesting that iron stores in patients with hepatitis C regulate hepcidin expression, and that iron loading in chronic hepatitis C is not due to inappropriate hepcidin expression[65]. However, there is still a controversial result concerning the hepcidin metabolism in chronic hepatitis C that serum pro-hepcidin is down-regulated[66]. The role of hepcidin in chronic hepatits C seems to need further consideration.
The role of iron on the hepatocellular carcinoma (HCC) development in patients with chronic hepatitis C is another major concern. In primary hemochromatosis, iron could be involved in the development of HCC in associated with cirrhosis, suggesting a strong link between heavy iron overload and HCC development. In cases of chronic hepatitis C, it is also known that HCC are developed 20 to 30 years after the infection of hepatitis C virus through the progression of the disease from chronic hepatitis and cirrhosis. In Long-Evans Cinnamon (LEC) rat, an animal model of human Wilson disease which spontaneously developed hepatitis and liver fibrosis, HCC is frequently developed after the rats have recovered from initial fulminant hepatitis and subsequent liver fibrosis. This is considered to relate to progressive iron accumulation in the animal[67], and iron depletion prevents their development of hepatic cancer[68]. Even though the iron deposition in chronic hepatitis C is mild compared with that in hemochromatosis, iron may be an independent factor on the risk of HCC. It is reported that liver fibrosis is a favorable environment of proliferation of cancer cells by releasing transforming growth factor β, and there is a strong link between liver fibrosis and liver iron deposition. In clinical trials of phlebotomy, the hepatic content of 8-OH deoxyguanosine is decreased and fibrotic score is improved. An important issue in hepatocaricinogenesis in chronic hepatitis C is the closely related sustained production of ROS during inflammation and fibrosis. Moriya et al[69] reported that HCC developed in HCV core transgenic mice after the age of 16 mo, and showed high hepatic lipid peroxidation levels in old (more than 16 mo) core transgenic mice, than in control. However, the association of HCV transgenic mice, and HCC development disappeared with advanced passaging of animals, suggesting that HCC development in HCV transgenic mice cannot be simply explained by HCV infection, but requires additional cofactors. A recent study by Furutani et al[70] clearly showed that hepatic iron overload induces HCC in transgenic mice expressing HCV polyprotein. Transgenic animal carrying full length polyprotein-coding region (core to NS5B, nts 342-9378) by using pAlb promoter/enhancer was fed with excess iron diet. After 6 mo feeding, the transgenic mice showed marked steatosis and increased 8 hydroxy-2’deoxyguanosine content in association with the hepatic iron accumulation. Twelve months after feeding, 45% of transgenic mice developed hepatic tumors including HCC. It is noteworthy that the steatosis does not accompany with inflammation but a remarkable ultrastructural alteration of mitochondria associated with decreased degradation activity of fatty acids.
Nonalcoholic steatohepatitis (NASH) is a clinical entity characterized by the development of histopathological changes in the liver that are nearly identical to those induced by excessive alcohol intake, but in the absence of alcohol abuse; the presence of macrovesicular steatosis and mixes inflammatory infiltrate associate with varying amounts of Mallory’s hyaline, glycogenated nuclei, and focal hepatocyte ballooning degeneration. Clinical features of NASH include obesity, hyperlipidemia, diabetes mellitus, and hypertension. In US population, approximately 25% is obese, and at least 20% of the obese individuals have hepatic steatosis. Thus, non-alcoholic liver disease (NAFLD) is the most common cause of liver dysfunction, and it is believed that NASH becomes a cause of cryptogenic cirrhosis and hepatocellular carcinoma (HCC). In patients with homozygote of HFE-related hemochromatosis, obesity and steatosis affect liver disease progression, and will be cofactors for iron overload. There is one study of Australia that showed that the prevalence of abnormal genotype of HFE in NASH is 31% compared to a normal prevalence of 13% in the general population, sugget that excess iron might be important. A study on North American subjects showed similar results that the prevalence of the HFE gene mutation associated with hereditary hemochromatosis are increasing in patients with NASH[71]. In the study dealing Japanese NASH patients, who had no HFE gene mutations, a significant staining of liver iron and increased level of thioredoxin, a marker of oxidative stress in addition to the increase of serum ferritin, was observed.
