Published online Aug 28, 2007. doi: 10.3748/wjg.v13.i32.4295
Revised: June 1, 2007
Accepted: June 4, 2007
Published online: August 28, 2007
Chronic hepatitis B virus (HBV) infection is the most common cause of hepatic fibrosis and hepatocellular carcinoma (HCC), mainly as a result of chronic necroinflammatory liver disease. A characteristic feature of chronic hepatitis B infection, alcoholic liver disease and nonalcoholic fatty liver disease (NAFLD) is hepatic steatosis. Hepatic steatosis leads to an increase in lipid peroxidation in hepatocytes, which, in turn, activates hepatic stellate cells (HSCs). HSCs are the primary target cells for inflammatory and oxidative stimuli, and these cells produce extracellular matrix components. Chronic hepatitis B appears to progress more rapidly in males than in females, and NAFLD, cirrhosis and HCC are predominately diseases that tend to occur in men and postmenopausal women. Premenopausal women have lower hepatic iron stores and a decreased production of proinflammatory cytokines. Hepatic steatosis has been observed in aromatase-deficient mice, and has been shown to decrease in animals after estradiol treatment. Estradiol is a potent endogenous antioxidant which suppresses hepatic fibrosis in animal models, and attenuates induction of redox sensitive transcription factors, hepatocyte apoptosis and HSC activation by inhibiting a generation of reactive oxygen species in primary cultures. Variant estrogen receptors are expressed to a greater extent in male patients with chronic liver disease than in females. These lines of evidence suggest that the greater progression of hepatic fibrosis and HCC in men and postmenopausal women may be due, at least in part, to lower production of estradiol and a reduced response to the action of estradiol. A better understanding of the basic mechanisms underlying the sex-associated differences in hepatic fibrogenesis and carciogenesis may open up new avenues for the prevention and treatment of chronic liver disease.
- Citation: Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF. Female hepatology: Favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 2007; 13(32): 4295-4305
- URL: https://www.wjgnet.com/1007-9327/full/v13/i32/4295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i32.4295
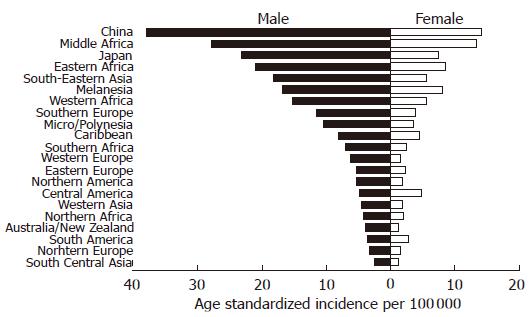
Worldwide chronic infection with hepatitis B virus (HBV)[1] and hepatitis C virus (HCV)[2] is seen in 350 million and 170 million people respectively. Chronic HBV infection is currently the most common cause of cirrhosis and hepatocellular carcinoma (HCC) in the world. HBV is transmitted by the prenatal, parenteral, and sexual routes. If an HBV infection is acquired at birth, there is a strong chance that the child will become chronically infected. However, if the infection is acquired during adulthood, the risk of chronic infection is relatively low (about 10%-20%)[3,4]. Fifteen to 40% of chronically infected people may develop cirrhosis and HCC[5]. The remaining individuals become inactive carriers, otherwise defined as asymptomatic or healthy carriers. Among individuals infected with HBV, those who express the HBV surface antigen (i.e., HBsAg carriers) are approximately 20 times more likely to develop HCC than those who do not[6]. Clinical observations and death statistics support the view that chronic hepatitis B and C appear to progress more rapidly in males than in females[7,8], and that cirrhosis is largely a disease of men and postmenopausal women, with the exception of autoimmune liver diseases, such as primary biliary cirrhosis and chronic autoimmune hepatitis[9,10]. The most clearly established risk factors for HCC are chronic infection with HBV and HCV, cirrhosis, male sex, older age, alcohol abuse, and exposure to dietary aflatoxin[11]. Liver injury in chronic hepatitis B is predominantly caused by the cellular immune response to the virus, and over time the balance between HBV and the alterations in the host immune response[12]. By contrast, the high frequency of chronicity (54%-86%)[13] in HCV infection and evidence of high rates of HCV mutations are perhaps the result of an ineffective immune response or immunological escape by HCV. According to the International Agency for Research on Cancer[14], the male:female ratio of the age-standardized incidence per 100 000 of liver cancer worldwide is 2.9:1. In Asia (particularly in China, Japan, and Taiwan), the incidence of liver cancer is high and accounts for one-half of all liver cancer cases in the world (Figure 1). In the Asian-Pacific region including China and Taiwan, and sub-Saharan Africa, HBV is hyperendemic, whereas in Japan, Western Europe and the USA, HBV infection is much less common, whereas HCV infection is more prevalent and is recognized as a major causative factor for HCC. The inshore region of the Yangtze River, including Qidong, in eastern China has the highest incidence of HCC, due to high levels of aflatoxin, which is associated with HCC because of its carcinogenic potential in HBsAg carriers[15].
Moreover, there is growing concern in clinical practice with regard to the development of nonalcoholic fatty liver disease (NAFLD). Although in most cases, fatty liver does not progress to more severe liver disease, approximately 15%-20% of patients have histological signs of fibrosis and necroinflammation, indicating the presence of nonalcoholic steatohepatitis (NASH). These patients are at a higher risk of developing cirrhosis, terminal liver failure, and HCC[16]. Most studies show a female predilection for NASH[16]; however, two recent reports indicate that NAFLD is more prevalent in men in each age group, and that there is a male predominance for NASH as well[17,18].
Differences in the social environment and the lifestyles of men and women may be involved in the basic mechanisms underlying the sex-associated differences in chronic liver diseases. In general, men have a greater risk of exposure to hepatitis viruses as well as greater opportunities for drinking and smoking. Environmental factors may result in a higher preponderance of nutritional and exercise-associated problems in men. However, it should be noted that some mechanisms related to sex-linked differences may be based on biological factors, including estrogen-related female sex hormones, such as estradiol, rather than simply gender differences in social environment and lifestyle. Hepatic estrogen receptors (ERs) mediate estrogen action in the liver. The present review summarizes the current knowledge of the biological functions of estrogens and the ER status as it relates to hepatic fibrogenesis and carcinogenesis.
