Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1477
Revised: January 9, 2007
Accepted: March 6, 2007
Published online: March 14, 2007
The gastrointestinal tract represents the largest mucosal membrane surface in the human body. The immune system in the gut is the first line of host defense against mucosal microbial pathogens and it plays a crucial role in maintaining mucosal homeostasis. Membranous or microfold cells, commonly referred to as microfold cells, are specialized epithelial cells of the gut-associated lymphoid tissues (GALT) and they play a sentinel role for the intestinal immune system by delivering luminal antigens through the follicle-associated epithelium to the underlying immune cells. M cells sample and uptake antigens at their apical membrane, encase them in vesicles to transport them to the basolateral membrane of M cells, and from there deliver antigens to the nearby lymphocytes. On the flip side, some intestinal pathogens exploit M cells as their portal of entry to invade the host and cause infections. In this article, we briefly review our current knowledge on the morphology, development, and function of M cells, with an emphasis on their dual role in the pathogenesis of gut infection and in the development of host mucosal immunity.
- Citation: Miller H, Zhang J, KuoLee R, Patel GB, Chen W. Intestinal M cells: The fallible sentinels? World J Gastroenterol 2007; 13(10): 1477-1486
- URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1477.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1477
The gastrointestinal (GI) tract, in addition to its role as an organ for nutrient absorption, represents a key interface between the host and its external environment. Since the GI tract has the highest recorded bacterial cell density of any microbial ecosystem[1], it is not surprising that the GI immune system is both extensive and complex. The GI tract contains more antibody-producing cells than in the spleen and lymph nodes combined, and it contributes the majority of the body’s immunoglobulin production in the form of IgA secreted into the intestinal lumen[2,3]. The GI mucosa, due to its large surface area (200 times greater than the skin)[4], requires consistent monitoring for potentially harmful agents (such as pathogens) while discriminating these from harmless food and non-pathogenic antigens. Gut-associated lymphoid tissue (GALT), consisting of Peyer’s patches (PP), appendix, and other lymphoid aggregates in the large intestine, plays crucial roles in the maintenance of homeostasis in the GI system. The membranous or microfold cell (M cell) in the Peyer’s patches is one of the primary cell types responsible for the capability of the intestinal immune system to mount both immunological and mucosal tolerogenic responses to foreign antigens.
This review will briefly summarize the current knowledge on intestinal M cells, with the emphasis of its potential role in GI infection and immunity. However, it is worth noting that M cells are also present in other mucosa-associated lymphoid tissues (MALT), such as the bronchus-associated lymphoid tissue (BALT) and nasal-associated lymphoid tissue (NALT)[5].
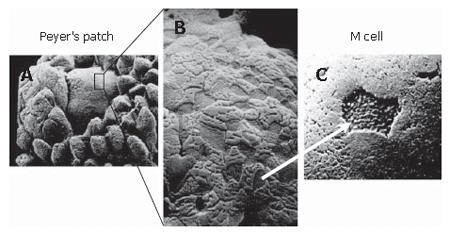
M cells are specialized epithelial cells forming part of the follicle-associated epithelium (FAE) which overlies the PP and other lymphoid aggregates. The most striking feature of the human M cell under light or electron microscopy is the absence of surface microvilli which are characteristic of the intestinal epithelial cells. Instead, the apical membrane of the M cell has a microfold (or membranous) topography (Figure 1)[6-8], and hence the name M cell. Like other epithelial cells, M cells form tight junctions to maintain a barrier function, albeit with different structural features and adhesion protein expression[9]. The basolateral membrane of M cells is invaginated, and forms many “pockets”, which harbor infiltrating lymphocytes[10]. The formation of these “pockets” greatly reduces the intracellular distance that antigens have to travel and allows M cells to rapidly transport (within 10 to 15 min) antigenic materials to the basolateral membrane[11,12].
The morphology of M cells varies greatly amongst different animal species, and within anatomic sites of a species. For instance, the microfold structure is present only in human M cells[7], and human M cells lack microvilli. In contrast, the microvilli are present on the surface of murine M cells, but these are short and irregular[13] in contrast to the microvilli on the M cells of rabbit caecal lymphoid patches which are longer than the neighboring enterocytes[14]. The M cells express a wide range of carbohydrate markers with diverse glycoconjugate profiles[15], which perhaps allows M cells to interact with a broad range of microbes[16,17]. For example, while Ulex europaeus agglutinin-1 (UEA-1), an α-L-fucose residue- specific lectin which selectively labels fucose, recognizes M cells and goblet cells overlying the mouse PP[18,19], it fails to react with M cells on the mouse caecum or colon[15,20]. Conversely, UEA-1 does not bind to M cells of rabbit PP but reacts with those in the caecal lymphoid patches[21]. As a result, studies of rabbit M cells have frequently used vimentin, instead of UEA-1, as histochemical markers[22-25]. On the other hand, human M cells are generally negative for specific lectin binding[26], but are positive for the sialyl Lewis A antigen[27]. M cells in rats, guinea pigs and cats share similar lectin-binding patterns to enterocytes, although the cytokeratins 8 and 18 are over-expressed in M cells of rats and pigs, respectively, compared to neighboring enterocytes[28,29]. Because of these variations and diversity in the morphology and lectin-binding patterns, multiple confirmatory characteristics are usually required for the positive identification and characterization of M cells. Although glycosylation patterns and lectin-binding properties remain commonly used identifiers of M cells due to their relative ease of analyses, electron microscopy currently remains the most definitive method for M cell identification[15,20,30,31].
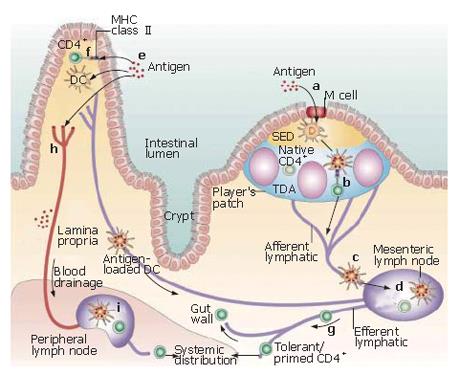
One of the major functions of M cells is believed to be the uptake and transport of antigens from the gut lumen to the underlying mucosal immune system (Figure 2)[32]. The apical membrane of M cells is specialized for the uptake and transport of antigens, featuring a reduced glycocalyx[33], and a general lack of membrane hydrolytic enzymes[34]. Additionally, the dramatic reduction of lysosomes may allow M cells to transport microorganisms into the lymphoid follicles without altering their antigenic properties[35]. M cells have been shown to be able to transport proteins[36,37], bacteria[31,38,39], viruses[40] and non-infectious particles[41,42] from the apical membrane to the basolateral surface. Bacteria and large particle transport is accomplished by phagocytosis, accompanied by apical membrane ruffling and actin cytosketeton rearrangements[38,43]. Under electron microscopy observation, M cells appear to reach out and engulf these large particles. Viral, and small adherent particles are endocytosed in clathrin-coated vesicles[41], while non-adherent antigens undergo fluid phase pinocytosis[6,11]. However, the role of M cells in antigen processing and presentation per se remains unclear. Although there have been several reports of M cells expressing major histocompatibility complex (MHC) class II molecules[44-46], these findings could not be confirmed by others[32,47,48]. However, M cells do express cathepsin E, which is typically expressed on antigen-presenting cells[49], and M cells can also produce the pro-inflammatory cytokine interleukin 1 (IL-1)[50]. In addition, M cells are the main producers of CC chemokine ligand (CCL) 9 and CCL20 in the FAE[51], and also produce CXC chemokine ligand (CXCL) 16[52].
During embryonic and postnatal development, each crypt in the intestine is a clonal unit[53,54] whose cells differentiate into multiple types as they migrate. Cells on the villous side of the crypt differentiate into absorptive enterocytes, goblet cells and enteroendocrine cells. The cells on the FAE side of the crypt acquire the phenotype of absorptive enterocytes, M cells and, rarely, goblet cells[12,55,56]. Within this framework, two hypotheses have been proposed for the development of M cells. The first hypothesis suggests that M cells originate from a distinct cell lineage following an independent differentiation program. Evidence supporting this hypothesis includes that M cell development in the PP is restricted to specialized dome-associated crypts, and that both M cell precursors and their developmental intermediates have been identified within these dome-associated crypts[34,57] with early commitment of M cells observed in the mid-crypt area of caecum, appendix and PP[58,59]. In addition, the arrangement of some M cells as radial strips on the FAE dome with a single, predominating glycosylation pattern also implies M cell commitment occurs in the dome-associated crypts[14,60,61]. However, even in this example, the M cell glycosylation pattern is heterogenous.
The second hypothesis postulates that M cells develop from FAE enterocytes either as a developmental/transient stage of enterocytes, or in response to local signal stimulations (such as contact with lymphocytes and chemokines/cytokines). Indeed, Caco-2 cells, a human intestinal adenocarcinoma cell line, differentiate into an M cell-like morphology and phenotype after in vitro co-culture with murine PP-derived lymphocytes[39] or human B lymphoma cell lines. Moreover, intravenous injection of PP lymphocytes or normal bone marrow transplantation into severe combined immunodeficient (SCID) mice correlates with the development of M cells in the FAE[62,63]. The hypothesis that M cells are derived from enterocytes[64] is also supported by ultrastructural studies of chicken caecal tonsils[65], and by cell division and apoptosis studies in mouse PP[66] and in rabbit ileal PP[61]. Furthermore, a possible intermediate M cell/enterocyte cell type has been recently identified in upper regions of the dome in pigs[59].
Kerneis and Pringault have merged these diverse observations together into a single postulation that intestinal cell differentiation is largely determined by the crypt stem cells[67]. However, with the proper stimuli, alternate differentiation pathways could be followed (which they term “intestinal cell plasticity”)[67]. In the case of M cells, enterocytes (perhaps immature) may convert into an M cell phenotype[67].
Although it is generally recognized that the mucosal lymphoid cells induce the development of the overlying specialized FAE, and that cell-to-cell contact and/or soluble factors provide important signals for the development of M cells[12], the events and signaling pathways directly involved in M cell differentiation and development remain poorly understood. The tumor necrosis factor (TNF) family of cytokines, particularly lymphotoxin (LT)-α,LT-β, and TNF-α produced by B cells, appear to play crucial roles in the development of Peyer's patches and FAE[68,69]. Their involvement in the development of M cells per se is, however, less clear. In the absence of LT-α and LT-β, the specialized areas of PP anlagen in the embryonic intestine are not formed[70-72]. Also, LT-β receptor-knockout mice lack PP[69]. However, mice whose B cells do not express LT-β do have normal FAE and M cells, although with smaller PP. Recombinase-activating gene (RAG)-1 -/- knockout mice, which lack mature B and T lymphocytes, have small PP-like aggregates having a normal M cell density[73]. When the LT-β receptor signaling is blocked by the antagonist lymphotoxin-β receptor-immunoglobulin G fusion protein in RAG-/- mice, the percentage of M cells in the PP-like aggregates decreases, suggesting that the LT-β signaling is essential for the differentiation and development of M cells, but LT-β signaling molecules could be supplied by other cell types in the absence of mature B and T lymphocytes[73]. On the other hand, mice having defective CD40 or IL-4 signaling, defective B-cell proliferation, or deficient in signal transducer and activator of transcription 6 (STAT6) have normal FAE and M cells[18,74]. Furthermore, although Toll-like receptors (TLRs) are expressed by the M cells[75-78], and exposure to bacteria can trigger TLR signaling resulting in induction of M cell proliferation[79] and up-regulation of transcytosis[80], TLR signaling does not appear to be essential for the development of M cells since MyD88-knockout, TLR-2 or TLR-4-knockout mice have normal M cell populations[18,79,81].
The notch signaling system is a recently characterized, highly conserved mechanism which regulates the differentiation, proliferation and apoptotic events at all stages of cell development, including the differentiation and renewal of intestinal epithelial cells and other types of intestinal cells, such as goblet cells, enteroendocrine cells, and Paneth cells[18,82,83]. Therefore, notch and notch ligands may play an important role in M cell developmental signaling. Indeed, the expression of Jagged-1 mRNA, a notch ligand, is increased in the in vitro M cell system compared to the parental epithelial cell line[84]. A subset of cells of the FAE in mice with a mutated Delta-3 gene, a notch ligand, showed abnormal apical membranes (dubbed as ‘C cells’), and it has been suggested that these cells are precursor M cells[18]. This ‘C cell’ morphology has also been observed in normal mice[64].