As diabetes and obesity were background conditions of NAFLD, and is thought to be a initial triggering factor, insulin resistance is now considered the fundamental operative mechanism. Insulin resistance is probably the "first step" in NASH, and a close correlation between insulin resistance and iron is speculated. Even though it is not still clear whether secondary iron accumulation increases insulin resistance, or vice versa, oxidative stress may be the elusive "second" hit of possibly multiple steps in the progression of steatosis to fibrosing steatohepatitis[72]. This may be due to the activation of stellate cells[73].
Because hepatic iron promotes oxidative stress, it seems that iron is a contributory cofactor in NASH. This proposal is strengthened by an association with hepatic fibrosis with NASH[74] and was confirmed by measuring serum markers of oxidative stress[75-77]. Excess hepatic iron also occur in insulin resistance-associated iron overload (IRHIO), characterized by hyperferritinemia with normal to mild increases in transferrin saturation. There is an interesting clinical study that venesections and restricted diet are effective in patients with IRHIO[78]. As in IRHIO, restriction of dietary calories, fat and iron improved NAFLD in addition the decrease of levels of serum aminotransferases and ferritin[79]. It seems that the simultaneous disorder of iron and glucose and/or lipid metabolism, in most cases associated with insulin resistance, is responsible for persistent hyperferritinemia and identifies patients at risk for NASH[80]. However, it is still unclear why iron is deposited in IRHIO and NAFLD. There is an interesting report by Bekri et al[81] that there is an increase of hepcidin in adipose tissue of the severely obese but of liver, suggesting that severe obesity itself cause hypoferremia due to the overproduction of hepcidin in the adipocytes. This finding may explain the hypoferremia in severe obese patients, but does not show the mechanism of hepatic iron deposition in IRHIO and NASH. Further studies are needed to clarify this issue, including an increase of transferrin iron influx into hepatocytes in NAFLD.
In patients with NASH, increased transferrin saturation correlated positively with the severity of fibrosis in univariate analysis, although it became insignificant when age, obesity, diabetes, and AST/ALT ratio were controlled. A recent study showed improvement in insulin sensitivity with the use of venesection in 11 patients with NASH. Biweekly phlebotomy until serum ferritin concentration became lower than or equal to 30 ng/mL reduced mean serum ALT activity without a significant change of body weight, suggesting that iron reduction therapy by phlebotomy will be one of the promising therapies for NASH[82], although this approach cannot be implemented without extensive review.
The natural history of NASH is still unclear, but some patients follow advanced liver fibrosis progressing to cirrhosis and sometimes HCC[83]. It is also known that diabetes increases the risk of hepatocellular carcinoma in US[84]. Further studies are needed to clarify this issue, especially the relation between hepatocarcinogenesis from mild iron accumulation in NASH.
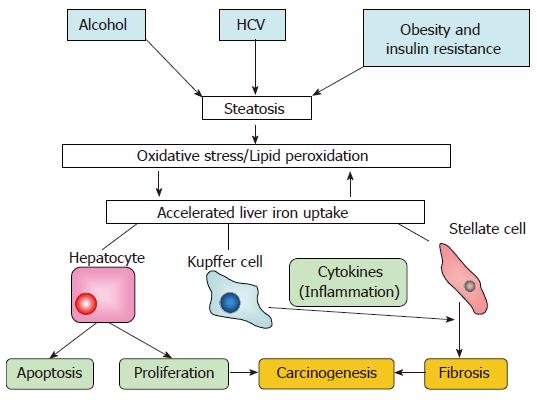
As shown in Figure 2, a common pathway through steatosis/oxidative stress may be present for the develop-ment of liver fibrosis and carcinogenesis by iron.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Alla V, Bonkovsky HL. Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005;25:461-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Chitturi S, George J. Interaction of iron, insulin resistance, and nonalcoholic steatohepatitis. Curr Gastroenterol Rep. 2003;5:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Ortiz V, Berenguer M, Rayón JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Barton JC, Edwards CQ. Hemochromatosis. Cambridge University Press. 2000;435-467. [DOI] [Cited in This Article: ] |
| 5. | Cohen AR. New advances in iron chelation therapy. Hematology Am Soc Hematol Educ Program. 2006;42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Kono S, Suzuki H, Takahashi K, Takahashi Y, Shirakawa K, Murakawa Y, Yamaguchi S, Miyajima H. Hepatic iron overload associated with a decreased serum ceruloplasmin level in a novel clinical type of aceruloplasminemia. Gastroenterology. 2006;131:240-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Powell LW. Normal human iron storage and its relation to ethanol consumption. Australas Ann Med. 1966;15:110-115. [PubMed] [Cited in This Article: ] |
| 8. | Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, Saito H, Kato J. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005;29:189S-193S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Takada A, Takase S, Tsutsumi M. Characteristic features of alcoholic liver disease in Japan: a review. Gastroenterol Jpn. 1993;28:137-148. [PubMed] [Cited in This Article: ] |
| 10. | Bacon BR, Britton RS. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990;11:127-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 294] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci USA. 1993;90:8742-8746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 267] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Niemelä O. Aldehyde-protein adducts in the liver as a result of ethanol-induced oxidative stress. Front Biosci. 1999;4:D506-D513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Pietrangelo A, Gualdi R, Casalgrandi G, Geerts A, De Bleser P, Montosi G, Ventura E. Enhanced hepatic collagen type I mRNA expression into fat-storing cells in a rodent model of hemochromatosis. Hepatology. 1994;19:714-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Ohhira M, Ohtake T, Matsumoto A, Saito H, Ikuta K, Fujimoto Y, Ono M, Toyokuni S, Kohgo Y. Immunohistochemical detection of 4-hydroxy-2-nonenal-modified-protein adducts in human alcoholic liver diseases. Alcohol Clin Exp Res. 1998;22:145S-149S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Shaw S, Jayatilleke E. The role of cellular oxidases and catalytic iron in the pathogenesis of ethanol-induced liver injury. Life Sci. 1992;50:2045-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kurose I, Higuchi H, Kato S, Miura S, Ishii H. Ethanol-induced oxidative stress in the liver. Alcohol Clin Exp Res. 1996;20:77A-85A. [PubMed] [Cited in This Article: ] |
| 17. | Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 363] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Xiong S, She H, Sung CK, Tsukamoto H. Iron-dependent activation of NF-kappaB in Kupffer cells: a priming mechanism for alcoholic liver disease. Alcohol. 2003;30:107-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S-54S. [PubMed] [Cited in This Article: ] |
| 21. | Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, Kato J, Niitsu Y. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 1993;18:874-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Kobune M, Kohgo Y, Kato J, Miyazaki E, Niitsu Y. Interleukin-6 enhances hepatic transferrin uptake and ferritin expression in rats. Hepatology. 1994;19:1468-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ganz T. Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol. 2006;306:183-198. [PubMed] [Cited in This Article: ] |
| 24. | Ikuta K, Zak O, Aisen P. Recycling, degradation and sensitivity to the synergistic anion of transferrin in the receptor-independent route of iron uptake by human hepatoma (HuH-7) cells. Int J Biochem Cell Biol. 2004;36:340-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54:980-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto Y, Kohgo Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26:26S-31S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Suzuki M, Fujimoto Y, Suzuki Y, Hosoki Y, Saito H, Nakayama K, Ohtake T, Kohgo Y. Induction of transferrin receptor by ethanol in rat primary hepatocyte culture. Alcohol Clin Exp Res. 2004;28:98S-105S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Shindo M, Torimoto Y, Saito H, Motomura W, Ikuta K, Sato K, Fujimoto Y, Kohgo Y. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatol Res. 2006;35:152-162. [PubMed] [Cited in This Article: ] |