During the early phase of chronic HBV infection, patients are positive for HBV e antigen (HBeAg), a surrogate marker of active HBV replication, and have frequent acute flares characterized by substantial increases in the serum aminotransferase levels as the result of specific, T-lymphocyte-mediated cellular responses to viral antigens and apoptosis of the hepatocytes. Some of the acute flares may be followed by seroconversion from HBeAg to its antibody (anti-HBe) and clinical remission[19,20], while other patients progress to cirrhosis and HCC, particularly elderly men[21,22]. A positive test for HBeAg is associated with higher inflammatory activity in the liver and an increased risk of HCC[23]. Long term cohort studies show that a higher percentage of females clear their HBeAg and become HBsAg negative compared to males[24]. Seroconversion from HBeAg to anti-HBe and from HBsAg to anti-HBs occurs more frequently in female subjects than in males[25]. Generally, females produce more vigorous cellular and humoral immune reactions, and have a higher incidence of autoimmune diseases than males[26,27]. The underlying mechanism by which females seem more likely to achieve seroconversion of HBeAg and HBsAg remains unclear. However, estradiol has been reported to induce the production of interferon (INF)-γ in lymphocytes[28], and augments an antigen-specific primary antibody response in human peripheral blood mononuclear cells[29]. IFN-γ is a potent cytokine with immunomodulatory and antiproliferative properties. Therefore, female subjects, particularly before menopause, may produce antibodies against HBeAg and HBsAg at a higher frequency than males with chronic HBV infection.
Immunization is the most effective method of preventing transmission of HBV. After immunization with HBV vaccine, approximately 90% of healthy adults and 95% of infants, children, and adolescents develop a protective serum level of anti-HBs. Predictors associated with a nonresponse to HBV vaccination, include male sex, older age, obesity and immunocompromising chronic diseases[30]. After an initial vaccination in newborns of Alaska Natives who were seronegative for HBsAg and anti-HBs at birth, the mean concentrations of anti-HBs were higher in females (975 mIU/mL) than males (722 mIU/mL), but after 15 years these values decreased to 23 mIU/mL in females compared to 32 mIU/mL in males[31]. In Taiwan, the chronic HBsAg carrier rate was lower in females than males (4.4% vs 10.7%) who were born to HBsAg carrier mothers, vaccinated against HBV at birth, and followed for over 18 years[32].
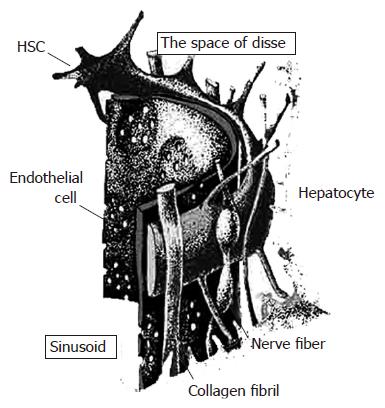
Damage to the parenchymal cell membranes produces reactive oxygen species (ROS) derived from the lipid peroxidative processes, which are a feature of sustained inflammatory response and liver injury, once the antioxidant mechanisms have been depleted. Cells are well equipped to neutralize the effects of ROS, by virtue of a series of antioxidant protective systems, including superoxide dismutase (SOD), glutathione peroxidase and glutathione. Although mild liver injury usually results in an almost complete resolution, persistence of the original insult causes prolonged activation of tissue repair mechanisms, leading to hepatic fibrosis rather than to effective tissue repair. Hepatic fibrosis, or collagen deposition, is associated with inflammation and cell death, which are a consequence of severe liver damage that occurs in many patients with chronic liver disease, regardless of the etiology such as HBV/HCV infection, alcohol abuse, and iron overload state[33]. In other words, collagen production predominates over hepatocellular regeneration. Collagens are mainly produced by cells known as hepatic stellate cells (HSCs). HSCs are located in the space of Disse in close contact with hepatocytes and sinusoidal endothelial cells[34] (Figure 2). In the injured liver, HSCs are regarded as the primary target cells for inflammatory and peroxidative stimuli and are transformed into myofibroblast-like cells. These HSCs are referred to as activated cells and their activation is accompanied with a loss of cellular retinoid, and the synthesis of α-smooth muscle actin (SMA), and large quantities of the major components of the extracellular matrix, including collagen typesIand III.
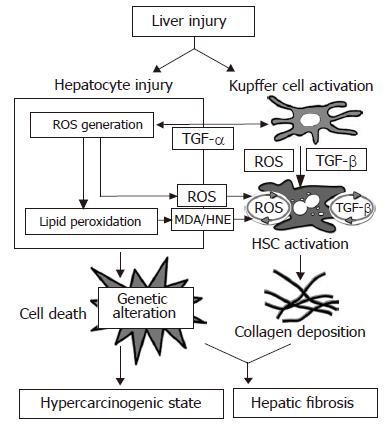
Transgenic mice expressing HBsAg exhibit oxidative stress and DNA damage, leading to the development of HCC[35,36]. In addition, HBV X (HBx) protein alters the mitochondrial transmembrane potential and enhances ROS production in the liver[37]. A primary source of ROS production in hepatocytes and HSCs is mitochondrial NADH/NADPH oxidase. Hydrogen peroxide is more stable and membrane permeable compared to other ROS, which has lead to the hypothesis that it acts as a second messenger causing the following reactions: (1) induction of gene expression of redox sensitive transcription factors, such as activator protein (AP)-1 and nuclear factor (NF)-κB[38], (2) stimulation of apoptosis[39], and (3) modulation of cell proliferation[40]. AP-1 and NF-κB induce the expression of multiple genes involved in inflammation, cell death and fibrogenesis, including cytokines and growth factors such as platelet-derived growth factor and transforming growth factor (TGF)-β. TGF-β is a major fibrogenic cytokine, acting as a paracrine and autocrine (from HSCs) mediator, which triggers and induces the activation of HSCs in vivo. Hydrogen peroxide is converted into the hydroxyl radical, a harmful and highly reactive ROS, in the presence of transition metals such as iron. The hydroxyl radical is able to induce DNA cleavage and lipid peroxidation in the structure of membrane phospholipids, leading to cell death. Malondialdehyde (MDA) and hydroxynonenal (HNE), end products of lipid peroxidation, are discharged from injured hepatocytes into the space of Disse. Paracrine stimuli derived from hepatocytes undergoing oxidative stress induce HSC proliferation and collagen synthesis, and these HSCs are activated by ROS as well as by MDA and HNE[41,42].
HSCs are also able to produce ROS through the activation of NADH/NADPH oxidase by stimuli from outside the cell[43]. Exogenous TGF-β increases ROS production by HSCs, whereas the addition of ROS induces TGF-β production and secretion by these cells[44]. This so-called autocrine loop of ROS by HSCs is regarded as the mechanism that corresponds to the autocrine loop of TGF-β which HSCs produce in response to this cytokine with an increased collagen expression in the injured liver[45] (Figure 3).
It should be noted that estradiol and its derivatives are strong endogenous antioxidants that reduce lipid peroxide levels in the liver and serum[46,47]. Recent studies show that estradiol suppresses iron (ferric nitrilotriacetate)-induced ROS generation, lipid peroxidation, activation of AP-1 and NF-κB, and the loss of SOD and glutathione peroxidase activities in cultured rat hepatocytes[48,49]. Estradiol also inhibits iron (FeSO4)-induced lipid peroxidation in isolated rat liver mitochondria[48]. These findings suggest that the inhibitory effect of estradiol on AP-1 and NF-κB activation may be produced by scavenging ROS and/or by reducing the intracellular production of ROS via antioxidant enzyme induction.
Many of the actions of estradiol are mediated through the ER subtypes, ERα and ERβ. In addition to the action of ER as a classical estrogen response element, ERα and ERβ also mediate gene transcription from an AP-1 enhancer element. Paech et al[50] reported that ERα and ERβ from an AP-1 site signaled in opposite ways when combined with estradiol: estradiol activated transcription with ERα, whereas estradiol inhibited transcription with ERβ. A high level of ERβ expression and a low level of ERα expression is seen in human and rat hepatocytes[49,51,52]. In addition, estradiol up-regulates the Bcl-2 expression in cultured rat hepatocytes undergoing oxidative stress[49]. The overexpression of Bcl-2 is known to suppress lipid peroxidation and to prevent apoptosis, leading to an increase in cellular longevity. These findings suggest that estradiol may protect hepatocytes from oxidative damage, inflammatory cell injury and cell death by the suppression of AP-1 and NF-κB activation and induction of Bcl-2 expression.
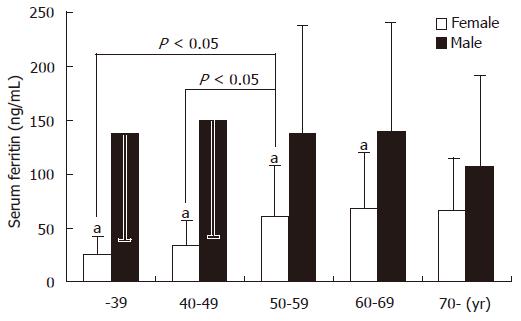
Iron is known to be a potent in vivo factor in the production of hydroxy radicals, leading to DNA mutagenesis[53]. In humans with iron overload due to genetic or acquired causes, fibrosis and cirrhosis are the predominant features of liver disease. The clinical expression of genetic hemochromatosis is 5 to 10 times more frequent in men than in women, possibly because of iron loss during menstruation and increased requirements during pregnancy. In the absence of hepatic inflammation, serum ferritin is a reliable marker of hepatic iron stores. In a study from Japan, serum ferritin levels were examined by gender and age in 305 healthy individuals. In men, the serum ferritin values reached the maximum level in the age group of 40 to 49 years and declined thereafter. By contrast, in women the serum ferritin remained relatively low until menopause, at a level which was one-fifth of that for men of comparable age, and reached the maximum level after menopause to approximately one-half of that for men of comparable age. These findings correlate with the data obtained from the third National Health and Nutrition Examination Survey in the USA[54] and indicate that women, especially before menopause, have lower iron stores in the liver (Figure 4).
Alizadeh et al[55] analyzed the liver histology of age- and sex-matched untreated patients with chronic hepatitis B (n = 20) and chronic hepatitis C (n = 43) and showed that the hepatocyte iron-staining score was similar in chronic hepatitis B and C. The frequency of iron-staining in chronic hepatitis B and C was significantly greater than that seen in alcoholic liver disease[56]. It should be noted that hepatic iron overload is an independent risk factor associated with hepatic fibrosis and cell death[10,57]. In Chinese patients with HBV-associated HCC, stainable iron in non-cancerous and cancerous liver specimens was preferentially enriched in HBV replicating hepatocytes, and was lower in 25% (1/4) of female patients compared to 71% (25/35) of males[58]. Deugnier et al[59] reported that HBsAg seropositivity in male patients with genetic hemochromatosis complicated with HCC was significantly higher (6.2%) than in sex- and age-matched male blood donors (0.075%) and female controls (0.04%), whereas female patients with and without HCC showed no serum HBV marker. These findings suggest that hepatic iron overload may facilitate viral replication in hepatocytes, or that the virus infected hepatocytes tend to accumulate iron. In addition, the lower hepatic iron stores in females may, in part, explain their greater cytoprotection against liver injury.
Chronic alcohol consumption in moderate to heavy quantities results in increased serum ferritin, which may result in increased hepatic iron stores[60]. Hepcidin is a circulatory peptide synthesized in the liver that appears to regulate iron absorption in the duodenum. Harrison-Findik et al[61] treated mice with ethanol added to drinking water for 7 d. Ethanol-treated mice showed significantly lower hepatic hepcidin mRNA expression compared to controls, and also demonstrated greater suppression of hepcidin gene expression in males than females. This effect was abrogated by an antioxidant, vitamin E, while ROS production by ethanol via the alcohol dehydrogenase- and CYP2E1-metabolizing system appeared to result in decreased expression of hepcidin. These findings indicate that alcohol-induced oxidative stress downregulates hepcidin transcription, leading to increased duodenal iron transport, and chronic alcohol consumption leads to increased hepatic iron stores, which is more likely to occur in males than in females. However, females are more vulnerable to alcohol-related damage because they have smaller volume of distribution and lower gastric alcohol dehydrogenase activity[62]. In addition, it has been reported that after alcohol consumption, estrogen stimulation of Kupffer cells increases their sensitivity to endotoxins and leads to higher levels of chemical mediators[63]. These findings suggest that chronic alcohol use may induce more rapid and more severe liver injury in females than in males[64].
