Published online Aug 28, 2007. doi: 10.3748/wjg.v13.i32.4306
Revised: April 10, 2007
Accepted: April 12, 2007
Published online: August 28, 2007
Unresectable pancreatic cancers have an extremely dismal prognosis and chemoresistant nature. The treatment of pancreatic cancer is still problematic. Gemcitabine is a promising new agent that has been studied recently for palliation of advanced pancreatic cancer. However, the response rates have been highly variable, and are often irreproducible. To improve this low response rate, various treatments are needed because no standard treatment exists. Intra-arterial chemotherapy is considered to take advantage of the first pass effect of the drug, generating higher local drug concentrations in tumor cells with lower toxicity. Regional intra-arterial chemotherapy may provide high levels of cytostatic concentrations within the tumor and, simultaneously, a low rate of systemic side effects compared with systemic administration of anti-neoplastic drugs. Intra-arterial chemotherapy has been introduced as an alternative treatment for advanced pancreatic cancer. Further clinical trials of this method should be subjected to a prospective randomized controlled study for advanced pancreatic cancer.
- Citation: Ishikawa T. Is it relevant that intra-arterial chemotherapy may be effective for advanced pancreatic cancer? World J Gastroenterol 2007; 13(32): 4306-4309
- URL: https://www.wjgnet.com/1007-9327/full/v13/i32/4306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i32.4306
Pancreatic cancer is a malignancy with a poor prognosis[1]. Surgery is considered the only curative therapy. However, the 5-year survival rate after resection of pancreatic cancer is still very low, even when radical surgery is performed[2,3]. The cause of death in patients with advanced pancreatic cancer is primarily local progression of cancer and distant metastasis. At diagnosis pancreatic cancer is most commonly locally advanced and often accompanied by distant metastases to the liver and peritoneum[4].
Unresectable pancreatic cancers have an extremely dismal prognosis and chemoresistant nature because systemic chemotherapy is of limited effectiveness for this disease[5-9].
The treatment of pancreatic cancer is still problematic and a major challenge for oncologists because of its highly dismal outcomes. Many chemotherapeutic agents have been tested as single or combination therapies for pancreas cancer.
Recently, it has been reported that gemcitabine (Gemzar: Eli Lilly Co Ltd., Tokyo, Japan) is an effective drug in the treatment of patients with metastatic pancreas cancer. Gemcitabine is a promising agent that improves the survival of patients with unresectable pancreas cancer, and now a standard first-line agent for these patients[10,11]. It is a nucleoside analogue with broad-spectrum antitumor activity. The major mechanism of action of gemcitabine is the inhibition of DNA synthesis. The primary endpoint of this trial was improvement in the clinical benefit response (CBR) score defined by performance status, weight gain, and pain control. However, the response rates have been highly variable, and are often irreproducible. To improve this low response rate, various treatments have been applied; however, no standard treatment exists. For these reasons, none of the reported treatment regimens can be considered to be standard treatment and in order to evaluate if intra-arterial regional chemotherapy is indeed superior to systemic chemotherapy, randomized trials must be conducted. In this paper, we review recent approaches to pancreas cancer in terms of regional chemotherapy.
Pancreas cancer is considered an almost chemoresistant tumor; the average tumor response rate with 5-FU alone or in combination with other agents is in the range of 7%-28%[3,12]. Systemic adjuvant chemotherapy for pancreatic cancer has not increased the 5-year survival rate.
Some researchers, therefore, have proposed that only symptomatic support should be performed for stage IV advanced pancreatic cancer[13]. Even though many chemotherapeutic agents have been evaluated in patients with pancreatic cancer, 5-fluorouracil (5-FU) has been the most important drug for 20 years[14].
Gemcitabine (Gemzar: Eli Lilly Co Ltd., Tokyo, Japan) is a promising new agent that has been recently studied for palliation of advanced (stage IV) unresectable pancreatic cancer and in pancreatic cancer it showed a response rate comparable to 5-FU, but with an improved clinical benefit[11]. Moreover, the action of gemcitabine seems to be synergetic with 5-FU. Both gemcitabine and 5-FU have shown well-recognized antitumor activity against pancreatic cancer and in in vitro assays showed synergistic activity[15].
It is clinically more beneficial in more patients than other chemotherapeutic agents such as 5-FU, but its efficacy is still insufficient even when combined with other agents.
Systemic chemotherapy is of limited effectiveness. Approaches beyond systemic chemotherapy are needed for advanced pancreas cancer.
Chemotherapy may not be effective because the sparse vascularity of pancreatic cancer may not allow for adequate drug accumulation in tumor tissues. The ineffectiveness of systemic chemotherapy is probably due to failure to reach a drug concentration within the tumor because of dose-limited toxicity produced in bone marrow and epithelial tissue.