| 31. | Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 383] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Müllner EW, Kühn LC. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988;53:815-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 452] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 33. | Hentze MW, Kühn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175-8182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 993] [Cited by in F6Publishing: 972] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 34. | Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4-hydroxynonenal-modified proteins in the livers of rats with alcoholic liver disease. Hepatology. 1997;26:650-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Cairo G, Pietrangelo A. Nitric-oxide-mediated activation of iron-regulatory protein controls hepatic iron metabolism during acute inflammation. Eur J Biochem. 1995;232:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Philpott CC, Haile D, Rouault TA, Klausner RD. Modification of a free Fe-S cluster cysteine residue in the active iron-responsive element-binding protein prevents RNA binding. J Biol Chem. 1993;268:17655-17658. [PubMed] [Cited in This Article: ] |
| 37. | Duane P, Raja KB, Simpson RJ, Peters TJ. Intestinal iron absorption in chronic alcoholics. Alcohol Alcohol. 1992;27:539-544. [PubMed] [Cited in This Article: ] |
| 38. | Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, Stremmel W. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735-743 DOI : 10.1136/gut.2003.022863 PMCid: PMC1774035. [Cited in This Article: ] |
| 39. | Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, Suzuki Y, Fujimoto Y, Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007;31:S2-S8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Available from: http: //www.healthyarkansas.com/services/C_Training/edu/Glossary/default.htm. [Cited in This Article: ] |
| 43. | Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40:834-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 287] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986-988. [PubMed] [Cited in This Article: ] |
| 46. | Yano M, Hayashi H, Yoshioka K, Kohgo Y, Saito H, Niitsu Y, Kato J, Iino S, Yotsuyanagi H, Kobayashi Y. A significant reduction in serum alanine aminotransferase levels after 3-month iron reduction therapy for chronic hepatitis C: a multicenter, prospective, randomized, controlled trial in Japan. J Gastroenterol. 2004;39:570-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Van Thiel DH, Friedlander L, Fagiuoli S, Wright HI, Irish W, Gavaler JS. Response to interferon alpha therapy is influenced by the iron content of the liver. J Hepatol. 1994;20:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 155] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, Levine RA, Fiarman G, Thiim M, Tavill AS. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31:730-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, Craxì A. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol. 2006;45:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Kohgo Y, Torimoto Y, Kato J. Transferrin receptor in tissue and serum: updated clinical significance of soluble receptor. Int J Hematol. 2002;76:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Mozer-Lisewska I, Mania A, Kowala-Piaskowska A, Figlerowicz M, Słuzewski W. Alterations of soluble transferrin receptor level in children with chronic hepatitis C during treatment with recombinant interferon-alpha and ribavirin. Hepatol Res. 2005;33:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Bonkovsky HL, Naishadham D, Lambrecht RW, Chung RT, Hoefs JC, Nash SR, Rogers TE, Banner BF, Sterling RK, Donovan JA. Roles of iron and HFE mutations on severity and response to therapy during retreatment of advanced chronic hepatitis C. Gastroenterology. 2006;131:1440-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Piperno A, Sampietro M, D'Alba R, Roffi L, Fargion S, Parma S, Nicoli C, Corbetta N, Pozzi M, Arosio V. Iron stores, response to alpha-interferon therapy, and effects of iron depletion in chronic hepatitis C. Liver. 1996;16:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Guyader D, Boucher E, André P, Even C, Cottereau J, Bianchi A, Gasser P, Mendler MH, Deugnier Y, Brissot P. A pilot study of iron depletion as adjuvant therapy in chronic hepatitis C patients not responding to interferon. Am J Gastroenterol. 1999;94:1696-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Theurl I, Zoller H, Obrist P, Datz C, Bachmann F, Elliott RM, Weiss G. Iron regulates hepatitis C virus translation via stimulation of expression of translation initiation factor 3. J Infect Dis. 2004;190:819-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Xiong S, She H, Takeuchi H, Han B, Engelhardt JF, Barton CH, Zandi E, Giulivi C, Tsukamoto H. Signaling role of intracellular iron in NF-kappaB activation. J Biol Chem. 2003;278:17646-17654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Saito H, Fujimoto Y, Ohtake T, Suzuki Y, Sakurai S, Hosoki Y, Ikuta K, Torimoto Y, Kohgo Y. Up-regulation of transferrin receptor 1 in chronic hepatitis C: Implication in excess hepatic iron accumulation. Hepatol Res. 2005;31:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Beutler E. Hemochromatosis: genetics and pathophysiology. Annu Rev Med. 2006;57:331-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826-20832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 432] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 60. | Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O'Kelly J, Umehara Y, Wano Y, Said JW, Koeffler HP. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Takeo M, Kobayashi Y, Fujita N, Urawa N, Iwasa M, Horiike S, Tanaka H, Kaito M, Adachi Y. Upregulation of transferrin receptor 2 and ferroportin 1 mRNA in the liver of patients with chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Mifuji R, Kobayashi Y, Ma N, Qiang QL, Urawa N, Horiike S, Iwasa M, Kaito M, Malavasi F, Adachi Y. Role of transferrin receptor 2 in hepatic accumulation of iron in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:144-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906-1910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 64. | Inamura J, Ikuta K, Jimbo J, Shindo M, Sato K, Torimoto Y, Kohgo Y. Upregulation of hepcidin by interleukin-1beta in human hepatoma cell lines. Hepatol Res. 2005;33:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Aoki CA, Rossaro L, Ramsamooj R, Brandhagen D, Burritt MF, Bowlus CL. Liver hepcidin mRNA correlates with iron stores, but not inflammation, in patients with chronic hepatitis C. J Clin Gastroenterol. 2005;39:71-74. [PubMed] [Cited in This Article: ] |
| 66. | Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36:288-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Kato J, Kohgo Y, Sugawara N, Katsuki S, Shintani N, Fujikawa K, Miyazaki E, Kobune M, Takeichi N, Niitsu Y. Abnormal hepatic iron accumulation in LEC rats. Jpn J Cancer Res. 1993;84:219-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Kato J, Kobune M, Kohgo Y, Sugawara N, Hisai H, Nakamura T, Sakamaki S, Sawada N, Niitsu Y. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Invest. 1996;98:923-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 895] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 70. | Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Bonkovsky HL, Jawaid Q, Tortorelli K, LeClair P, Cobb J, Lambrecht RW, Banner BF. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31:421-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714-2724. [PubMed] [Cited in This Article: ] |
| 73. | Washington K, Wright K, Shyr Y, Hunter EB, Olson S, Raiford DS. Hepatic stellate cell activation in nonalcoholic steatohepatitis and fatty liver. Hum Pathol. 2000;31:822-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 483] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 75. | Nakashima T, Sumida Y, Furutani M, Hirohama A, Okita M, Mitsuyoshi H, Itoh Y, Okanoue T. Elevation of serum thioredoxin levels in patients with nonalcoholic steatohepatitis. Hepatol Res. 2005;33:135-137. [PubMed] [Cited in This Article: ] |
| 76. | Konishi M, Iwasa M, Araki J, Kobayashi Y, Katsuki A, Sumida Y, Nakagawa N, Kojima Y, Watanabe S, Adachi Y. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J Gastroenterol Hepatol. 2006;21:1821-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Piperno A, Vergani A, Salvioni A, Trombini P, Viganò M, Riva A, Zoppo A, Boari G, Mancia G. Effects of venesections and restricted diet in patients with the insulin-resistance hepatic iron overload syndrome. Liver Int. 2004;24:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Yamamoto M, Iwasa M, Iwata K, Kaito M, Sugimoto R, Urawa N, Mifuji R, Konishi M, Kobayashi Y, Adachi Y. Restriction of dietary calories, fat and iron improves non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22:498-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 82. | Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K, Nakashima T, Okanoue T. Effect of iron reduction by phlebotomy in Japanese patients with nonalcoholic steatohepatitis: A pilot study. Hepatol Res. 2006;36:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1036] [Cited by in F6Publishing: 995] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 84. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |