INTRODUCTION
5-Fluorouracil (5-FU) is an antimetabolite that is widely used in the treatment of solid tumors. The metabolism and pharmacokinetics of 5-FU play important roles in determining its efficacy and toxicity. Cardiac events related to 5-FU have been described since 1975[1]. Recent prospective clinical trials have demonstrated that 2% to 10% of the patients exposed to 5-FU developed cardiovascular complications[2,3]. Capecitabine is a fluoropyrimidine carbamate active in several solid tumors. Its use will be probably increased in the coming years in patients with colorectal cancer due to the recent findings of a randomized trial[4]. This clinical trial demonstrated that capecitabine is an effective alternative to intravenous fluorouracil (5-FU) plus leucovorin in the adjuvant treatment of colon cancer. Capecitabne is converted to the active 5-FU by the action of a series of enzymes. One of these enzymes, thymidine phosphorylase (TP), has higher concentrations in tumor tissue than in normal tissue[5]. This suggests that the activation occurs preferentially in tumor tissue, providing a favorable ratio for toxicity and radiosensitization. Only a few cases of capecitabine-induced cardiotoxicity have been reported to date[6-10]. The first case described occurred in a patient with a prior history of 5-FU-related cardiotoxicity[7]. Another report described a case of coronary spasm after drug administration in a woman with moderate arterial hypertension[9]. To our knowledge, capecitabine-induced coronary vasospasm has been only once reported in patients without previous history of cardiologic events[8]. A common etiology for capecitabine and 5-FU-induced coronary vasospasm has recently been suggested in a colon cancer patient[6].
Walko et al recently compiled a review of the literature reporting adverse effects observed in capecitabine-treated patients[10]. Chest pain occurred in 6% of 758 breast and colorectal cancer patients treated with capecitabine at a dose of 2500 mg/m2 per day in 2 divided doses for 14 d followed by 1 wk rest. They concluded that this complication is more frequent in patients who have a history of coronary artery disease and recommended close monitoring for cardiac abnormalities during therapy.
The present case report describes several episodes of angina after oral administration of capecitabine in a patient without prior cardiac history and without previous treatment with fluoropyrimidines.
CASE REPORT
A 71 year-old male patient with a moderately differentiated adenocarcinoma of the rectum (uT3N0) was to be treated with intensity modulated radiation therapy (48, 34 Gy to the 95% of the Gross Tumor Volume) with Oxaliplatin 60 mg/m2 on d 1, 8 and 15 and Capecitabine 825 mg/m2 bid given on the radiation days. Surgery was to follow 4-6 wk later.
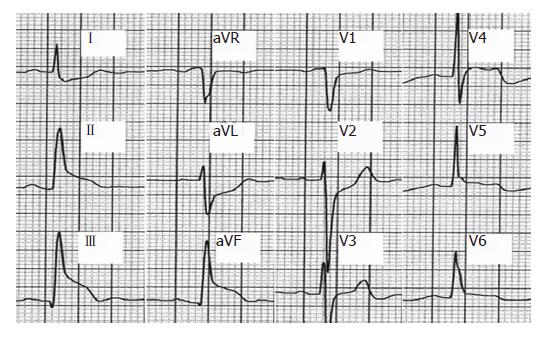
On March 16, 2005, the patient started radiotherapy; he received the first cycle of Oxaliplatin and was placed on Capecitabine. On radiation d 8, he received the second chemotherapy cycle without any complications. On d 9, he complained of stomach pain and nausea, and gastric protection and antiemetics were increased. On d 15, the third cycle of Oxaliplatin was given, and the patient continued with oral capecitabine. He continued to complain of the same stomach pain which improved with vomiting. On d 17 he came to the emergency room complaining of thoracic pressure, sweat, and dyspnea during exercise on two different occasions. He had had two previous episodes of chest pain at rest, and one of these episodes woke him up. A cardiac check-up was undertaken. The electrocardiogram showed no abnormalities. The echocardiogram demonstrated a normal systolic function with an ejection fraction of 0.55. During the stress echocardiography with exercise the patient had oppressive thoracic pain, ST segment elevation in precordial and inferior electrocardiographic leads (Figure 1) and contractility changes (akinesia and severe hypokinesia) in the territory of the right and anterior descending coronary arteries respectively. Cardiac catheterization did not reveal significant coronary artery disease and intracoronary injection of ergonovine failed to induce coronary spasm in the epicardial arteries. Capecitabine administration was discontinued, and the patient completed the planned irradiation course uneventfully.
Figure 1 EKG during the stress ecocardiography.
ST elevation in anterior (V4-V6) and inferior leads (DII, DIII, aVF).
Dihydropyrimidine dehydrogenase (DPD) defficiency, which may cause accumulation of potentially cardiotoxic 5-FU metabolites, was ruled out. DNA was isolated from purified lymphocytes by standard procedures and PCR amplification of exon 14 and its flanking intronic regions was performed. Further sequence analysis was carried out on an Applied Biosystems model 377 automated DNA sequencer using the dye-terminator method. DNA sequence was clearly wild-type (Figure 2).
Figure 2 Wild-type sequence at 5'-splice recognition site of intron 14 in DPD gene.
DISCUSSION
Capecitabine is converted to 5´-deoxy-5-fluorocytudine (5'DFCR) by carboxylesterase in the liver, then to 5´DFUR by cytidine deaminase, and finally to 5-FU in tumor tissue by pyrimidine nucleoside phosphorylase. TP, an angiogenic substance identical to the angiogenic agent “platelet derived endothelial cell growth factor” (PD-ECGF)[11,12], is required for the final activation step of the prodrug. TP has been found to be expressed in atherosclerotic plaques[13]. This fact could explain the higher risk in patients with a prior history of coronary disease.
Deficiency of dihydropyrimidine dehydrogenase (DPD), the rate-limiting enzyme in fluoropyrimidines catabolism, has been linked to toxic side effects of these drugs. The most prominent mutation of the DPD gene resulting in severe DPD deficiency is a G to A mutation in the GT 5’-splice recognition site of intron 14 (exon 14-skipping mutation). The corresponding mRNA lacks exon 14, and the enzymatic activity of the translated DPD protein is virtually absent (Figure 2).
Myocardial injury, thrombogenic effects, immunoallergic reaction, and ischemia secondary to coronary artery spasm have all been implicated in the mechanism of 5-FU-induced cardiac toxicity, although coronary spasm is thought to be the main one[14-17]. Capecitabine toxicity is thought to have the same etiology as 5-FU, although Capecitabine and its metabolites are minimally cytotoxic in vitro compared with 5-FU[18].
Coronary spasm is defined as an abnormal contraction of an epicardial coronary artery resulting in myocardial ischemia. The commonly associated manifestations of myocardial ischemia are oppressive chest pain and ST-segment alterations on the electrocardiogram[19]. However, the most sensitive marker of myocardial ischemia is the appearance of a wall motion abnormality, which can be detected by echocardiography[20].
Our patient presented with rest and exercise-induced angina, and the stress echocardiogram revealed exercise-induced cardiac ischemia in the territory of the right and anterior descending coronary arteries. The coronary angiography was normal, both in baseline conditions and during intracoronary ergonovine injection. The absence of epicardial coronary stenosis ruled out atherosclerotic coronary disease as the cause of ischemia. The expected finding in this case was ergonovine-induced coronary spasm, but it failed to occur. Sometimes the test is negative in patients with spontaneous spasm because the sensitivity of ergonovine to induce spasm is below 100%. Another explanation for the discrepancy between the exercise stress test and the angiographic findings is the occurrence of spasm at the microvascular level, in small coronary vessels.
In conclusion, capecitabine should be considered a drug with cardiotoxic potential even in the absence of prior cardiac history. It can induce coronary spasm at the macro or microvascular level.