Intratumoral blood vessels are immature, lacking both smooth muscle cells and immunoreactive nerves[16]. Therefore, tumor vessels are unable to react to vasocon-stricting agents[17,18].
Angiotensin-II (AT-II) causes arteriolar constriction in normal blood vessels. It is a powerful vasoconstrictor which has been shown to alter the distribution of blood flow in favor of intrahepatic tumor perfusion during short (3-4 min) intra-arterial infusions of the compound[19]. We reported previously that chemotherapy with AT-II was effective in some cancers[20-25]. So, we supposed that it is important for the strategy of pancreas cancer treatment to comprehend the hemodynamics of this cancer.
We showed that administration of AT-II enhanced the metastatic liver tumor as compared with normal tissue. Intratumoral blood flow increased in all patients with malignant tumors due to the pressure effect of AT-II[26].
Furthermore, high detection rate of liver metastasis revealed by pharmacoangiographic CT administrating AT-II suggests the improvement of diagnosis on preoperative staging[26].
A relative increase in tumor blood flow may enable higher doses of regional chemotherapy to be given while avoiding hepatotoxicity. Thus, selective increase in tumor blood flow by AT-II induces a marked increase in the tumor vascular area, which may improve not only the chemotherapeutic effects, but also the diagnostic effects in cancer patients. These results support that the delivery of anticancer drugs could be selectively enhanced in tumor tissue under AT-II induced hypertension. This condition for drug delivery to tumor tissue may play a major role in enhancing therapeutic effects of chemotherapy.
Intra-arterial regional chemotherapy has improved the response rates and quality of life in patients with liver metastases from colorectal cancer[27].
Pancreas cancer is considered a chemoresistant tumor and up to now an individual drug with a high level of activity has been lacking. The most important reasons for this drug resistance are the presence of both a biological and a mechanical barrier. The first is the multidrug resistance gene (MDR1) product and the second a very dense, poorly vasculized, fibrotic envelope that is almost impenetrable by drugs[28]. Furthermore, the chemotherapy drugs are often quickly eliminated by a vigorous multidrug resistance mechanism in pancreas cancer[29]. In addition, pancreas cancer is highly resistant because it expresses moderate to high levels of P-170 glycoprotein.
P-glycoprotein is part of a drug or toxin efflux enzyme system that rapidly clears chemotherapeutic agents from the tumor cell[30].
However, it is expected that the drug dose delivered by regional chemotherapy must be increased at least five-fold to overcome the tumor cell resistance derived from the P-170 drug efflux enzyme system.
Intra-arterial chemotherapy for pancreatic cancer is still in its infancy and the ideal schedule does not yet exist. To improve the effect of chemotherapeutic agents against pancreatic cancers, effective methods for drug delivery into tumor tissues should be developed. Intra-arterial infusion allows higher drug concentrations to reach the tumor, overcoming the problem of poor blood flow to the tumor mass in comparison with healthy tissue. The dose-dependent sensitivity of pancreatic cancer to locoregional chemotherapy has been shown in previous studies[29].
Although the purpose of intra-arterial chemotherapy is to deliver a high dose of anticancer agents into the cancer tissue and a low dose into the non-cancerous tissues, the conventional intra-arterial chemotherapy methods[31] for pancreatic cancer have not succeeded in this goal. Moreover, intra-arterial infusion is considered to take advantage of the first pass effect of the drug, generating higher local drug concentrations in tumor cells with lower toxicity.
The chemotherapy is usually given through catheters placed in celiac/hepatic artery or portal vein. Early reports of intraarterial adjuvant therapy have been published[32,33]. In these studies, catheters were placed in the main tributaries of the celiac trunk and portal vein postoperatively to administer 5-FU. In the long-term follow-up, 1-, 3- and 5- year survivals were 92%, 54% and 39%, respectively[33]. Noteworthy in this study is the significantly lower rate of death from hepatic metastases, 8%, with intraarterial adjuvant therapy compared with controls, and the authors suggest that this effect improved survival. Further studies using intraarterial chemotherapy through a transcutaneous catheter via the femoral artery have been published by Beger et al[34,35]. In the long-term follow-up[34], this treatment resulted in a mean survival of 23 mo and 3-year survival of 48%. In the control group, the mean survival was a mere 10.5 mo.
As adjuvant chemotherapy, a significantly better survival was found with intraarterial treatment compared with historical controls. The calculated 1-, 2-, 3-year survivals were 95%, 59% and 43% in 19 patients with active treatment compared with 50%, 10% and 5% in controls. Hence, the therapy seems safe, but the intravascular catheters have caused some problems, including intimal damage in 2% and one case of pseudoaneurysm[34].
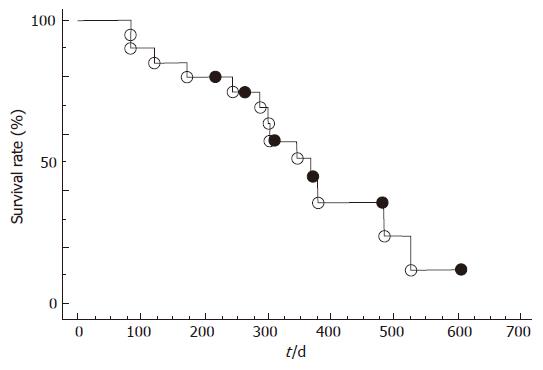
The results of intra-arterial chemotherapy for advanced pancreatic cancer are displayed in Table 1. Recently, Mambrini et al[36] also reported that intra-arterial infusion of 5-fluorouracil, leucovorin, epirubicin and carboplatin (FLEC regimen) is well-tolerated and effective in patients with unresectable pancreatic cancer. According to the definition of the World Health Organization (WHO), the objective response rate includes the reporting complete and partial remissions (Table 1). A complete remission is defined as the disappearance of any evidence of tumor (either primary tumor or metastases). A partial remission is defined as a minimum of a 50% reduction in tumor size without the reappearance of any new lesions[31,37-42]. Moreover, we reported that intra-arterial chemotherapy including changes in distribution of blood flow induced by AT-II appears to be an effective palliative treatment for advanced pancreatic cancer not only for prolonging patient survival but also for improving the quality of life even for high aging patients (Figure 1)[43].
| Author | n | Cytostatic agents | Responserate (%) | Survival time (mo) |
| Mambrini[35] | 211 | 5-FU, Leucovorin | 7.2 | 9.2 |
| Epirubicin, Carboplatin | ||||
| Theodors[29] | 19 | 5-FU, MMC, Doxorubicin | 42.0 | 5.2 |
| Maurer[37] | 12 | MXT, 5-FU | 8.0 | 6.0 |
| Homma[38] | 31 | 5-FU, CDDP | 73.9 | 18.0 |
| Cantone[39] | 96 | 5-FU/Folic Acid, Carboplatin | 15.0 | 9.9 |
| Gausauge[40] | 32 | MXT | 19.0 | 7.5 |
| Lorenz[41] | 17 | GEM, MMC | 24.0 | 9.1 |
| Author's data[43] | 20 | GEM, 5-FU, CDDP (Tegafur/Uracil) | 25.0 | 12.0 |
| Angiotensin-II |
Intra-arterial chemotherapy is suggested to take advantage of the first pass effect of chemotherapeutics, generating higher local drug concentrations at the tumor cell membrane and therefore enhancing cellular drug uptake as compared to intravenous infusion. Therefore, changing the route of drug delivery, which can increase the concentration of the drug in the regional area, thus reducing systemic side-effects, is an effective approach to solving these problems. In addition, this may lead to an increase of tumor response rate and prolongation of survival time.
We suggest that, in the near future, regional chemo-therapy method should be subjected to a prospective randomized controlled study for advanced pancreatic cancer. Further clinical trials of this method for pancreatic cancer are needed.
Regional intra-arterial chemotherapy may provide high levels of cytostatic concentrations within the tumor and, simultaneously, a low rate of systemic side effects compared with systemic administration of anti-neoplastic drugs. In order to achieve higher local drug concentrations in the tumor without causing side-effects of a comparable level of systemic treatment, this method has been introduced as an alternative treatment for advanced pancreatic cancer.
S- Editor Zhu LH L- Editor Zhu LH E- Editor Lu W
| 1. | Gold EB, Cameron JL. Chronic pancreatitis and pancreatic cancer. N Engl J Med. 1993;328:1485-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120-124; discussion 120-124;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 403] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg. 1994;81:102-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 147] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Carter SK, Comis RL. The integration of chemotherapy into a combined modality approach for cancer treatment. VI. Pancreatic adenocarcinoma. Cancer Treat Rev. 1975;2:193-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Zimmerman SE, Smith FP, Schein PS. Chemotherapy of pancreatic carcinoma. Cancer. 1981;47:1724-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 7. | Douglass HO. Current approaches to multimodality management of advanced pancreatic cancer. Hepatogastroenterology. 1993;40:433-442. [PubMed] [Cited in This Article: ] |
| 8. | Nicolson M, Webb A, Cunningham D, Norman A, O'Brien M, Hill A, Hickish T. Cisplatin and protracted venous infusion 5-fluorouracil (CF)--good symptom relief with low toxicity in advanced pancreatic carcinoma. Ann Oncol. 1995;6:801-804. [PubMed] [Cited in This Article: ] |
| 9. | Evans TR, Lofts FJ, Mansi JL, Glees JP, Dalgleish AG, Knight MJ. A phase II study of continuous-infusion 5-fluorouracil with cisplatin and epirubicin in inoperable pancreatic cancer. Br J Cancer. 1996;73:1260-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Moore M. Activity of gemcitabine in patients with advanced pancreatic carcinoma. A review. Cancer. 1996;78:633-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 11. | Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA, Green MR, Tarassoff PG, Brown TD, Casper ES. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 417] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Fung MC, Sakata T. What's new in pancreatic cancer treatment? J Hepatobiliary Pancreat Surg. 2002;9:61-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [Cited in This Article: ] |
| 14. | Bosch X. Fluorouracil and mitomycin for pancreatic cancer. Lancet Oncol. 2005;6:644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Whitehouse PA, Cooper AJ, Johnson CD. Synergistic activity of gamma-linolenic acid and cytotoxic drugs against pancreatic adenocarcinoma cell lines. Pancreatology. 2003;3:367-373; discussion 373-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ashraf S, Loizidou M, Crowe R, Turmaine M, Taylor I, Burnstock G. Blood vessels in liver metastases from both sarcoma and carcinoma lack perivascular innervation and smooth muscle cells. Clin Exp Metastasis. 1997;15:484-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Peterson HI, Mattson J. Vasoactive drugs and tumor blood flow. Biorheology. 1984;21:503-508. [PubMed] [Cited in This Article: ] |
| 18. | Mattson J, Appelgren L, Karlsson L, Peterson HI. Influence of vasoactive drugs and ischaemia on intra-tumour blood flow distribution. Eur J Cancer. 1978;14:761-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sasaki Y, Imaoka S, Hasegawa Y, Nakano S, Ishikawa O, Ohigashi H, Taniguchi K, Koyama H, Iwanaga T, Terasawa T. Changes in distribution of hepatic blood flow induced by intra-arterial infusion of angiotensin II in human hepatic cancer. Cancer. 1985;55:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 20. | Ishikawa T, Mizuno K, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. A case of advanced gastric cancer with bone metastasis and severe DIC responding to hypertensive subselective chemotherapy with pharmacokinetic modulating chemotherapy. Gan To Kagaku Ryoho. 2005;32:523-527. [PubMed] [Cited in This Article: ] |
| 21. | Ishikawa T, Mizuno K, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. Modified pharmacokinetic modulating chemotherapy for progressive gastric cancer accompanied by peritoneal dissemination. Gan To Kagaku Ryoho. 2005;32:469-472. [PubMed] [Cited in This Article: ] |
| 22. | Ishikawa T, Mizuno K, Watanabe K, Baba Y, Oota H, Yoshida T, Kamimura T. A case of successful management of nonresectable pancreas cancer with liver metastasis by intra-arterial infusion chemotherapy with gemcitabine hydrochloride, 5-FU, CDDP and administration of tegafur/uracil. Gan To Kagaku Ryoho. 2004;31:1555-1558. [PubMed] [Cited in This Article: ] |
| 23. | Ishikawa T, Nomura K, Baba Y, Hayashi S, Oota H, Yoshida T, Kamimura T. A case of advanced gastric cancer with liver and intra-abdominal lymph node metastasis treated by hypertensive selective chemotherapy with pharmacokinetic modulating chemotherapy. Gan To Kagaku Ryoho. 2003;30:1151-1155. [PubMed] [Cited in This Article: ] |
| 24. | Ishikawa T, Mita Y, Kobayashi M, Tashiro K, Tashiro S, Matsuki H. A case of nonresectable scirrhous type gastric cancer treated by hypertensive subselective chemotherapy with pharmacokinetic modulating chemotherapy. Gan To Kagaku Ryoho. 2001;28:1137-1140. [PubMed] [Cited in This Article: ] |
| 25. | Ishikawa T, Sato S, Matsuzawa J, Mita Y, Matsui S, Tashiro K, Tashiro S, Matsuki H. A case of successful management of nonresectable pancreas cancer with liver metastasis by intra-arterial infusion chemotherapy with angiotensin-II and administration of tegafur/uracil. Gan To Kagaku Ryoho. 2001;28:521-525. [PubMed] [Cited in This Article: ] |
| 26. | Ishikawa T, Ushiki T, Kamimura H, Togashi T, Tsuchiya A, Watanabe K, Seki K, Ohta H, Yoshida T, Takeda K. Angiotensin-II administration is useful for the detection of liver metastasis from pancreatic cancer during pharmacoangiographic computed tomography. World J Gastroenterol. 2007;13:3080-3083. [PubMed] [Cited in This Article: ] |
| 27. | Rougier P, Laplanche A, Huguier M, Hay JM, Ollivier JM, Escat J, Salmon R, Julien M, Roullet Audy JC, Gallot D. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112-1118. [PubMed] [Cited in This Article: ] |
| 28. | Goldstein LJ. MDR1 gene expression in solid tumours. Eur J Cancer. 1996;32A:1039-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Muchmore JH, Preslan JE, George WJ. Regional chemotherapy for inoperable pancreatic carcinoma. Cancer. 1996;78:664-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 30. | Goldstein LJ, Galski H, Fojo A, Willingham M, Lai SL, Gazdar A, Pirker R, Green A, Crist W, Brodeur GM. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989;81:116-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 889] [Cited by in F6Publishing: 965] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Theodors A, Bukowski RM, Hewlett JS, Livingston RB, Weick JK. Intermittent regional infusion of chemotherapy for pancreatic adenocarcinoma. Phase I and II pilot study. Am J Clin Oncol. 1982;5:555-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Ishikawa O, Ohigashi H, Sasaki Y, Furukawa H, Kabuto T, Kameyama M, Nakamori S, Hiratsuka M, Imaoka S. Liver perfusion chemotherapy via both the hepatic artery and portal vein to prevent hepatic metastasis after extended pancreatectomy for adenocarcinoma of the pancreas. Am J Surg. 1994;168:361-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Ishikawa O, Ohigashi H, Sasaki Y, Masao K, Kabuto T, Furukawa H, Imaoka S. Adjuvant therapies in extended pancreatectomy for ductal adenocarcinoma of the pancreas. Hepatogastroenterology. 1998;45:644-650. [PubMed] [Cited in This Article: ] |
| 34. | Beger HG, Link KH, Gansauge F. Adjuvant regional chemotherapy in advanced pancreatic cancer: results of a prospective study. Hepatogastroenterology. 1998;45:638-643. [PubMed] [Cited in This Article: ] |
| 35. | Beger HG, Gansauge F, Büchler MW, Link KH. Intraarterial adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic cancer: significant reduction in occurrence of liver metastasis. World J Surg. 1999;23:946-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Mambrini A, Sanguinetti F, Pacetti P, Caudana R, Iacono C, Guglielmi A, Guadagni S, Del Freo A, Fiorentini G, Cantore M. Intra-arterial infusion of 5-fluorouracil, leucovorin, epirubicin and carboplatin (FLEC regimen) in unresectable pancreatic cancer: results of a ten-year experience. In Vivo. 2006;20:751-755. [PubMed] [Cited in This Article: ] |
| 37. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 38. | Maurer CA, Borner MM, Läuffer J, Friess H, Z'graggen K, Triller J, Büchler MW. Celiac axis infusion chemotherapy in advanced nonresectable pancreatic cancer. Int J Pancreatol. 1998;23:181-186. [PubMed] [Cited in This Article: ] |
| 39. | Homma H, Doi T, Mezawa S, Takada K, Kukitsu T, Oku T, Akiyama T, Kusakabe T, Miyanishi K, Niitsu Y. A novel arterial infusion chemotherapy for the treatment of patients with advanced pancreatic carcinoma after vascular supply distribution via superselective embolization. Cancer. 2000;89:303-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 40. | Cantore M, Pederzoli P, Cornalba G, Fiorentini G, Guadagni S, Miserocchi L, Frassoldati A, Ceravolo C, Smerieri F, Muchmore JH. Intra-arterial chemotherapy for unresectable pancreatic cancer. Ann Oncol. 2000;11:569-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Gansauge F, Link KH, Rilinger N, Kunz R, Beger HG. Regional chemotherapy in advanced pancreatic carcinoma. Med Klin. 90:501-505. [Cited in This Article: ] |
| 42. | Lorenz M, Heinrich S. Regional chemotherapy. Hematol Oncol Clin North Am. 2002;16:199-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Ishikawa T, Kamimura H, Tsuchiya A, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Takeda K, Kamimura T. Clinical Efficacy of Intra-arterial Pharmacokinetic Chemotherapy with 5-Fluorouracil, CDDP, Gemcitabine, and Angiotensin-II in Patients with Advanced Pancreatic Cancer. Hepato-Gastroenterology. 2007;in press. [Cited in This Article: ] |