During ongoing HBV replication irrespective of the HBeAg seroconversion status, hepatic fibrosis eventually progresses to the stage of cirrhosis. The incidence of cirrhosis in chronic HBV infection, based on data mostly from tertiary care centers, ranges from 2% to 7% annually[20,65-68], although, in a study on 1506 asymptomatic HBV carriers, the cirrhosis incidence rate was 0.7% annually[69]. Activated HSCs are responsible for much of the collagen synthesis observed during hepatofibrogenesis. Male gender and older age are associated with a more rapid progression of hepatic fibrosis [64].
Using multivariate analysis, Iloeje et al[70] in Taiwan, China and Zarski et al[71] in France observed that the independent predictors for cirrhosis in patients with chronic HBV infection were male sex and age > 50 years. McMahon also demonstrated that male sex and old age were the most important factors associated with disease progression in chronic hepatitis B[72]. Patients infected with HBV genotype C have delayed HBeAg seroconversion, a longer duration of viremia, higher risk of progressive disease, and a correspondingly higher rate of cirrhosis and HCC[73]. Genotypes C and B are prevalent in Asia, whereas genotypes A and D are common in Western countries. In addition, the significance of older age as a risk factor may reflect a more prolonged duration of the liver disease and accumulated exposure to environmental risk factors such as aflatoxin, in highly endemic areas[74]. An increased prevalence of HBeAg negative chronic hepatitis B has recently been observed in several countries[75,76]. Patients with HBeAg negative chronic hepatitis B are usually males who are older than those with HBeAg positive chronic hepatitis B[76]. Moreover, the age at the time of acute infection plays an important role in the development of chronicity. Neonatal infection, common in areas of high or intermittent HBV prevalence, is associated with high rates of chronicity, while exposure at an adult age, only occasionally results in chronic infection. An ideal method of assessment of disease progression would be to prospectively follow a large cohort of untreated patients with all the major HBV genotypes (A to D) from infection until death, with repeated liver biopsy and measurement of HBV serological markers. However, such a study would be ethically and pragmatically impossible to perform in an unselected population.
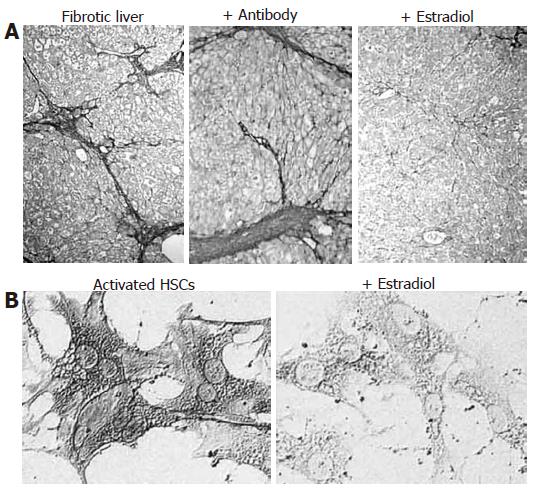
In the hepatic fibrosis model in male rats, estradiol results in suppression of early apoptosis and hepatic fibrosis. These changes are accompanied with reduced collagen content and lower levels of procollagen typeIand III mRNA and α-SMA expression as well as induced Bcl-2 expression[77-79]. In addition, the use of neutralizing antibody against estradiol in male rats (Figure 5A) and ovarectomy in female rats leads to enhanced fibrogenesis[77]. Rat HSCs possess functional ERβ, but not ERα, which respond directly to estradiol exposure; estradiol attenuates the production of collagen typeI, α-SMA expression and cell proliferation in cultured rat HSCs[51,77,78] (Figure 5B). A recent report indicates that estradiol inhibits ROS generation and antioxidant enzyme loss via the suppression of NADH/NADPH oxidase activity, and blocks hydrogen peroxide-induced TGF-β expression, activation of AP-1 and NF-κB, and proliferation and transformation of cultured rat HSCs[45]. These findings suggest that, by suppressing NADH/NADPH oxidase activity, estradiol prevents the autocrine loop of ROS and TGF-β by HSCs (Figure 3) as well as HSC activation, and has a cytoprotective effect against hepatocyte injury.
Hepatic steatosis is a characteristic feature of chronic HBV and HCV infections. The frequency of hepatic steatosis in chronic hepatitis B varies from 27% to 51%, and in chronic hepatitis C from 31% and 72%[80-83]. It has been suggested that hepatic steatosis may reflect a direct cytopathic effect of HCV and plays a role in disease progression. In support of this hypothesis is a transgenic mouse model that expresses HCV core gene and develops progressive hepatic steatosis and HCC[84,85]. It is conceivable that following hepatocyte injury, hepatic steatosis leads to increased lipid peroxidation, which contributes to HSC activation by releasing soluble mediators[86], and thereby induces hepatic fibrosis. In contrast to HCV, there is little information on the correlation between HBV-associated hepatic steatosis and hepatic fibrosis. Furthermore, the molecular mechanism by which HBV mediates hepatic steatosis has not been clearly characterized. Although a cross-sectional study from Australia failed to confirm the impact of steatosis on hepatic fibrosis in chronic hepatitis B (but not in hepatitis C)[87], another cross-sectional analysis in Taiwanese adults revealed that liver damage, evaluated by serum alanine aminotransferase (ALT) levels, was independently associated with HBV carrier status, ultrasonographic fatty liver and male sex[88]. Fatty liver, also termed hepatic steatosis, is the result of the deposition of triglycerides via the accumulation of free fatty acids in hepatocytes. Fatty liver is detected by ultrasonography based on the comparative assessment of image brightness relative to the kidneys[89]. Recently Kim et al[90] reported that HBx protein induces hepatic lipid accumulation mediated by sterol regulatory element binding protein 1 and peroxisome proliferator-activated receptor γ, leading to hepatic steatosis. There is mounting evidence that hepatic lipid accumulation is related to hepatic fibrosis, inflammation, apoptosis, and cancer[91,92].
Increased lipid peroxidation and accumulation are also commonly observed in alcoholic liver disease and NAFLD based on studies in human alcohol-related liver injury and animal models of diet-induced hepatic steatosis and drug-induced steatohepatitis[93-95]. In the progression of fatty liver disease, lipid peroxidation products are generated because of impaired oxidation of the accumulated fatty acids. The key mediators of impaired β-oxidation include increased activity of cytochrome P450 and reduced electron transport in hepatocyte mitochondria. The secretion of inflammatory mediators including TNF-α and ROS by Kupffer cells resulting in HSC activation with disordered collagen production, are believed to play an important role in NASH-associated cryptogenic cirrhosis.
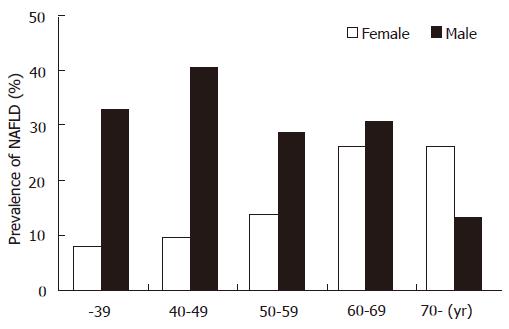
In Japan, Australia, Western Europe and the USA, ultrasonographic surveys of the general population indicate that nearly one-quarter of the adult population has hepatic steatosis[96,97]. NAFLD is more common in males than females, particularly in Asians[98]. In a study on the gender differences in NAFLD among Asians, the prevalence of ultrasonographic NAFLD was examined in 3229 Japanese adults in a health screening center in Tokushima. Prevalence of NAFLD was 2.5-fold higher in males than females (31.5% vs 12.4%). The biggest difference in NAFLD prevalence between females and males was observed in individuals < 50 years (Figure 6).
Recent studies have shown that visceral fat is an independent predictor of fatty liver, even in patients with a normal body mass index, and is much more harmful than subcutaneous accumulation of adipose tissue[99,100]. Human adipose tissue contains ERα and ERβ. Low estrogen levels in menopausal women are associated with a loss in subcutaneous fat and gain in visceral fat[101]. Estrogen treatment of male-to-female transsexuals increases the amount of subcutaneous adipose tissue. Thus, estrogen alters the male type of visceral fat distribution into the female type[102]. It has been reported that women treated with estrogen have a lower visceral accumulation of adipose tissue[103].
An animal study showed that hepatic steatosis becomes evident spontaneously in aromatase-deficient mouse, which lack the ability to produce estrogen and are impaired with respect to hepatocellular fatty acid β-oxidation. Estradiol replacement reduces hepatic steatosis and restores the impairment in mitochondrial and peroxisomal fatty acid β-oxidation to the wild-type level[104]. In addition, tamoxifen an antiestrogen compound used in the treatment of ER positive breast cancer, is associated with an increased risk of fatty liver and NASH[105,106]. Therefore, the greater progression of liver injury with steatosis in males, regardless of the etiology, may be due at least in part to the decreased production of estradiol and/or the lower response to the actions of estradiol.
In inflammatory and oxidative liver injury, the accumulation of leukocytes and macrophages including Kupffer cells at sites of inflammation and injury is thought to be mediated by chemokines, such as macrophage chemotactic protein (MCP)-1 and interleukin (IL)-8. The mononuclear cells and macrophages are in turn able to release proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β and IL-6, leading to persistent liver injury. There is a large body of evidence indicating that the decline in ovarian function with menopause is associated with spontaneous increase in TNF-α, IL-1β and IL-6[107]. Estradiol, at physiological concentrations, inhibits the spontaneous secretion of proinflammatory cytokines in whole blood cultures[108] or peripheral blood mononuclear cells (PBMCs)[109]. The spontaneous production of TNF-α and IL-1β by PBMCs is higher in patients with chronic hepatitis C than in healthy subjects[110]. Endotoxin-stimulated TNF-α production by PBMCs is also higher in HBsAg carriers with elevated ALT levels than in HBsAg carriers with normal ALT levels[111]. Moreover, TNF-α production by hepatocytes in patients with chronic HBV infection is reported to be transcriptionally up-regulated by HBx protein[112,113]. Estradiol is able to attenuate IL-1β in ER expressing HepG2 cells[114], and to ameliorate burn-induced increase in serum TNF-α levels in rats[115]. In vivo studies show that transdermal administration of estradiol in postmenopausal women decreases spontaneous IL-6 production by PBMCs after 12 mo of therapy[109]. A preliminary study has also shown that hydrogen peroxide-induced TNF-α and MCP-1 expressions are attenuated by estradiol in the peritoneal macrophages of female mice[116]. These findings suggest that estradiol has a hepatoprotective effect against inflammation and oxidative stress, at least in part, by preventing macrophage accumulation and inhibiting proinflammatory cytokine production.
However, it appears that macrophages respond differently to endotoxins compared to Kupffer cells as far as the signaling pathways are concerned[117]. Estrogen increases the sensitivity of Kupffer cells to endotoxin[63], while estradiol augments the increase in the serum levels of TNF-α after endotoxin treatment in animals[118].
Both direct and indirect carcinogenic mechanisms are involved in the pathogenesis of HCC induced by chronic HBV infection. HBV may induce HCC indirectly by causing chronic necroinflammatory liver disease[23]. When HBV replication is sustained, hepatocytes undergo a process of continuous damage and regeneration. Chronic necroinflammation may induce a malignant transformation by producing mutagenic ROS during the inflammatory process along with hepatic fibrosis, leading to the development of cirrhosis and HCC (Figure 3). The active replication of HBV may also initiate malignant transformation through a direct carcinogenic mechanism by increasing the probability of insertion of viral DNA in or near proto-oncogenes, tumor-suppressor genes, or their regulatory elements in the cellular DNA[119]. The integration of viral DNA may increase the production of transactivator protein HBx antigen, which may induce the malignant transformation of hepatocytes, and bind to the p53 tumor-suppressor gene and disrupt its activity[23,120].
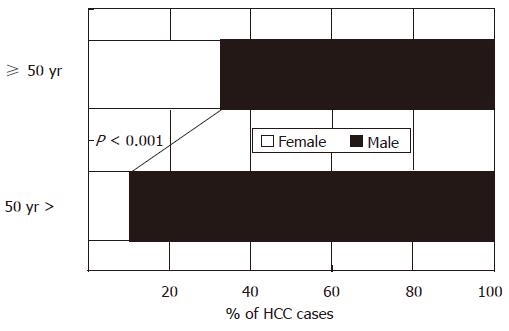
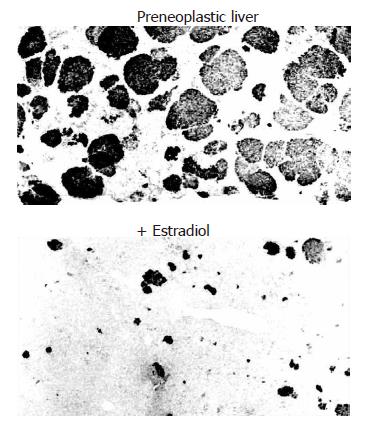
As with the risk factors for hepatic fibrosis, male sex and age > 50 years are important risk factors for HCC[121], although it is unclear whether the susceptibility to the integration of viral DNA inducing malignant transformation of hepatocytes is different between males and females. Conversely, premenopausal women, without the risk factors of male sex and older age, are least vulnerable to HCC. The age-specific male-to-female ratio was examined in 901 Japanese patients with HBV-associated HCC in Tokushima. When the subjects were divided into two age groups, based on whether they were younger or older than the menopausal age of 50 years, the younger group had a significantly lower proportion of females (10.5%) than the older group (32.8%, Figure 7). Moreover, variant ERs are expressed in HCC patients and, to a greater extent, in male patients than in female patients, even at an early stage of chronic liver disease[122,123]. The occurrence of variant ERs leads to the loss of estrogen responsiveness. Experimentally induced carcinomas using carcinogens, as well as spontaneous neoplasms, occur at a higher rate in male rats and mice. Another study demonstrated the suppressive effect of estradiol on chemical hepatocarcinogenesis in rats induced by dimethylnitrosamine (DEN)-2-acetylaminofluorene (AAF)-partial hepatectomy (PH)[124] (Figure 8). Taken together, these lines of evidence suggest that both estradiol and the ER status play a critical role in the biological defense against hepatocarcinogenesis.
Gender-associated differences are not limited to chronic liver disease and are of potential interest in other chronic fibrogenic disorders as well. The predominance of atherosclerosis and the higher rate of renal fibrosis progression in men are excellent lines of evidence that point to the role of estrogen in the wound healing/fibrogenic process[125]. It has been reported that estradiol inhibits the proliferation of vascular smooth muscle cells (VSMCs)[126]. VSMCs are anatomically analogous to HSCs, and are reported to express ERβ at a higher level after vascular injury, without any accompanying changes in ERα expression[127]. Several studies have documented the antifibrogenic effect of estrogen on VSMCs[128,129]. Moreover, renal mesangial cells which are analogous to HSC, have similar properties including playing a prominent role in fibrogenesis. The cell proliferation and collagen synthesis by mesangial cells have also been shown to be modulated by estradiol[130].
The present review indicates that estradiol may have a beneficial effect in the progression of chronic liver disease. However, it should be noted that administration of estradiol in women has potential problems, including an increased risk of breast cancer and endometrial abnormalities[131,132]. In addition, estradiol and ER subtypes have been reported to play a role in the modulation of cholangiocyte proliferation[133], which is a hallmark for the progression of cholestatic liver disease.
Being a male or a female is an important basic human variable that affects health and liver disease throughout ones life span. A better understanding of the biological mechanisms underlying the gender-associated differences observed in chronic HBV infection may provide valuable information on more effective treatment modalities in liver disease in both males and females.
S- Editor Ma N L- Editor Anand BS E- Editor Yin DH
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1728] [Cited by in F6Publishing: 1678] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 2. | Hepatitis C: global prevalence. Wkly Epidemiol Rec. 1997;72:341-344. [PubMed] [Cited in This Article: ] |
| 3. | Seeff LB, Beebe GW, Hoofnagle JH, Norman JE, Buskell-Bales Z, Waggoner JG, Kaplowitz N, Koff RS, Petrini JL, Schiff ER. A serologic follow-up of the 1942 epidemic of post-vaccination hepatitis in the United States Army. N Engl J Med. 1987;316:965-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 175] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 642] [Cited by in F6Publishing: 626] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 5. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Yu MW, Chen CJ. Hepatitis B and C viruses in the development of hepatocellular carcinoma. Crit Rev Oncol Hematol. 1994;17:71-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med. 1990;150:1051-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 9. | Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res. 2007;37:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Kao JH, Chen DS. Recent research progress in hepatocellular carcinoma. J Formos Med Assoc. 2002;101:239-248. [PubMed] [Cited in This Article: ] |
| 12. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1171] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 13. | Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 387] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Cancer Incidence in Five Continents Vol. VIII. IARC Scientific Publications No. 155. Lyon, France: International Agency for Research on Cancer 2002; 566-568. [Cited in This Article: ] |
| 15. | Kensler TW, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nat Rev Cancer. 2003;3:321-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714-2724. [PubMed] [Cited in This Article: ] |
| 17. | Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 423] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 476] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Bortolotti F, Jara P, Crivellaro C, Hierro L, Cadrobbi P, Frauca E, Camarena C, De La Vega A, Diaz C, De Moliner L. Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol. 1998;29:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 924] [Cited by in F6Publishing: 870] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 24. | Alward WL, McMahon BJ, Hall DB, Heyward WL, Francis DP, Bender TR. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J Infect Dis. 1985;151:604-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Zacharakis GH, Koskinas J, Kotsiou S, Papoutselis M, Tzara F, Vafeiadis N, Archimandritis AJ, Papoutselis K. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I-II/EC-project). J Med Virol. 2005;77:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985;121:531-551. [PubMed] [Cited in This Article: ] |
| 27. | Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 766] [Cited by in F6Publishing: 686] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362-4367. [PubMed] [Cited in This Article: ] |
| 29. | Clerici E, Bergamasco E, Ferrario E, Villa ML. Influence of sex steroids on the antigen-specific primary antibody response in vitro. J Clin Lab Immunol. 1991;34:71-78. [PubMed] [Cited in This Article: ] |
| 30. | Sjogren MH. Prevention of hepatitis B in nonresponders to initial hepatitis B virus vaccination. Am J Med. 2005;118 Suppl 10A:34S-39S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Su FH, Chen JD, Cheng SH, Lin CH, Liu YH, Chu FY. Seroprevalence of Hepatitis-B infection amongst Taiwanese university students 18 years following the commencement of a national Hepatitis-B vaccination program. J Med Virol. 2007;79:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 438] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Wake K. Cell-cell organization and functions of 'sinusoids' in liver microcirculation system. J Electron Microsc (Tokyo). 1999;48:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Hagen TM, Huang S, Curnutte J, Fowler P, Martinez V, Wehr CM, Ames BN, Chisari FV. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:12808-12812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 240] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Nakamoto Y, Suda T, Momoi T, Kaneko S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 2004;64:3326-3333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem. 1996;271:13422-13429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 358] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Clément MV, Pervaiz S. Reactive oxygen intermediates regulate cellular response to apoptotic stimuli: an hypothesis. Free Radic Res. 1999;30:247-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 398] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 42. | Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 230] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Cui X, Shimizu I, Lu G, Itonaga M, Inoue H, Shono M, Tamaki K, Fukuno H, Ueno H, Ito S. Inhibitory effect of a soluble transforming growth factor beta type II receptor on the activation of rat hepatic stellate cells in primary culture. J Hepatol. 2003;39:731-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem. 1999;274:33881-33887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, Fukuno H, Honda H, Okamura Y, Ito S. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Yoshino K, Komura S, Watanabe I, Nakagawa Y, Yagi K. Effect of estrogens on serum and liver lipid peroxide levels in mice. J Clin Biochem Nutr. 1987;3:233-239. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Lacort M, Leal AM, Liza M, Martín C, Martínez R, Ruiz-Larrea MB. Protective effect of estrogens and catecholestrogens against peroxidative membrane damage in vitro. Lipids. 1995;30:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Omoya T, Shimizu I, Zhou Y, Okamura Y, Inoue H, Lu G, Itonaga M, Honda H, Nomura M, Ito S. Effects of idoxifene and estradiol on NF-kappaB activation in cultured rat hepatocytes undergoing oxidative stress. Liver. 2001;21:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Inoue H, Shimizu I, Lu G, Itonaga M, Cui X, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M. Idoxifene and estradiol enhance antiapoptotic activity through estrogen receptor-beta in cultured rat hepatocytes. Dig Dis Sci. 2003;48:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1669] [Cited by in F6Publishing: 1580] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 51. | Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M, Ito S. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Cheng X, Shimizu I, Yuan Y, Wei M, Shen M, Huang H, Urata M, Sannomiya K, Fukuno H, Hashimoto-Tamaoki T. Effects of estradiol and progesterone on tumor necrosis factor alpha-induced apoptosis in human hepatoma HuH-7 cells. Life Sci. 2006;79:1988-1994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Berger M, de Hazen M, Nejjari A, Fournier J, Guignard J, Pezerat H, Cadet J. Radical oxidation reactions of the purine moiety of 2'-deoxyribonucleosides and DNA by iron-containing minerals. Carcinogenesis. 1993;14:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Mohammad Alizadeh AH, Fallahian F, Alavian SM, Ranjbar M, Hedayati M, Rahimi F, Khedmat H, Etemadi A, Zali MR, Azizi F. Insulin resistance in chronic hepatitis B and C. Indian J Gastroenterol. 2006;25:286-289. [PubMed] [Cited in This Article: ] |
| 56. | Kaji K, Nakanuma Y, Sasaki M, Unoura M, Kobayashi K, Nonomura A. Hemosiderin deposition in portal endothelial cells: a novel hepatic hemosiderosis frequent in chronic viral hepatitis B and C. Hum Pathol. 1995;26:1080-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Rigamonti C, Andorno S, Maduli E, Capelli F, Boldorini R, Sartori M. Gender and liver fibrosis in chronic hepatitis: the role of iron status. Aliment Pharmacol Ther. 2005;21:1445-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Zhou XD, DeTolla L, Custer RP, London WT. Iron, ferritin, hepatitis B surface and core antigens in the livers of Chinese patients with hepatocellular carcinoma. Cancer. 1987;59:1430-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 59. | Deugnier Y, Battistelli D, Jouanolle H, Guyader D, Gueguen M, Loréal O, Jacquelinet C, Bourel M, Brissot P. Hepatitis B virus infection markers in genetic haemochromatosis. A study of 272 patients. J Hepatol. 1991;13:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Rouault TA. Hepatic iron overload in alcoholic liver disease: why does it occur and what is its role in pathogenesis? Alcohol. 2003;30:103-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 62. | Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 293] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 63. | Ikejima K, Enomoto N, Iimuro Y, Ikejima A, Fang D, Xu J, Forman DT, Brenner DA, Thurman RG. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol. 1998;274:G669-G676. [PubMed] [Cited in This Article: ] |
| 64. | Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 428] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 66. | Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, Realdi G, Ruol A. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 281] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29:971-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 312] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 68. | Huo T, Wu JC, Hwang SJ, Lai CR, Lee PC, Tsay SH, Chang FY, Lee SD. Factors predictive of liver cirrhosis in patients with chronic hepatitis B: a multivariate analysis in a longitudinal study. Eur J Gastroenterol Hepatol. 2000;12:687-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Yu MW, Hsu FC, Sheen IS, Chu CM, Lin DY, Chen CJ, Liaw YF. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145:1039-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1164] [Cited by in F6Publishing: 1101] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 71. | Zarski JP, Marcellin P, Leroy V, Trepo C, Samuel D, Ganne-Carrie N, Barange K, Canva V, Doffoel M, Cales P. Characteristics of patients with chronic hepatitis B in France: predominant frequency of HBe antigen negative cases. J Hepatol. 2006;45:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24 Suppl 1:17-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Fung SK, Lok AS. Hepatitis B virus genotypes: do they play a role in the outcome of HBV infection? Hepatology. 2004;40:790-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Kensler TW, Egner PA, Wang JB, Zhu YR, Zhang BC, Lu PX, Chen JG, Qian GS, Kuang SY, Jackson PE. Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology. 2004;127:S310-S318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 252] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, Inoue H, Honda H, Ito S. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004;74:897-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of hepatitis C. Hepatology. 1992;15:567-571. [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 387] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 81. | Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 306] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 82. | Lefkowitch JH, Schiff ER, Davis GL, Perrillo RP, Lindsay K, Bodenheimer HC, Balart LA, Ortego TJ, Payne J, Dienstag JL. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology. 1993;104:595-603. [PubMed] [Cited in This Article: ] |
| 83. | Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824-1832. [PubMed] [Cited in This Article: ] |
| 84. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] [Cited in This Article: ] |
| 85. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 893] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 86. | Gressner AM, Lotfi S, Gressner G, Lahme B. Identification and partial characterization of a hepatocyte-derived factor promoting proliferation of cultured fat-storing cells (parasinusoidal lipocytes). Hepatology. 1992;16:1250-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Gordon A, McLean CA, Pedersen JS, Bailey MJ, Roberts SK. Hepatic steatosis in chronic hepatitis B and C: predictors, distribution and effect on fibrosis. J Hepatol. 2005;43:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Lin YC, Hsiao ST, Chen JD. Sonographic fatty liver and hepatitis B virus carrier status: synergistic effect on liver damage in Taiwanese adults. World J Gastroenterol. 2007;13:1805-1810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1368] [Cited by in F6Publishing: 1353] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 90. | Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, Kim HH, Yang US, Yu DY, Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. 2007;132:1955-1967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 91. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 92. | Powell EE, Jonsson JR, Clouston AD. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 93. | Lettéron P, Duchatelle V, Berson A, Fromenty B, Fisch C, Degott C, Benhamou JP, Pessayre D. Increased ethane exhalation, an in vivo index of lipid peroxidation, in alcohol-abusers. Gut. 1993;34:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 193] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 95. | Berson A, De Beco V, Lettéron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 96. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 752] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 97. | Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124:248-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 98. | Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 840] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 100. | Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 101. | Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24:226-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 317] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 102. | Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:E317-E325. [PubMed] [Cited in This Article: ] |
| 103. | Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 328] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 104. | Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105:1819-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Van Hoof M, Rahier J, Horsmans Y. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1996;124:855-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Oien KA, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, MacSween RN. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353:36-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 521] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 108. | Rogers A, Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Rachoń D, Myśliwska J, Suchecka-Rachoń K, Wieckiewicz J, Myśliwski A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol. 2002;172:387-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Kishihara Y, Hayashi J, Yoshimura E, Yamaji K, Nakashima K, Kashiwagi S. IL-1 beta and TNF-alpha produced by peripheral blood mononuclear cells before and during interferon therapy in patients with chronic hepatitis C. Dig Dis Sci. 1996;41:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Hsu HY, Chang MH, Ni YH, Lee PI. Cytokine release of peripheral blood mononuclear cells in children with chronic hepatitis B virus infection. J Pediatr Gastroenterol Nutr. 1999;29:540-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | González-Amaro R, García-Monzón C, García-Buey L, Moreno-Otero R, Alonso JL, Yagüe E, Pivel JP, López-Cabrera M, Fernández-Ruiz E, Sánchez-Madrid F. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179:841-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 223] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 113. | Lara-Pezzi E, Majano PL, Gómez-Gonzalo M, García-Monzón C, Moreno-Otero R, Levrero M, López-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor alpha gene expression in hepatocytes. Hepatology. 1998;28:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 114. | Kilbourne EJ, Scicchitano MS. The activation of plasminogen activator inhibitor-1 expression by IL-1beta is attenuated by estrogen in hepatoblastoma HepG2 cells expressing estrogen receptor alpha. Thromb Haemost. 1999;81:423-427. [PubMed] [Cited in This Article: ] |
| 115. | Ozveri ES, Bozkurt A, Haklar G, Cetinel S, Arbak S, Yeğen C, Yeğen BC. Estrogens ameliorate remote organ inflammation induced by burn injury in rats. Inflamm Res. 2001;50:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Shiraishi T, Shimizu I, Itagaki T, Urata M, Tamaki K, Fukuno H, Yano H, Kataoka K, Wada S, Ito S. Inhibitory effects of estrogen on chemokine and proinflammatory cytokine production in mononuclear cells and macrophages [abstract]. Gastroenterology. 2006;150:A-793. [Cited in This Article: ] |
| 117. | Schultze RL, Gangopadhyay A, Cay O, Lazure D, Thomas P. Tyrosine kinase activation in LPS stimulated rat Kupffer cells. Cell Biochem Biophys. 1999;30:287-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Zuckerman SH, Bryan-Poole N, Evans GF, Short L, Glasebrook AL. In vivo modulation of murine serum tumour necrosis factor and interleukin-6 levels during endotoxemia by oestrogen agonists and antagonists. Immunology. 1995;86:18-24. [PubMed] [Cited in This Article: ] |
| 119. | Matsubara K, Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990;7:243-260. [PubMed] [Cited in This Article: ] |
| 120. | Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Stürzbecher HW, Hoeijmakers JH, Harris CC. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55:6012-6016. [PubMed] [Cited in This Article: ] |
| 121. | Tanaka Y, Mukaide M, Orito E, Yuen MF, Ito K, Kurbanov F, Sugauchi F, Asahina Y, Izumi N, Kato M. Specific mutations in enhancer II/core promoter of hepatitis B virus subgenotypes C1/C2 increase the risk of hepatocellular carcinoma. J Hepatol. 2006;45:646-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498-500. [PubMed] [Cited in This Article: ] |
| 123. | Villa E, Dugani A, Moles A, Camellini L, Grottola A, Buttafoco P, Merighi A, Ferretti I, Esposito P, Miglioli L. Variant liver estrogen receptor transcripts already occur at an early stage of chronic liver disease. Hepatology. 1998;27:983-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Shimizu I, Yasuda M, Mizobuchi Y, Ma YR, Liu F, Shiba M, Horie T, Ito S. Suppressive effect of oestradiol on chemical hepatocarcinogenesis in rats. Gut. 1998;42:112-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Pinzani M, Romanelli RG, Magli S. Progression of fibrosis in chronic liver diseases: time to tally the score. J Hepatol. 2001;34:764-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Vargas R, Wroblewska B, Rego A, Hatch J, Ramwell PW. Oestradiol inhibits smooth muscle cell proliferation of pig coronary artery. Br J Pharmacol. 1993;109:612-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 130] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res. 1998;83:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 128. | Bayard F, Clamens S, Meggetto F, Blaes N, Delsol G, Faye JC. Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture. Endocrinology. 1995;136:1523-1529. [PubMed] [Cited in This Article: ] |
| 129. | Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Lubahn DB, O'Donnell TF, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3:545-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 325] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 130. | Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, Silbiger S. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 104] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer FE. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1061] [Cited by in F6Publishing: 1094] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 132. | Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1512] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 133. | Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |