Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2007; 13(1): 48-64
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.48
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.48
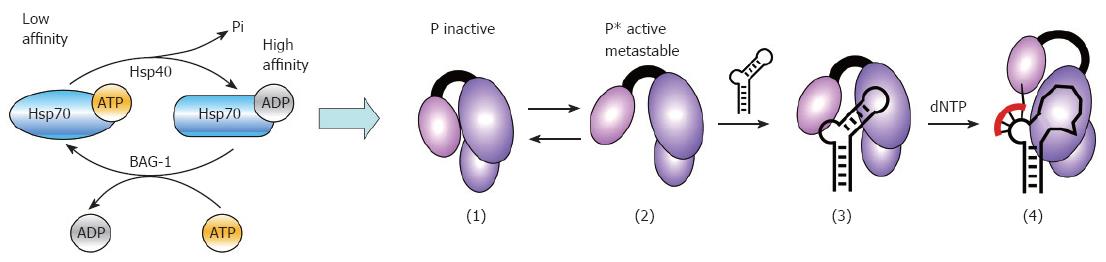
Figure 6 Model for Hsp40/Hsp70 mediated in vitro activation of P protein.
The low ATPase activity of Hsp70 is stimulated by Hsp40, yielding high substrate affinity Hsp70/ADP. A new cycle of substrate release and folding requires exchange of the ADP for new ATP which is stimulated by nucleotide exchange factors such as BAG-1. This ATP-dependent Hsp70 cycling applies to the chaperone´s global folding activities but likely also to P activation: in the inactive state of P (1), the ε RNA binding pocket is inaccessible; Hsp40/Hsp70 activation creates active P* (2) which is able to bind ε RNA (3); P* is metastable, and decays to the inactive state (1) within minutes. Maintaining a steady-state level of P* requires constant re-activation by Hsp40/Hsp70 and thus a continuous supply of fresh ATP. Complexes containing priming-competent ε RNAs (3) undergo induced-fit rearrangements in the RNA and the protein, enabling them to initiate DNA synthesis upon dNTP addition (4). Several Dε variants bind P but do not act as template; most likely they are trapped at stage (3). The same may hold for human HBV P protein complexes with HBV ε RNA.
- Citation: Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol 2007; 13(1): 48-64
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/48.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.48