In the human GI system, M cells are mainly found on the FAE overlying the dome structure of Peyer’s patches in the small intestine[85,86]. The FAE, aside from having M cells, is distinguished by a reduced number of goblet cells and enterocytes[87,88]. Beneath the FAE lies the sub-epithelial dome (SED), a diffuse region of dendritic cells (DCs), naive B cells, CD4+ and CD8+ T cells, and macrophages[55]. Particles transported by the M cells from the lumen can be captured in SED by immature DCs[89], which then migrate to B-cell follicles and parafollicular T-cell zones and become mature DCs[90].
However, M cells are also present over lymphoid follicles in the colon and rectum[91]. These follicles in the colonic crypts have a specialized epithelium with a greater proportion of goblet cells than PP, but fewer than the surrounding colonic regions. Similar to PP M cells, colonic M cells have transport vesicles, a thin glycocalyx, and a basolateral invagination containing pockets of lymphocytes[13].
The percentage of M cells comprising FAE varies substantially among host species and their anatomical locations, ranging from 5% to 10% in the human and murine PP[92] to about 50% in the rabbit and human caecal lymphoid aggregates[27,93].
The accessibility of M cells on the mucosal surface and their ability to transcytose particulate material make the M cells an ideal entry point for potential pathogens. Indeed, it has been demonstrated that M cells can transport a diverse array of mucosal microorganisms across the intestinal epithelial barrier, including bacteria (Vibrio cholerae[94], Campylobacter jejuni[95], Mycobacterium tuberculosis[13], Shigella spp.[96,97], Salmonella spp.[98,99], Escherichia coli[100,101], Yersinia spp.[102]), viruses (MMTV virus[74,103], polioviruses[104], reoviruses[105-107], prions[108] and HIV[40,109]) and parasites (Cryptosporidium[110]). In fact, many pathogens exploit the M cells as a conduit to invade the host and establish an infection. In this regard, enteropathogens, such as Salmonella typhimurium, S. typhi, Shigella sp. and Yersinia spp., are capable of directly invading and destroying M cells and spreading the infection to neighboring enterocytes. For example, S. typhimurium initially invades the M cells[98,99], and induces a spotty and diffuse infection pattern with small groups of infected M cells[111]. Experimental infection in calves have shown that S. typhimurium is ingested by M cells within 5 min of contact[99,112]. The process ends with the exfoliation of majority of the infected M cells within 30 min, and cell death within an hour. This disruption of M cells allows the pathogen access to the neighboring enterocytes, and results in the sloughing off of the FAE[113,114]. Although these results have not been directly confirmed in humans, ulcerations are nevertheless present in regions corresponding to PP in cases of typhoid infection[115]. Similarly, free HIV particles use both, M cells and DCs, as conduits to infect local CD4+ T cells[40,109,116]. In addition, it has been shown that the success of host adaptation of Salmonella in pigs is closely associated with the increased number of pathogens per M cell, as compared to the parental strain[117].
Other enteropathogens, such as Shigella species, are capable of attaching and adhering to M cells, but do not necessarily induce any cytotoxicity to the infected cells[97,118,119]. Instead, it induces membrane ruffling[96], and the afflicted M cells increase in size, rather than proliferating, to accommodate increased numbers of mononuclear cells in their basolateral pocket[96].
The interaction between intestinal pathogens and M cells are likely influenced and controlled by factors deriving from both the pathogen and the host. In this regard, the long polar fimbria (LPF) produced by the lpf operon and Salmonella pathogenicity island-1 (SPI-1) encoding the type III secretory system play important roles in selective adherence of Salmonella to M cells[120,121]. LpfC or SPI-1 mutants of Salmonella show reduced colonization, decreased virulence, are not cytotoxic to M cells and are not disruptive to the FAE[99,122]. Transformation of the lpf operon into non-piliated E coli increased their uptake in PP[120]. Similarly, the uptake of Yersinia and Shigella by M cells is mediated by invasin or mechanisms encoded by a 30-kb virulence plasmid, respectively[102]. The presence or absence of these M cell-targeting gene products in pathogens might explain the differences seen amongst different strains of the pathogen in their attachment to M cells. For example, the rabbit diarrheagenic E. coli (RDEC)-1 strain is selective for adherence to M cells[100,101], whereas enterohemorrhagic E. coli (EHEC), such as strain O157:H7, has been found to attach to the FAE of human PP[123]. On the other hand, enteropathogenic E. coli (EPEC) is not transcytosed by the M cells and remains in the gut lumen.
It appears that most of the bacterial genes and their products identified to date for their invasive role represent the primary, but not the exclusive, mechanism for the entry of pathogens in M cells[124]. In Salmonella cases, some Salmonella serotypes, which are M cell selecting, lack the lpf operon, and others with the lpf operon do not target M cells[120,125]. Similarly, invasin-deficient Y. pseudotuberculosis mutants have delayed uptake of 3 to 5 d in vivo, but are nonetheless found in the spleen and liver at the same time and produce the same LD50 values, as the wild-type strain[126]. Perhaps, M cells can also recognize other Yersinia adhesins, such as pH 6 antigen and the plasmid encoded YadA, but with less affinity than for invasin[127]. In addition, the expression of the lpf operon has been found to cycle between ‘off’ and ‘on’, being referred to as phase variation[128,129]. It is probably an adaptation to avoid host defence. In this regard, cultivation in Lauria Bertani (LB) broth appears to increase the proportion of S. typhimurium in the lpf operon ‘on’phase[128].
It is now recognized that the entry of M cells by intestinal pathogens is also mediated by a number of surface adhesion molecules, particularly those within the integrin family, of the host cells. In this regard, enteropathogenic Y. pseudotuberculosis can attach and invade murine M cells viaβ1-integrins expressed by the apical M cell membranes[130-132]. Studies have postulated that β1-integrins were the receptor for Yersinia invasin protein[131]. In vitro studies have demonstrated that α2β1 integrins are the exclusive heterodimer form found on the M cell apical membrane[133], although others have found that this heterodimer does not normally interact with invasin[134]. Other studies have shown that inhibition of α5β1 integrin expression on the apical membranes of Caco-2 cells and M cells in vitro abolished the abilities of these cells to transport microbes[77,135]. In addition, lymphotropic (X4) HIV transport by M cells is CXC4 receptor-mediated and is lactosyl cerebroside-dependent in vitro[109]. Finally, the variation of M cell glycocalyx has led to speculation about its role in pathogen tissue tropism[19,136].
Just as pathogens can exploit M cells as the portal of entry for infection, biomedical researchers have, for many years, investigated the potential of using M cell-specific mechanisms for drug or vaccine delivery to the mucosal immune system[137-140]. Compared to parenteral routes, mucosal administration of drugs and vaccines is relatively simple, safe and inexpensive[141]. An additional benefit of mucosal immunization is its capability of priming and inducing both systemic and mucosal immune responses in the host[142,143]. Mucosal vaccination is necessary for protection against mucosal pathogens because parenteral immunization is generally ineffective for the development of protective mucosal immunity[144], and optimal vaccination strategies for many pathogens may require both mucosal and systemic delivery components[145].
Successful mucosal vaccines must circumvent the same barriers that mucosal pathogens have, i.e. mucus, proteases, nucleases, secreted antibodies, and the epithelial glycocalyx. Mucosal pathogens themselves have so far been the most effective models exploited for mucosal vaccination. The advantages of attenuated, live vaccines include their ability to activate multiple, innate immune responses. Currently, most effective oral vaccines are live attenuated poliovirus and live attenuated S. typhi, both of which exhibit selective binding to M cells and exploit M cell transport to the mucosal lymphoid tissue[99,104]. For this reason, other recombinant bacteria, including attenuated Lactococcus spp., Listeria monocytogenes, and Yersinia spp., have been constructed as delivery vectors for heterologous antigens[146-148].
M cells actively transport microparticles up to 1 μm in size, and those that are adherent to M cells are effectively transcytosed[12,33,149]. Thus, formulations that are multimeric or particulate and adhere to the mucosal surfaces, especially if there are some M cell specificities, seem most effective[145] while soluble, non-adherent antigens are frequently poorly internalized and hence generally induce weak immune responses or even immune tolerance[150]. The packaging of drugs and antigen microparticles on polystyrene or latex mircospheres protects them from degradation within the GI tract as well as allows them to be transcytosed by M cells[42,151-155]. Chitosan microparticles have shown promise both for oral vaccines and intranasal application[156-158]. Others have examined the potential of using copolymeric microparticles[159], proteosomes[160], liposomes[161], virus-like particles[164,163], and viral vectors, such as poliovirus and adenovirus[164,165]. However, these formulations can also bind to enterocytes[161], and are readily taken up by mucosal DCs. In addition, small vesicles derived from outer membrane components of bacteria[160,166] are interesting because of their uptake by M cells and DCs and their potential to induce an innate immune response through the activation of TLR pathways. Unfortunately, their tendency to become trapped in mucus necessitates large doses[145]. There are also regulatory concerns regarding the use of live, attenuated vectors for vaccine delivery, especially for use in immunocompromised population and the risk of reversion of the attenuated strain to full virulence.
Several recent studies have elegantly demonstrated the feasibility to specifically exploit M cells for mucosal vaccine development[167,168]. Manocha et al[167] have shown that HIV peptide bearing microparticles targeted to M cells, using UEA-1 lectins, are more immunogenic when administered mucosally than systemically. Wang et al[169] have used the adhesin protein sigma-1 from the enteric pathogen reovirus, which infects PP M cells, to direct DNA vaccines to the mucosal immune system[168]. Three expression plasmids encoding the genes for HIV gp160, cytoplasmic gp140, and secreted gp140 were conjugated to sigma-1 with poly-L-lysine and individually tested in mice. Intranasal immunization of mice showed specific, long-term CTL responses to gp160[168]. Upon challenge using a standard HIV surrogate test, these mice showed significant antiviral protection.
However, the relative importance of M cells in the induction of protective immunity by mucosal immunization remains unknown. For example, the antigen-specific immune responses as measured by IgG production is not substantially altered in the absence of PP[31]. Although M cells are capable of uptaking and transporting antigens, their role in antigen processing and presentation is less well characterized. In addition, M cells consist of only a small percentage of intestinal epithelial cells, raising the question of their overall efficiency in antigen uptake in the GI system. Moreover, there are redundancies at multiple levels of the mucosal immune system to ensure its continuing functionality. In this regard, intestinal DCs can migrate between mucosal epithelial cells, and directly sample the luminal antigens by forming transepithelial dendrites[170,171]. Other cell types, such as villous enterocytes, also express MHC class II molecules and are capable of sampling and presenting intestinal antigens[172,173]. The difficulty in determining the precise role of M cells in the induction of mucosal immune responses is further confounded by the lack of availability of animal models which are completely and specifically deficient in M cells, making studies of intestinal antigen sampling by alternate cell types impossible.
Although M cells were initially believed to be exclusively located within the FAE region in the GI tract, this notion has been challenged by the recent identification and characterization of the intestinal villous M cells[31]. Intestinal villous M cells share all the known features of traditional M cells, but are independent of PP and not associated with the FAE. Instead, intestinal villous M cells lie on the intestinal villi either as small dense clusters (50 to 60 per animal) or diffusely. Intestinal villous M cells are more common in the terminal ileum than in other areas of the small intestine. Although the role and potential significance of these M cells remain to be elucidated, evidence to date indicates that they are functionally analogous to the PP M cells[31] and may compensate for PP M cell functions. Indeed, GALT-deficient mice produce antigen-specific IgG comparable with that produced in wild-type animals upon non-invasive bacterial challenge, and the population of UEA-1+ cells increased, perhaps the result of villous M cells developing from epithelial cells upon exposure to foreign antigens or pathogens, such as S. typhimurium[31].
More than three decades have passed since the first description of the M cell as the antigen shuttle for the mucosal immune system[6,7]. Current knowledge has highlighted the dynamic and complex role that M cells play in entry/invasion of pathogens, in antigen sampling, and in facilitating eliciting of immunity to GI infections. The advent of new technologies, such as confocal laser scanning microscopy and the intracellular visualization by use of fluorescence techniques, have supplemented the initial static electron microscopy studies in the characterization of M cells. The ability to cultivate M cells in vitro has complemented the in vivo models, and makes the molecular analysis of M cell functionality possible. The host-pathogen interactions have shown the varying strategies of the pathogen in exploiting M cells as conduits to initiate an infection, while at the same time evading or circumventing host immune surveillances. However, much work remains to be done to clarify the cellular and molecular mechanisms of the attachment to and the uptake of pathogens by M cells, and the interaction between the M cell and the pathogen, particularly the downstream events are evoked by the M cell antigen transport. Also, how does this transport lead to both mucosal and systemic immune responses? The presence of functional redundancies in the mucosal immune system and the lack of suitable animal models have further hindered the clarification of the precise role of M cells in the induction of mucosal immune responses and the rationale of targeting M cells for mucosal immunization. Further understanding and characterization of the mechanisms involved in the interaction between M cells and microorganisms, in the development and activation of M cells, and in the development of novel M cell targeting approaches will be needed for the development of a new generation of mucosal vaccines. In this regard, the recent identification of intestinal villous M cells and the rapid progress in our understanding of the role of TLR in the regulation of bacterial antigen uptake by M cells are likely to accelerate the development of M cell-based mucosal vaccines.
We would like to thank Dao Ly for her assistance during the preparation of the draft manuscript.
S- Editor Wang J L- Editor Kumar M E- Editor Chin GJ
| 1. | Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578-6583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3323] [Cited by in F6Publishing: 2187] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 2. | Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, Nilsen EM, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999;20:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285-296. [PubMed] [Cited in This Article: ] |
| 4. | Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 360] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Gebert A, Pabst R. M cells at locations outside the gut. Semin Immunol. 1999;11:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973;136:455-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 412] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Owen RL, Jones AL. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189-203. [PubMed] [Cited in This Article: ] |
| 8. | Kato TO, Owen RL. Structure and function of intestinal mucosal epithelium. Mucosal immunology, 2nd ed. San Diego: Academic Press Inc 1999; 115-132. [Cited in This Article: ] |
| 9. | Clark MA, Hirst BH. Expression of junction-associated proteins differentiates mouse intestinal M cells from enterocytes. Histochem Cell Biol. 2002;118:137-147. [PubMed] [Cited in This Article: ] |
| 10. | Regoli M, Bertelli E, Borghesi C, Nicoletti C. Three-dimensional (3D-) reconstruction of M cells in rabbit Peyer's patches: definition of the intraepithelial compartment of the follicle-associated epithelium. Anat Rec. 1995;243:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Owen RL. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977;72:440-451. [PubMed] [Cited in This Article: ] |
| 12. | Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 13. | Owen RL. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer's patches--a personal and historical perspective. Semin Immunol. 1999;11:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Jepson MA, Clark MA, Simmons NL, Hirst BH. Epithelial M cells in the rabbit caecal lymphoid patch display distinctive surface characteristics. Histochemistry. 1993;100:441-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108-G1121. [PubMed] [Cited in This Article: ] |
| 16. | Neutra MR, Giannasca PJ, Giannasca KT. M cells and microbial pathogens. Infections of the GI tract. New York: Raven Press 1995; 163-178. [Cited in This Article: ] |
| 17. | Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005;206:177-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 171] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Clark MA, Jepson MA, Hirst BH. Lectin binding defines and differentiates M-cells in mouse small intestine and caecum. Histochem Cell Biol. 1995;104:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Jepson MA, Mason CM, Clark MA, Simmons NL, Hirst BH. Variations in lectin binding properties of intestinal M cells. J Drug Target. 1995;3:75-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gebert A, Hach G. Vimentin antibodies stain membranous epithelial cells in the rabbit bronchus-associated lymphoid tissue (BALT). Histochemistry. 1992;98:271-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH. Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J. 1992;24:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Iwatsuki H, Ogawa C, Suda M. Vimentin-positive cells in the villus epithelium of the rabbit small intestine. Histochem Cell Biol. 2002;117:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Lelouard H, Reggio H, Roy C, Sahuquet A, Mangeat P, Montcourrier P. Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2. Infect Immun. 2001;69:1061-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Sharma R, van Damme EJ, Peumans WJ, Sarsfield P, Schumacher U. Lectin binding reveals divergent carbohydrate expression in human and mouse Peyer's patches. Histochem Cell Biol. 1996;105:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67:946-953. [PubMed] [Cited in This Article: ] |
| 28. | Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA. Immunocytochemical characterization of the follicle-associated epithelium of Peyer's patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol. 1996;71:363-370. [PubMed] [Cited in This Article: ] |
| 29. | Gebert A, Rothkötter HJ, Pabst R. Cytokeratin 18 is an M-cell marker in porcine Peyer's patches. Cell Tissue Res. 1994;276:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Buda A, Sands C, Jepson MA. Use of fluorescence imaging to investigate the structure and function of intestinal M cells. Adv Drug Deliv Rev. 2005;57:123-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110-6115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 32. | Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 936] [Cited by in F6Publishing: 880] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 33. | Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 277] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Bye WA, Allan CH, Trier JS. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984;86:789-801. [PubMed] [Cited in This Article: ] |
| 35. | Owen RL, Apple RT, Bhalla DK. Morphometric and cytochemical analysis of lysosomes in rat Peyer's patch follicle epithelium: their reduction in volume fraction and acid phosphatase content in M cells compared to adjacent enterocytes. Anat Rec. 1986;216:521-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Gebert A. Identification of M-cells in the rabbit tonsil by vimentin immunohistochemistry and in vivo protein transport. Histochem Cell Biol. 1995;104:211-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Kabok Z, Ermak TH, Pappo J. Microdissected domes from gut-associated lymphoid tissues: a model of M cell transepithelial transport in vitro. Adv Exp Med Biol. 1995;371A:235-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Fujimura Y. Functional morphology of microfold cells (M cells) in Peyer's patches--phagocytosis and transport of BCG by M cells into rabbit Peyer's patches. Gastroenterol Jpn. 1986;21:325-335. [PubMed] [Cited in This Article: ] |
| 39. | Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 453] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 40. | Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760-765. [PubMed] [Cited in This Article: ] |
| 41. | Neutra MR, Phillips TL, Mayer EL, Fishkind DJ. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987;247:537-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 187] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J. Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer's patch M cells. Cell Tissue Res. 1995;279:433-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Borghesi C, Regoli M, Bertelli E, Nicoletti C. Modifications of the follicle-associated epithelium by short-term exposure to a non-intestinal bacterium. J Pathol. 1996;180:326-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 44. | Allan CH, Mendrick DL, Trier JS. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology. 1993;104:698-708. [PubMed] [Cited in This Article: ] |
| 45. | Jarry A, Robaszkiewicz M, Brousse N, Potet F. Immune cells associated with M cells in the follicle-associated epithelium of Peyer's patches in the rat. An electron- and immuno-electron-microscopic study. Cell Tissue Res. 1989;255:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Nagura H, Ohtani H, Masuda T, Kimura M, Nakamura S. HLA-DR expression on M cells overlying Peyer's patches is a common feature of human small intestine. Acta Pathol Jpn. 1991;41:818-823. [PubMed] [Cited in This Article: ] |
| 47. | Brandtzaeg P, Bjerke K. Immunomorphological characteristics of human Peyer's patches. Digestion. 1990;46 Suppl 2:262-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Brandtzaeg P, Bjerke K. Human Peyer's patches: lympho-epithelial relationships and characteristics of immunoglobulin-producing cells. Immunol Invest. 1989;18:29-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Finzi G, Cornaggia M, Capella C, Fiocca R, Bosi F, Solcia E, Samloff IM. Cathepsin E in follicle associated epithelium of intestine and tonsils: localization to M cells and possible role in antigen processing. Histochemistry. 1993;99:201-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Pappo J, Mahlman RT. Follicle epithelial M cells are a source of interleukin-1 in Peyer's patches. Immunology. 1993;78:505-507. [PubMed] [Cited in This Article: ] |
| 51. | Hase K, Ohshima S, Kawano K, Hashimoto N, Matsumoto K, Saito H, Ohno H. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA Res. 2005;12:127-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, Kawano K, Ohshima S, Chihara R, Itoh K. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol. 2006;176:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Ponder BA, Schmidt GH, Wilkinson MM, Wood MJ, Monk M, Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 259] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Rubin DC, Ong DE, Gordon JI. Cellular differentiation in the emerging fetal rat small intestinal epithelium: mosaic patterns of gene expression. Proc Natl Acad Sci USA. 1989;86:1278-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Debard N, Sierro F, Kraehenbuhl JP. Development of Peyer's patches, follicle-associated epithelium and M cell: lessons from immunodeficient and knockout mice. Semin Immunol. 1999;11:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Wolf JL, Bye WA. The membranous epithelial (M) cell and the mucosal immune system. Annu Rev Med. 1984;35:95-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 144] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Gebert A, Fassbender S, Werner K, Weissferdt A. The development of M cells in Peyer's patches is restricted to specialized dome-associated crypts. Am J Pathol. 1999;154:1573-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Lelouard H, Sahuquet A, Reggio H, Montcourrier P. Rabbit M cells and dome enterocytes are distinct cell lineages. J Cell Sci. 2001;114:2077-2083. [PubMed] [Cited in This Article: ] |
| 59. | Miyazawa K, Aso H, Kanaya T, Kido T, Minashima T, Watanabe K, Ohwada S, Kitazawa H, Rose MT, Tahara K. Apoptotic process of porcine intestinal M cells. Cell Tissue Res. 2006;323:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Gebert A, Posselt W. Glycoconjugate expression defines the origin and differentiation pathway of intestinal M-cells. J Histochem Cytochem. 1997;45:1341-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Takeuchi T, Gonda T. Cellular kinetics of villous epithelial cells and m cells in rabbit small intestine. J Vet Med Sci. 2004;66:689-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Savidge TC, Smith MW. Evidence that membranous (M) cell genesis is immuno-regulated. Adv Exp Med Biol. 1995;371A:239-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Sharma R, Schumacher U, Adam E. Lectin histochemistry reveals the appearance of M-cells in Peyer's patches of SCID mice after syngeneic normal bone marrow transplantation. J Histochem Cytochem. 1998;46:143-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Smith MW, Peacock MA. "M" cell distribution in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980;159:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Kitagawa H, Hosokawa M, Takeuchi T, Yokoyama T, Imagawa T, Uehara M. The cellular differentiation of M cells from crypt undifferentiated epithelial cells into microvillous epithelial cells in follicle-associated epithelia of chicken cecal tonsils. J Vet Med Sci. 2003;65:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Sierro F, Pringault E, Assman PS, Kraehenbuhl JP, Debard N. Transient expression of M-cell phenotype by enterocyte-like cells of the follicle-associated epithelium of mouse Peyer's patches. Gastroenterology. 2000;119:734-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Kernéis S, Pringault E. Plasticity of the gastrointestinal epithelium: the M cell paradigm and opportunism of pathogenic microorganisms. Semin Immunol. 1999;11:205-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302-9307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 306] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 69. | Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 572] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 70. | Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol. 1997;9:507-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 767] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 72. | Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 493] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 73. | Debard N, Sierro F, Browning J, Kraehenbuhl JP. Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer's patches. Gastroenterology. 2001;120:1173-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965-1968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 75. | Shimosato T, Tohno M, Kitazawa H, Katoh S, Watanabe K, Kawai Y, Aso H, Yamaguchi T, Saito T. Toll-like receptor 9 is expressed on follicle-associated epithelia containing M cells in swine Peyer's patches. Immunol Lett. 2005;98:83-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Tohno M, Shimosato T, Kitazawa H, Katoh S, Iliev ID, Kimura T, Kawai Y, Watanabe K, Aso H, Yamaguchi T. Toll-like receptor 2 is expressed on the intestinal M cells in swine. Biochem Biophys Res Commun. 2005;330:547-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006;74:625-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176:4275-4283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Savidge TC, Smith MW, James PS, Aldred P. Salmonella-induced M-cell formation in germ-free mouse Peyer's patch tissue. Am J Pathol. 1991;139:177-184. [PubMed] [Cited in This Article: ] |
| 80. | Gebert A, Steinmetz I, Fassbender S, Wendlandt KH. Antigen transport into Peyer's patches: increased uptake by constant numbers of M cells. Am J Pathol. 2004;164:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Smith MW, James PS, Tivey DR. M cell numbers increase after transfer of SPF mice to a normal animal house environment. Am J Pathol. 1987;128:385-389. [PubMed] [Cited in This Article: ] |
| 82. | Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155-2158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 682] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 83. | Beckers J, Clark A, Wünsch K, Hrabé De Angelis M, Gossler A. Expression of the mouse Delta1 gene during organogenesis and fetal development. Mech Dev. 1999;84:165-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Lo D, Tynan W, Dickerson J, Scharf M, Cooper J, Byrne D, Brayden D, Higgins L, Evans C, O'Mahony DJ. Cell culture modeling of specialized tissue: identification of genes expressed specifically by follicle-associated epithelium of Peyer's patch by expression profiling of Caco-2/Raji co-cultures. Int Immunol. 2004;16:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Gray HH. Anatomy of the human body. Philadelphia: Lea & Febiger 1918; . [Cited in This Article: ] |
| 86. | Makala LH, Suzuki N, Nagasawa H. Peyer's patches: organized lymphoid structures for the induction of mucosal immune responses in the intestine. Pathobiology. 2002;70:55-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Owen RL, Bhalla DK. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat. 1983;168:199-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Smith MW. Selective expression of brush border hydrolases by mouse Peyer's patch and jejunal villus enterocytes. J Cell Physiol. 1985;124:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Hopkins SA, Niedergang F, Corthesy-Theulaz IE, Kraehenbuhl JP. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell Microbiol. 2000;2:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 464] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 91. | Owen RL, Piazza AJ, Ermak TH. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991;190:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Jepson MA, Clark MA. Studying M cells and their role in infection. Trends Microbiol. 1998;6:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Mason CM, Jepson MA, Simmons NL, Hirst BH. Heterogenous Na+, K(+)-ATPase expression in the epithelia of rabbit gut-associated lymphoid tissues. Pflugers Arch. 1994;427:343-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Owen RL, Pierce NF, Apple RT, Cray WC. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 283] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 95. | Walker RI, Schmauder-Chock EA, Parker JL, Burr D. Selective association and transport of Campylobacter jejuni through M cells of rabbit Peyer's patches. Can J Microbiol. 1988;34:1142-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Sansonetti PJ, Arondel J, Cantey JR, Prévost MC, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752-2764. [PubMed] [Cited in This Article: ] |
| 97. | Jensen VB, Harty JT, Jones BD. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer's patches. Infect Immun. 1998;66:3758-3766. [PubMed] [Cited in This Article: ] |
| 98. | Kohbata S, Yokoyama H, Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30:1225-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 673] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 100. | Siebers A, Finlay BB. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 101. | Gebert A, Rothkötter HJ, Pabst R. M cells in Peyer's patches of the intestine. Int Rev Cytol. 1996;167:91-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 244] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Ménard R, Dehio C, Sansonetti PJ. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Karapetian O, Shakhov AN, Kraehenbuhl JP, Acha-Orbea H. Retroviral infection of neonatal Peyer's patch lymphocytes: the mouse mammary tumor virus model. J Exp Med. 1994;180:1511-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Siciński P, Rowiński J, Warchoł JB, Jarzabek Z, Gut W, Szczygieł B, Bielecki K, Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990;98:56-58. [PubMed] [Cited in This Article: ] |
| 105. | Amerongen HM, Wilson GA, Fields BN, Neutra MR. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J Virol. 1994;68:8428-8432. [PubMed] [Cited in This Article: ] |
| 106. | Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, Nibert ML, Neutra MR. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol. 2003;77:7964-7977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Wolf JL, Rubin DH, Finberg R, Kauffman RS, Sharpe AH, Trier JS, Fields BN. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212:471-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 377] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 108. | Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A. Transepithelial prion transport by M cells. Nat Med. 2001;7:976-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 109. | Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP. Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci USA. 2002;99:9410-9414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Landsverk T. Cryptosporidiosis and the follicle-associated epithelium over the ileal Peyer's patch in calves. Res Vet Sci. 1987;42:299-306. [PubMed] [Cited in This Article: ] |
| 111. | Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res Microbiol. 1994;145:543-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 112. | Frost AJ, Bland AP, Wallis TS. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet Pathol. 1997;34:369-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Phalipon A, Sansonetti PJ. Microbial-host interactions at mucosal sites. Host response to pathogenic bacteria at mucosal sites. Curr Top Microbiol Immunol. 1999;236:163-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Raupach B, Mecsas J, Heczko U, Falkow S, Finlay BB. Bacterial epithelial cell cross talk. Curr Top Microbiol Immunol. 1999;236:137-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Owen RL. M cells--entryways of opportunity for enteropathogens. J Exp Med. 1994;180:7-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087-6095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 117. | Meyerholz DK, Stabel TJ. Comparison of early ileal invasion by Salmonella enterica serovars Choleraesuis and Typhimurium. Vet Pathol. 2003;40:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11:193-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Nhieu GT, Sansonetti PJ. Mechanism of Shigella entry into epithelial cells. Curr Opin Microbiol. 1999;2:51-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Bäumler AJ, Tsolis RM, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer's patches. Proc Natl Acad Sci USA. 1996;93:279-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 121. | Galán JE. Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr Opin Microbiol. 1999;2:46-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 122. | Penheiter KL, Mathur N, Giles D, Fahlen T, Jones BD. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol Microbiol. 1997;24:697-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 123. | Phillips AD, Navabpour S, Hicks S, Dougan G, Wallis T, Frankel G. Enterohaemorrhagic Escherichia coli O157: H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut. 2000;47:377-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 124. | van der Velden AW, Bäumler AJ, Tsolis RM, Heffron F. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Infect Immun. 1998;66:2803-2808. [PubMed] [Cited in This Article: ] |
| 125. | Pascopella L, Raupach B, Ghori N, Monack D, Falkow S, Small PL. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun. 1995;63:4329-4335. [PubMed] [Cited in This Article: ] |
| 126. | Pepe JC, Miller VL. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci USA. 1993;90:6473-6477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 198] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 127. | Marra A, Isberg RR. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect Immun. 1997;65:3412-3421. [PubMed] [Cited in This Article: ] |
| 128. | Kingsley RA, Weening EH, Keestra AM, Bäumler AJ. Population heterogeneity of Salmonella enterica serotype Typhimurium resulting from phase variation of the lpf operon in vitro and in vivo. J Bacteriol. 2002;184:2352-2359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Norris TL, Kingsley RA, Bümler AJ. Expression and transcriptional control of the Salmonella typhimurium Ipf fimbrial operon by phase variation. Mol Microbiol. 1998;29:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102:427-436. [PubMed] [Cited in This Article: ] |
| 131. | Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237-1243. [PubMed] [Cited in This Article: ] |
| 132. | Schulte R, Kerneis S, Klinke S, Bartels H, Preger S, Kraehenbuhl JP, Pringault E, Autenrieth IB. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell Microbiol. 2000;2:173-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 133. | Hamzaoui N, Kernéis S, Caliot E, Pringault E. Expression and distribution of beta1 integrins in in vitro-induced M cells: implications for Yersinia adhesion to Peyer's patch epithelium. Cell Microbiol. 2004;6:817-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 679] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 135. | Tyrer P, Ruth Foxwell A, Kyd J, Harvey M, Sizer P, Cripps A. Validation and quantitation of an in vitro M-cell model. Biochem Biophys Res Commun. 2002;299:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 136. | Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | Jepson MA, Clark MA, Hirst BH. M cell targeting by lectins: a strategy for mucosal vaccination and drug delivery. Adv Drug Deliv Rev. 2004;56:511-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Brayden DJ, Baird AW. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Adv Drug Deliv Rev. 2004;56:721-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 139. | Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3:217-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 140. | Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004;20:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 141. | Man AL, Prieto-Garcia ME, Nicoletti C. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology. 2004;113:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 381] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 143. | Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709-1714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 144. | Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 570] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 145. | Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 858] [Cited by in F6Publishing: 877] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 146. | Robinson K, Chamberlain LM, Lopez MC, Rush CM, Marcotte H, Le Page RW, Wells JM. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect Immun. 2004;72:2753-2761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 147. | Guo CC, Ding J, Pan BR, Yu ZC, Han QL, Meng FP, Liu N, Fan DM. Development of an oral DNA vaccine against MG7-Ag of gastric cancer using attenuated salmonella typhimurium as carrier. World J Gastroenterol. 2003;9:1191-1195. [PubMed] [Cited in This Article: ] |
| 148. | Bowe F, Pickard DJ, Anderson RJ, Londoño-Arcila P, Dougan G. Development of attenuated Salmonella strains that express heterologous antigens. Methods Mol Med. 2003;87:83-100. [PubMed] [Cited in This Article: ] |
| 149. | Mantis NJ, Frey A, Neutra MR. Accessibility of glycolipid and oligosaccharide epitopes on rabbit villus and follicle-associated epithelium. Am J Physiol Gastrointest Liver Physiol. 2000;278:G915-G923. [PubMed] [Cited in This Article: ] |
| 150. | Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113:998-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 151. | Pappo J, Ermak TH. Uptake and translocation of fluorescent latex particles by rabbit Peyer's patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989;76:144-148. [PubMed] [Cited in This Article: ] |
| 152. | Jepson MA, Simmons NL, O'Hagan DT, Hirst BH. Comparison of poly(DL-lactide-co-glycolide) and polystyrene microsphere targeting to intestinal M cells. J Drug Target. 1993;1:245-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 153. | Jepson MA, Simmons NL, Savidge TC, James PS, Hirst BH. Selective binding and transcytosis of latex microspheres by rabbit intestinal M cells. Cell Tissue Res. 1993;271:399-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 154. | Smith MW, Thomas NW, Jenkins PG, Miller NG, Cremaschi D, Porta C. Selective transport of microparticles across Peyer's patch follicle-associated M cells from mice and rats. Exp Physiol. 1995;80:735-743. [PubMed] [Cited in This Article: ] |
| 155. | Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine. 1998;16:536-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 156. | McNeela EA, O'Connor D, Jabbal-Gill I, Illum L, Davis SS, Pizza M, Peppoloni S, Rappuoli R, Mills KH. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19:1188-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 157. | van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials. 2001;22:687-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 158. | van der Lubben IM, van Opdorp FA, Hengeveld MR, Onderwater JJ, Koerten HK, Verhoef JC, Borchard G, Junginger HE. Transport of chitosan microparticles for mucosal vaccine delivery in a human intestinal M-cell model. J Drug Target. 2002;10:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 159. | Seong SY, Cho NH, Kwon IC, Jeong SY. Protective immunity of microsphere-based mucosal vaccines against lethal intranasal challenge with Streptococcus pneumoniae. Infect Immun. 1999;67:3587-3592. [PubMed] [Cited in This Article: ] |
| 160. | Chabot S, Brewer A, Lowell G, Plante M, Cyr S, Burt DS, Ward BJ. A novel intranasal Protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine. 2005;23:1374-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 161. | Clark MA, Blair H, Liang L, Brey RN, Brayden D, Hirst BH. Targeting polymerised liposome vaccine carriers to intestinal M cells. Vaccine. 2001;20:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 162. | Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 163. | Choi WS, Pal-Ghosh R, Morrow CD. Expression of human immunodeficiency virus type 1 (HIV-1) gag, pol, and env proteins from chimeric HIV-1-poliovirus minireplicons. J Virol. 1991;65:2875-2883. [PubMed] [Cited in This Article: ] |
| 164. | Malkevitch NV, Robert-Guroff M. A call for replicating vector prime-protein boost strategies in HIV vaccine design. Expert Rev Vaccines. 2004;3:S105-S117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 165. | Levine MM. Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr. 2000;31:336-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 166. | Haneberg B, Herland Berstad AK, Holst J. Bacteria-derived particles as adjuvants for non-replicating nasal vaccines. Adv Drug Deliv Rev. 2001;51:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Manocha M, Pal PC, Chitralekha KT, Thomas BE, Tripathi V, Gupta SD, Paranjape R, Kulkarni S, Rao DN. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine. 2005;23:5599-5617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 168. | Wang X, Hone DM, Haddad A, Shata MT, Pascual DW. M cell DNA vaccination for CTL immunity to HIV. J Immunol. 2003;171:4717-4725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 169. | Lee PW, Hayes EC, Joklik WK. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981;108:156-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 261] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 170. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1870] [Cited by in F6Publishing: 1767] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 171. | Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1209] [Cited by in F6Publishing: 1182] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 172. | Zimmer KP, Büning J, Weber P, Kaiserlian D, Strobel S. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology. 2000;118:128-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 173. | Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, Nepom GT. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |