Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2758
Revised: February 6, 2007
Accepted: March 1, 2007
Published online: May 21, 2007
Although spinal tumors are uncommon, they may reduce survival or cause serious functional disorders in the extremities. Metastatic spinal tumors from malignant tumors can induce symptoms of spinal cord compression, such as paraplegia, quadriplegia, and vesicorectal disturbance, which are aggravated with progression of the diseases and time. We report a patient with hepatocellular carcinoma (HCC) who was suspected of having spinal lesions based on neurological findings, and a metastatic spinal tumor was found by imaging examination. Assuming that metastasis had occurred at the time lumbar pain developed, the patient reached the level of gait disturbance within only 4 mo, showing a rapid advancement of symptoms. If early diagnosis had been possible, treatment could be performed before acute myelopathy progressed to complete paralysis. We speculate that the terminal stage of HCC is not only liver failure associated with intrahepatic lesions but also metastasis to other regions, treatment for individual pathologies therefore, will be needed, which constitutes an important issue.
- Citation: Tamaki K, Shimizu I, Urata M, Kohno N, Fukuno H, Ito S, Sano N. A patient with spinal metastasis from hepatocellular carcinoma discovered from neurological findings. World J Gastroenterol 2007; 13(19): 2758-2760
- URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2758.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2758
Although spinal tumors are uncommon, they may reduce survival or cause serious functional disorders in the extremities. In the established differential diagnosis of spinal tumors, location is the most important feature, but clinical symptoms and the patient’s age and gender should also be considered. Metastatic spinal tumors from malignant tumors can induce symptoms of spinal cord compression, such as paraplegia, quadriplegia, and vesicorectal disturbance, which are aggravated with their progression and time. Thus, early clinical and imaging diagnoses may enable the early initiation of treatment. Maintenance of the patient’s QOL is also important. We report herein a patient with hepatocellular carcinoma (HCC) who was suspected of having spinal lesions based on neurological findings, and a metastatic spinal tumor could be diagnosed by imaging examination. Since there have been very few case reports of spinal metastasis from HCC[1-3], we report this case with discussion of the literature.
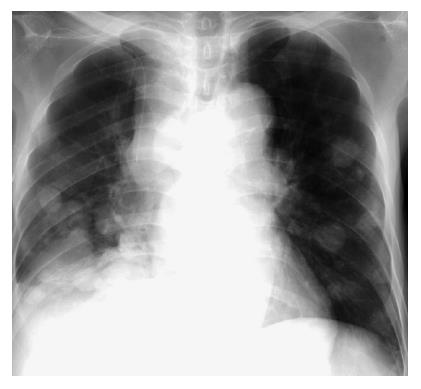
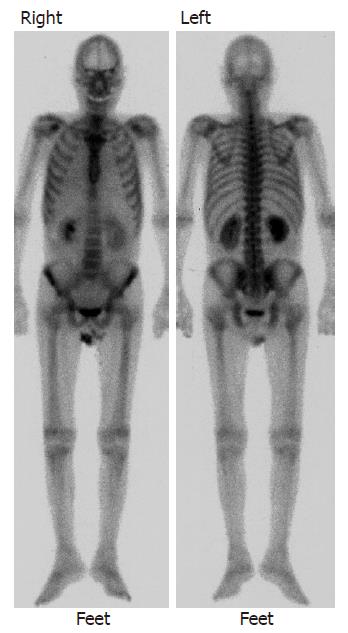
A 67-year-old male was diagnosed with chronic hepatitis C in 1994, and had been under treatment at the outpatient clinic of our hospital. In August 2000, HCC was found in the right hepatic lobe, and radiofrequency ablation (RFA) was performed. Treatments, such as transcatheter hepatic arterial embolization (TAE) and RFA, were performed thereafter on a regular basis. In December 2002, multiple metastases were noted in the lung (Figure 1), and systemic chemotherapy was initiated. Lumbar pain appeared at this time, but no abnormality, such as bone metastasis, was detected by imaging examination including bone scintigraphy (Figure 2) and CT. As the lumbar pain became slowly aggravated, and weakness of the lower limbs and gait disturbance progressed, the patient was admitted for examination and treatment on April 2, 2003 (Table 1).
| Hematology | Blood chemistry | ||
| WBC | 3100/mm3 | AST | 196 IU/L |
| RBC | 316 × 104/mm3 | ALT | 78 IU/L |
| Hb | 10.6 g/dL | LDH | 533 IU/L |
| Hct | 33.5 g/dL | T-Bil | 1.7 mg/dL |
| Plt | 8.0 × 104 /mm3 | D-Bil | 0.8 mg/dL |
| ALP | 290 IU/L | ||
| Coagulation | γ-GTP | 158 IU/L | |
| HPT | 63.7% | TP | 6.6 g/dL |
| Serology | Alb | 2.9 g/dL | |
| CRP | 2.01 mg/dL | T-Cho | 133 mg/dL |
| TG | 78 mg/dL | ||
| Virus marker | BUN | 12 mg/dL | |
| HBs-Ag | (-) | Cr | 0.6 mg/dL |
| HBs-Ab | (-) | Na | 139 mEq/L |
| HBc-Ab | (-) | K | 4.0 mEq/L |
| HBc-IgM | (-) | Cl | 103 mEq/L |
| HBe-Ag | (-) | Glu | 110 mg/dL |
| HBe-Ab | (-) | Tumor marker | |
| HCV-Ab | (+) | AFP | 220150 |
| PIVKA-II | 459000 mAU/mL |
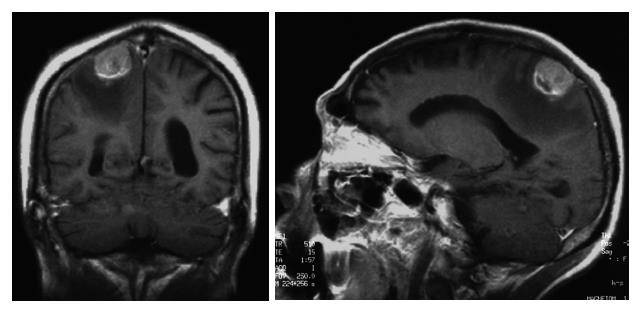
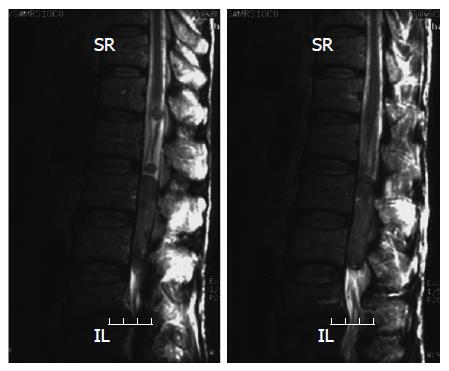
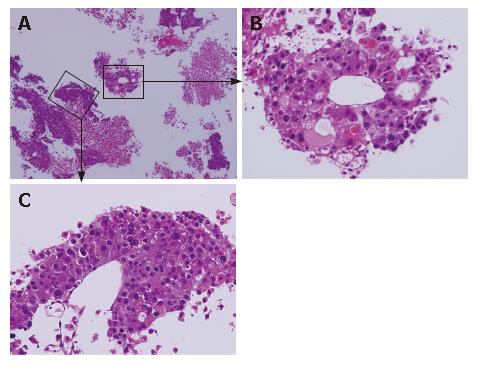
Head MRI on admission detected a 2.5-cm tumor, which may have metastasized from the HCC, and surrounding edema in the right parietal lobe (Figure 3). Symptoms of increased intracranial pressure appeared on the day following admission, and conservative treatment was initiated. Since paralysis of the bilateral legs, imperception, and vesicorectal disturbance appeared, a spinal cord MRI was performed, suspecting spinal lesions, and tumorous lesions were diffusely present from the medullary cone to cauda equina (Figure 4). Based on the rapid aggravation of the symptoms, a metastatic spinal tumor from the HCC was diagnosed. Aggressive treatment was considered not possible due to the general condition of the patient. Symptomatic treatment was therefore performed, but the patient died of respiratory insufficiency on April 14. An autopsy of the spinal tumor was performed to establish a definite diagnosis after consent was obtained from his family, and the pathological diagnosis was confirmed as metastasis of HCC (Figure 5).
Among the symptoms of spinal tumors are pain and abnormal sensations in the region innervated by the tumor-impaired region and subsequent muscle weakness, imperception, muscular atrophy, and in many cases vesicorectal disturbance. Okamoto et al[4] reported that weakness/paraplegia occurred in 85%, numbness/loss of sensation in 65%, bladder/bowel dysfunction in 75%, and local pain (backache, lumbago, and root pain) in 65% as symptoms of intradural parenchymal involvement (IPI).
Spinal metastasis is known to cause early aggravation or neurological symptoms. Edema and hemorrhage of the tumor and rapid tumor growth or secondary obstruction of spinal blood vessels may rapidly aggravate the symptoms[5]. Assuming that metastasis had occurred at the time lumbar pain developed, the patient would reach the level of gait disturbance within only 4 mo, showing a rapid advancement of symptoms. If early diagnosis had been made, treatment, such as decompression, could be performed before acute myelopathy progressed to complete paralysis.
The pathogenic mechanism of spinal metastasis might be as follows: (1) The coexistence of pulmonary and brain metastases supports the conclusion of dissemination through the arterial route; (2) the other is spread through the vertebral venous plexus, extending from the pelvis to the cranial venous sinuses, enabling retrograde transportation to the spinal cord; (3) direct invasion from contiguous structures; and (4) intraspinal dissemination.
This patient also had a metastatic brain tumor. HCC rarely metastasizes to the brain. In autopsy series, brain metastases were found in 1.3% in Japan[6]. In contrast, an American survey of 482 cases of intracranial metastases by the University of Maryland and John Hopkins University did not find a single case of metastatic HCC[7]. With the diffuse presence of tumors around the spinal cauda equina, intraspinal dissemination from the metastatic brain tumor appeared to be likely. Edelson et al[8] reported that more tumors are involved in the lumbar cord and cauda equina than it is expected from its length because the spinal cord is a common site of meningeal seeding.
Spinal tumors are classified into 3 types based on their localization: extradural, intradural-extramedullary, and intramedullary. Generally, extradural lesions most frequently metastasize. Intradural lesions are rare. Most lesions are extrameduallary, such as schwannoma and neurofibroma. Among spinal tumors, intramedullary tumors are uncommon, and most cases are astrocytoma and ependymoma[9]. MRI plays a central role in the diagnosis of these tumors[10], and readily permits their classification. However, aggressive examinations are not applicable in many cases because spinal tumors usually appear in the advanced stage. And spinal tumors are collectively handled with metastatic brain tumors and carcinomatous meningitis in many cases. Thus, a definite diagnosis before death is difficult and proper use of imaging examination will greatly assist in screening for tumors from other spinal diseases.
In conclusion, PI is usually one of the late manifestations in patients with widespread diseases. Thus, the prognosis of patients with IPI is poor.
To reduce pain in patients with a widespread disease, an early recognition of IPI symptoms and signs, and palliative treatment are necessary.
Advances in medical techniques have enabled the diagnosis and treatment of HCC in early stages, and have significantly prolonged the survival of the patients. We speculate that the terminal stage of HCC will be not only liver failure associated with intrahepatic lesions but also metastasis to other regions, therefore, the prognosis of the patients and the treatment for individual pathologies should be determined , which constitutes an important issue in this area.
S- Editor Liu Y L- Editor Ma JY E- Editor Che YB
| 1. | Cho KB, Sohn JH, Park KS, Kwon DY, Lee YS, Hwang JS, Hur JW, Ahn SH, Park SK. A case of primary hepatocellular carcinoma with metastasis to the spinal cord. Taehan Kan Hakhoe Chi. 2002;8:218-222. [PubMed] [Cited in This Article: ] |
| 2. | Kim M, Na DL, Park SH, Jeon BS, Roh JK. Nervous system involvement by metastatic hepatocellular carcinoma. J Neurooncol. 1998;36:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Friedman HD. Hepatocellular carcinoma with central nervous system metastasis: a case report and literature review. Med Pediatr Oncol. 1991;19:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Okamoto H, Shinkai T, Matsuno Y, Saijo N. Intradural parenchymal involvement in the spinal subarachnoid space associated with primary lung cancer. Cancer. 1993;72:2583-2588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Grem JL, Burgess J, Trump DL. Clinical features and natural history of intramedullary spinal cord metastasis. Cancer. 1985;56:2305-2314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 6. | Okuda K. Clinical aspects of hepatocellular carcinoma: Analysis of 134 cases. Hepatocellular Carcinoma. New York: John Wiley & Sons 1976; 387-436. [Cited in This Article: ] |
| 7. | Brunner DR, Dunne MG, Rao KC, Sutherland JC, Cisternino SJ. Hepatoma presenting as a cerebellar metastasis. J Comput Tomogr. 1982;6:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Edelson RN, Deck MD, Posner JB. Intramedullary spinal cord metastases. Clinical and radiographic findings in nine cases. Neurology. 1972;22:1222-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 143] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Van Goethem JW, van den Hauwe L, Ozsarlak O, De Schepper AM, Parizel PM. Spinal tumors. Eur J Radiol. 2004;50:159-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459-469. [PubMed] [Cited in This Article: ] |