Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2007; 13(1): 22-38
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.22
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.22
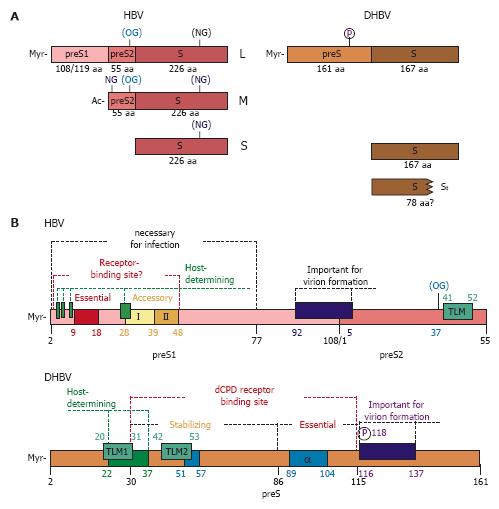
Figure 2 Surface proteins of HBV and DHBV.
(A) Schematic organisation of the three surface proteins of HBV and the two surface proteins of DHBV. For HBV, used N- and O-glycosylation sites (NG and OG) are shown, (parenthesis indicate partial glycosylation). DHBV surface proteins are non-glycosylated, but the L-protein is phosphorylated at position 118 within its preS-domain. The L-protein of HBV and DHBV is myristoylated (Myr) at Gly-2 of the N-terminus of preS1 or preS, respectively. In case of HBV the preS1-domain encodes for 108 or 119 amino acids, depending on the genotype. The preS2-domain of MHBs is N-terminally acetylated (Ac) and is 55 amino acids long, while a preS2-domain and therefore a special M-protein is missing in DHBV. (B) Schematic presentation of preS-domains of HBV and DHBV important for virion formation and viral entry. The host-determining regions are depicted in green. The potential receptor binding site within the preS1-domain of HBV is shown with the essential domain (aa 9-18) and the two accessory domains (aa 28-39 and 39-48, respectively). For DHBV, the carboxypeptidase D (dCPD) binding site is depicted with the essential and stabilizing domains. Alpha helical domains are shown in blue (α). Reported trans-location motives (TLM) are marked in boxes. In DHBV preS, two TLMs are predicted (TLM1 and TLM2). TLM1 overlaps with the host-determining region, while TLM2 is located within the stabilizing sequence of the dCPD receptor binding site. In the case of HBV, the carboxyterminus of the 55 aa long preS2-domain was reported to contain a single TLM. The numbering of HBV preS1 is for genotype D (108 aa). P, phosphorylation site; OG, O-glycosylation; myr, myristoylation.
- Citation: Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol 2007; 13(1): 22-38
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/22.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.22