Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2007; 13(1): 48-64
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.48
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.48
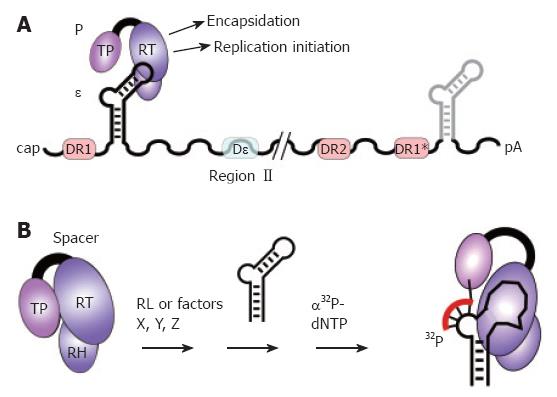
Figure 3 The pgRNA as substrate for P protein.
A: pgRNA organization. The pgRNA is shown with some major cis-elements, i.e. ε (hairpin structure), DR1, DR2, and DR1*; DHBV, but not HBV, requires for encapsidation an additional cis-element (Dε region II). Binding of P protein to 5´ ε, but not 3´ ε, initiates pgRNA encapsidation and replication; B: In vitro priming assay. P protein with its Terminal protein (TP), reverse transcriptase (RT), and RNase H (RH) domains can be activated by reticulocyte lysate (RL), or individual factors (X, Y, Z), to bind ε; ε may be supplied as small RNA covering just the hairpin structure. Upon addition of α32P-dNTPs, P uses ε as template to copy 3 to 4 nt from the ε bulge region; by the covalent linkage of the 5´ nt to a tyrosine residue in TP, P protein becomes radioactively labeled, providing a sensitive assay for activity. In vitro priming does thus far not work with human HBV P protein and ε RNA.
- Citation: Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol 2007; 13(1): 48-64
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/48.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.48