Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7497
Revised: October 25, 2006
Accepted: September 3, 2006
Published online: December 14, 2006
AIM: To evaluate the pharmaceutical safety of a Chinese herbal formula, Chunggan extract (CGX), traditionally prescribed as a hepatotherapeutic drug via systemic acute and subacute toxicological study.
METHODS: Twenty male dogs and 20 female dogs were fed doses 50 times and 4 times greater than the clinically-recommended drug dosages in an acute and a subacute toxicological study, respectively. Adverse effects were examined by comparing the differences between normal and drug-administered groups using clinical signs, necropsies, histopathologic findings, haematology, urinalysis, and biochemical analysis.
RESULTS: In the acute study no change in the body weight, diarrhoea, apetite, mortality rate and histopathology of major organs was observed in male or female dogs with a single administration of CGX at 5 g/kg. No drug-induced abnormalities at analysis of histopathology, haematology, urinalysis, and biochemistry were found with any dose of this drug.
CONCLUSION: CGX is supposed to be very safe when used in a clinical application with a wide therapeutic index.
- Citation: Choi WJ, Shin JW, Son JY, Seo DS, Park HS, Han SH, Sung HJ, Cho JH, Cho CK, Yoo HS, Lee YW, Son CG. Toxicological study of the hepatotherapeutic herbal formula, Chunggan extract, in beagle dogs. World J Gastroenterol 2006; 12(46): 7497-7502
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7497
Worldwide use of complementary and alternative medicines, including herbal products for various health benefits, has recently increased[1,2]. Botanical compounds and medical plants have also attracted attention among drug investigators and the pharmaceutical industry. Many herbal formulae have been traditionally prescribed for patients with hepatic disorders, and researchers have recently studied many compounds from natural resources as potential hepatotherapeutic candidates[3-5].
However, many issues related to a lack of scientific evidence about the efficacy and safety of herbal remedies remain unresolved[6,7]. Many reports and warnings have been published, particularly about the potential hepatotoxicity of herbal products, because the liver is a prime target for the toxic effects of general drugs[8-10].
Chunggan extract (CGX) is a potentially hepatotherapeutic drug derived from natural herbs. It has hepatoprotective effects on alcohol-, dimethylnitrosamine (DMN)-, and D-galactosamine-induced liver injury, a therapeutic effect on chronic liver disease, and an inhibitory effect on intestinal absorption and storage of cholesterol[11-14]. However, no animal-based high-dose toxicological study has been performed in conjunction with tests to confirm the drug’s safety and efficacy in the appropriate dosages.
This study applied acute and subacute toxicological tests using beagle dogs to evaluate the wide-range tolerance and safety of Chunggan extract. This report aims to provide vital information about the efficacy and safety of multi-herbal plant-derived traditional Chinese medicine.
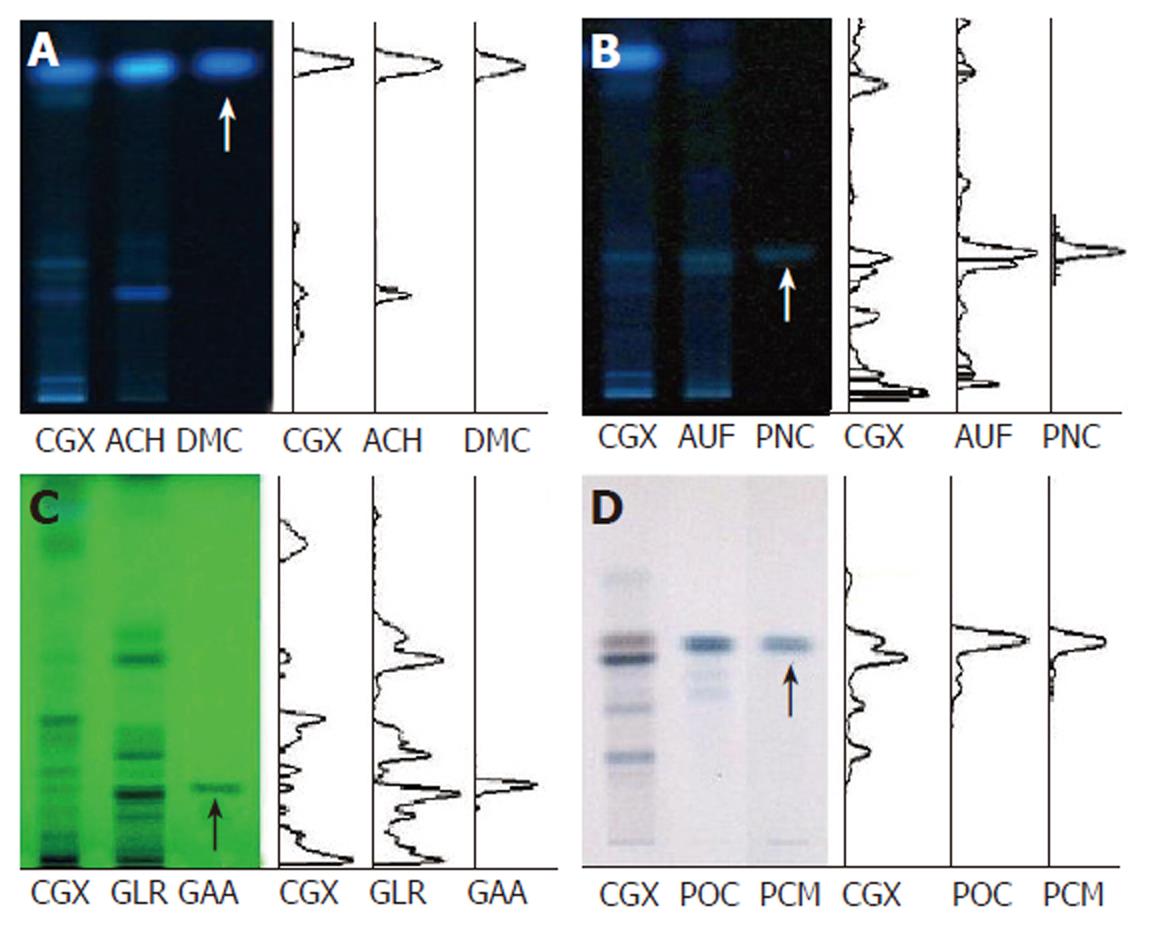
The ingredients of CGX include 5 g each of Artemisia capillaris Herba, Carapax Trionycis, Semen Raphani; 3 g each of Rhizoma Atractylodis Macrocephalae, Poria, Alismatis Rhizoma, Atractylodis Rhizoma, Salviae Miltiorrhizae Radix; 2 g each of Polyporus, Amomi Fructus, Aurantii Fructus, and 1 g of Glycyrrhizae Radix or Helenii Radix. The 10.71% (w/w) lyophilized water-extract was obtained from the initial dried mixture according to the Korean standard over-the-counter (OTC) monographs, and its high-performance thin layer chromatography (HPTLC)-based fingerprint was produced by the CAMAG application system (Muttenz, Switzerland) (Figure 1A-D). For the HPTLC analysis, water extracts of CGX, Artemisia capillaris Herba, Aurantii Fructus, Glycyrrhizae Radix, Poria cocos and their standard components, 6,7-dimethoxycoumarin (Sigma Chemical Co., St. Louis, MO, USA), Poncirin (Fluka, St. Louis, MO, USA), Glycyrrhizic acid ammonium salt (Sigma Chemical Co., St. Louis, MO, USA) and Pachyman (Megazyme Co., Wicklow, Ireland) were dissolved in HPLC-grade methanol and applied to pre-washed silica gel 60 F254 HPTLC plates (size 20 cm × 10 cm, thickness of the silica gel 0.2 mm; Merck, Darmstadt, Germany) with an automated applicator (Linomat IV; CAMAG). The samples were then separated (migration distance 80 mm) using HPLC-grade ethyl acetate/formic acid/acetic acid/water (15:1:1:2) except for Poria cocos (migration distance 70 mm, n-butanol/methanol/water as 50:25:20). The migrated components were visualized under UV radiation at 366 nm or 254 nm using Reprostar 3 with a digital camera (CAMAG). Only Poria coco was visualized under white light after derivatization with aniline-diphenylamine-phosphoric acid solution.
In total, 40 beagle dogs (20 males and 20 females) were obtained from Jung-Ang Lab Inc. (Seoul, Korea). Each dog was acclimatized to conditions in kennel units (1.08 m2) and was subjected to routine examination and acceptance procedures for 6 wk. Animal housing was maintained at 22 ± 3°C with a 12-h light/dark cycle, and air was exchanged approximately 13-18 times/h. Each dog was fed 400 g/d of a standard dry diet (Gold Pet; Agribrands Purina Korea Inc., Seoul, Korea) and had free access to automatically filtered tap water that was checked using routine chemical monitoring (Korea Chemicals Inspection & Testing Institute). At the beginning of treatment, the dogs averaged approximately 5 mo of age and 8.5-11.5 kg in weight.
Prior to the experiment, a veterinarian performed an animal health review. This study complied with the Testing Guidelines of the Korea Food and Drug Administration (KFDA; notification no. 1999-61).
After 12 h of fasting, two males and two females in each of four groups received oral doses (5 000, 2 500, 1 250, and zero mg/kg respectively) of CGX dissolved in distilled water through a catheter. Clinical observations were performed hourly for 6 h, after which mortality or clinical signs of toxicity were monitored daily for the following 2 wk. Body weight was measured weekly, and necropsy-gross findings were recorded on the final day.
Over 4 wk, three male and three female dogs received orally administered CGX in one of four dosages: 400, 200, 100, and zero mg/kg as a control. They were subsequently checked and measured carefully for mortality and clinical signs of toxicity (daily), body weight and food intake (weekly), ocular fundus examination, and slit lamp examination (before drug administration and 2 d before the final day). On the final day, all dogs were deprived of food, but not water, for 16 h. Urinalysis, hematologic, and various biochemical parameters of all dogs were measured using blood samples after collection via the jugular vein (before and on the final day of the experiment). After necropsy, organs (brain, hypophysis, heart, liver, spleen, kidneys, adrenal glands, prostate, testes, and ovaries) were weighed. Microscopic examination of the following organs was also done: lungs, trachea, heart, thymus, liver, kidneys, pancreas, spleen, thyroid, adrenal glands, testes, epididymes, ovaries, uterus, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, urinary bladder, aorta, brain, pituitary, prostate gland, and tongue. A histopathologist performed a complete examination of the tissue samples after they were stained with haematoxylin-eosin (H&E) following a 10% formalin fixation and embedded in paraffin wax.
Distributions of body and organ weight, food intake, and haematological and biochemical parameters were analysed using Levene’s test of equality of variance. When variance was homogenous, a one-way ANOVA was applied. Statistical differences in the means among groups were analysed using Dunnett’s multiple comparison test[15,16]. When variance was not homogenous, Dunnett’s t-test was performed. Statistical analyses were conducted using SPSS Base 10.0 (SPSS, Inc., Chicago, IL, USA).
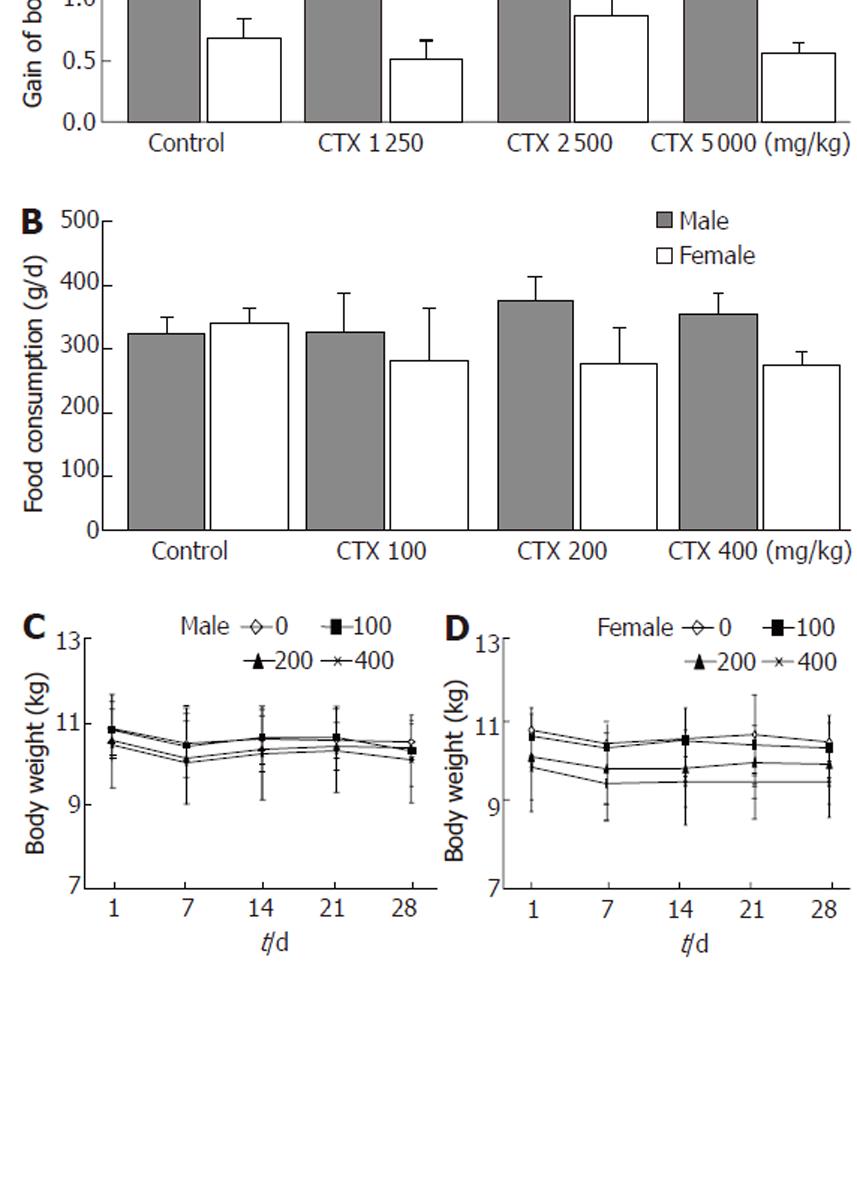
After administration of CGX and distilled water (DW) as a control, all dogs exhibited decreased activity for 1 h. No clinical symptoms including diarrhoea, anorexia, or death were observed in any of the dogs during the remaining 2 wk of the experiment. Each group exhibited a normal range in weight gain (Figure 2A), and no abnormal findings were seen at necropsy in the major organs of males or female dogs treated with CGX (data not shown).
Food consumption and body weight: Food consumption did not appear to markedly differ in any CGX-treated group compared to the control over 4 wk (Figure 2B), although females in the CGX-treated groups consumed less food than the control group. This result may have been due to a lower body weight among females at the starting point, leading to reduced food consumption. Similarly, no groups exhibited significant gains or losses in body weight during the 4-wk experimental period (Figure 2C and D).
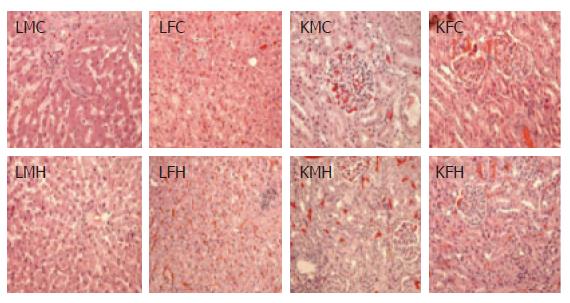
Clinical signs, necropsy, and histopathological findings: No clinical signs including anorexia or soft faeces were observed in any dogs during the 4 wk of the experiment. No tested groups exhibited any changes in ophthalmologic signs (summarised in Table 1). Macroscopic autopsy examination did not reveal any pathological findings in any organs, and no significant changes to body weight ratio were found in any of the 13 tested organs of either treated or control groups: liver, kidneys, spleen, adrenals, ovaries, brain, pituitary gland, lungs, heart, thymus, thyroid, uterus, and urinary bladder (data not shown). However, microscopic examination detected rare pathological findings, such as focal necrosis with macrophage, lymphocyte, plasma cells, hyperaemia, and subcutaneous edema in livers, bronchopneumonia in lungs, glomerular hyperaemia in kidneys, white pulp atrophy in spleens, and Helicobacter in stomachs; similar findings were exhibited by groups treated with CGX and the control group. However, no systematic histopathological differences were observed between groups. Figure 3 presents the only observed focally pathological features in kidneys and livers between the control group and the group treated with the highest levels of CGX (400 mg/kg per day).
| CGX (mg/kg per day) | 0 | 100 | 200 | 400 |
| Mortality | - | - | - | - |
| Clinical signs | NAD | NAD | NAD | NAD |
| Anorexia | - | - | - | - |
| Soft feces | - | - | - | - |
| Food intake | NAD | NAD | NAD | NAD |
| Ophthalmologic findings | NAD | NAD | NAD | NAD |
| Relative organ weight | NS | NS | NS | NS |
| Necropsy findings | NAD | NAD | NAD | NAD |
| Histopathological findings | Rarely in some samples | Rarely in some samples | ||
| Liver | Focal necrosis | Subcutaneous necrosis | ||
| Lung | Yellow pigmentation | Yellow pigmentation | ||
| Kidney | Glomerular hyperemia | Glomerular hyperemia | ||
| Spleen | White pulp atrophy | White pulp atrophy | ||
| Stomach | Helicobacter | Helicobacter | ||
Haematology and urinalysis: Before the experiment began, tests indicated that all dogs (12 males and 12 females) had normal haematological values (data not shown). Haematology was measured again on the final day of the experiment, and results revealed a significant decrease in mean corpuscular haemoglobin concentration (MCHC) only in male dogs treated with 100 mg/kg per day CGX compared to control dogs; 200 or 400 mg/kg per day male and all female dogs produced normal values (Table 2). No other haematological indicators revealed any significant change.
| Number of dogs | Male (12) | Female (12) | ||||||
| CGX (mg/kg•d) | 0 | 100 | 200 | 400 | 0 | 100 | 200 | 400 |
| Erythrocyte (M/UL) | 6.4 ± 0.6 | 6.80 ± 0.4 | 6.56 ± 0.4 | 6.70 ± 0.1 | 6.22 ± 0.5 | 6.70 ± 0.5 | 6.77 ± 0.5 | 6.59 ± 0.2 |
| Hemoglobin (g/dL) | 18.6 ± 2.1 | 17.6 ± 0.4 | 18.2 ± 1.0 | 18.1 ± 1.3 | 16.5 ± 1.7 | 17.5 ± 1.8 | 17.8 ± 0.4 | 17.7 ± 0.1 |
| Hematocrit (%) | 41.3 ± 4.1 | 42.8 ± 1.8 | 42.8 ± 2.2 | 42.2 ± 1.0 | 39.2 ± 2.8 | 40.1 ± 2.1 | 43.3 ± 3.9 | 41.6 ± 0.9 |
| MCV (fL) | 64.1 ± 0.3 | 63.1 ± 2.4 | 65.4 ± 2.8 | 62.9 ± 1.0 | 63.1 ± 0.1 | 60.1 ± 4.1 | 63.9 ± 1.4 | 63.1 ± 1.9 |
| MCH (pg) | 28.9 ± 1.1 | 26.0 ± 1.4 | 27.8 ± 2.3 | 27.0 ± 1.7 | 26.5 ± 1.2 | 26.1 ± 2.7 | 26.4 ± 2.4 | 26.8 ± 0.7 |
| MCHC (g/dL) | 45.2 ± 1.5 | 41.7 ± 0.6a | 42.4 ± 2.2 | 42.9 ± 2.2 | 42.1 ± 2.2 | 43.5 ± 2.5 | 41.3 ± 4.6 | 42.6 ± 0.7 |
| RDW (%) | 15.1 ± 0.1 | 15.9 ± 0.3 | 15.3 ± 0.4 | 15.9 ± 0.3 | 15.7 ± 0.1 | 15.9 ± 0.6 | 15.7 ± 0.5 | 15.1 ± 0.1 |
| Reticulocytes (%) | 0.30 ± 0.1 | 0.23 ± 0.1 | 0.37 ± 0.2 | 0.43 ± 0.2 | 0.77 ± 0.2 | 0.50 ± 0.4 | 0.53 ± 0.6 | 0.50 ± 0.2 |
| Platelets (k/UL) | 349 ± 13 | 320 ± 125 | 334 ± 50 | 344 ± 46 | 347 ± 94 | 333 ± 90 | 239 ± 87 | 245 ± 18 |
| MPV (fL) | 12.8 ± 0.3 | 15.1 ± 1.2 | 14.6 ± 2.2 | 14.0 ± 1.3 | 13.4 ± 1.6 | 12.8 ± 1.6 | 16.4 ± 4.2 | 15.0 ± 4.7 |
| PT (s) | 6.73 ± 0.2 | 7.20 ± 0.6 | 6.63 ± 0.3 | 6.77 ± 0.2 | 6.73 ± 0.3 | 7.00 ± 0.4 | 6.77 ± 0.1 | 6.93 ± 0.5 |
| aPTT (s) | 14.2 ± 0.5 | 19.3 ± 5.2 | 15.4 ± 0.7 | 15.2 ± 0.9 | 15.7 ± 0.2 | 15.4 ± 0.9 | 16.1 ± 2.6 | 16.5 ± 0.7 |
| Leukocytes (k/UL) | 9.86 ± 1.2 | 9.51 ± 1.3 | 8.56 ± 1.3 | 11.4 ± 1.4 | 9.64 ± 1.3 | 10.6 ± 0.2 | 11.8 ± 3.8 | 8.9 ± 1.6 |
| Neutrophil (%) | 61.0 ± 10 | 59.8 ± 15 | 65.7 ± 14 | 66.9 ± 10 | 57.0 ± 13 | 60.9 ± 14 | 49.2 ± 2.3 | 62.8 ± 13 |
| Lymphocyte (%) | 19.5 ± 4.1 | 23.7 ± 4.2 | 21.4 ± 4.7 | 21.8 ± 2.6 | 28.2 ± 5.2 | 26.7 ± 8.5 | 35.5 ± 13 | 27.1 ± 7.9 |
| Monocytes (%) | 7.5 ± 2.0 | 8.0 ± 3.2 | 6.9 ± 1.2 | 5.3 ± 0.9 | 7.2 ± 1.0 | 7.6 ± 5.7 | 10.4 ± 5.1 | 7.8 ± 2.2 |
| Eosinophil (%) | 11.6 ± 9.1 | 8.41 ± 4.2 | 5.96 ± 5.8 | 5.70 ± 1.7 | 7.47 ± 11 | 4.25 ± 1.9 | 4.15 ± 1.7 | 2.70 ± 2.4 |
| Basophil (%) | 0.30 ± 0.3 | 0.11 ± 0.1 | 0.12 ± 0.3 | 0.26 ± 0.2 | 0.31 ± 0.6 | 0.13 ± 0.1 | 0.34 ± 0.5 | 0.11 ± 0.1 |
No other unusual results were revealed by urinalysis before and after administration of CGX (data not shown).
Serum biochemistry: The serum chemistry in all dogs (12 males and 12 females) was checked before beginning CGX administration. Another chemistry analysis was conducted on the final day. Results did not reveal any significant changes to biochemistry values between the control and CGX groups, except the BUN levels in female dogs who were administered 400 mg/kg per day CGX when compared to the control group (Table 3).
| Number of dogs | Male (12) | Female (12) | ||||||
| CGX (mg/kg per day) | 0 | 100 | 200 | 400 | 0 | 100 | 200 | 400 |
| AST (IU/L) | 37.0 ± 3.4 | 36.3 ± 2.0 | 40.3 ± 14.2 | 41.3 ± 8.3 | 40.6 ± 8.5 | 34.3 ± 5.7 | 37.0 ± 4.3 | 40.0 ± 9.5 |
| ALT (IU/L) | 57.6 ± 7.3 | 48.6 ± 8.1 | 49.3 ± 29.7 | 65.6 ± 24.0 | 38.0 ± 7.0 | 46.6 ± 16.2 | 44.0 ± 1.7 | 33.6 ± 9.0 |
| ALP (IU/L) | 328 ± 46 | 299 ± 102 | 390 ± 221 | 238 ± 74 | 271 ± 104 | 391 ± 93 | 377 ± 159 | 174 ± 43 |
| BUN (mg/dL) | 18.2 ± 1.3 | 20.1 ± 3.1 | 22.8 ± 3.6 | 19.3 ± 3.5 | 13.4 ± 1.9 | 17.6 ± 2.6 | 13.9 ± 3.9 | 24.4 ± 4.8a |
| Creatinine (mg/dL) | 1.05 ± 0.1 | 1.20 ± 0.1 | 1.23 ± 0.1 | 1.18 ± 0.1 | 1.03 ± 0.2 | 1.15 ± 0.1 | 1.09 ± 0.1 | 1.11 ± 0.1 |
| Glucose (mg/dL) | 99.0 ± 15.5 | 115.6 ± 5.1 | 112.3 ± 11.9 | 110.7 ± 4.2 | 114 ± 14 | 121 ± 10.2 | 117 ± 5.6 | 116 ± 26.1 |
| Total chol. (mg/dL) | 252 ± 30 | 223 ± 34 | 260 ± 25 | 217 ± 51 | 299 ± 73 | 296 ± 34 | 263 ± 24 | 261 ± 58 |
| Total bilirubin (mg/dL) | 0.23 ± 0.1 | 0.27 ± 0.1 | 0.23 ± 0.1 | 0.30 ± 0.1 | 0.30 ± 0.1 | 0.30 ± 0.0 | 0.30 ± 0.1 | 0.33 ± 0.1 |
| Total protein (g/dL) | 7.83 ± 0.2 | 7.37 ± 0.2 | 7.33 ± 0.2 | 7.37 ± 0.3 | 7.60 ± 0.3 | 7.47 ± 0.6 | 7.40 ± 0.4 | 7.53 ± 0.2 |
| Albumin (g/dL) | 4.73 ± 0.3 | 4.33 ± 0.2 | 4.47 ± 0.1 | 4.60 ± 0.4 | 4.77 ± 0.3 | 4.50 ± 0.3 | 4.63 ± 0.3 | 4.57 ± 0.2 |
| AG ratio | 1.53 ± 0.2 | 1.43 ± 0.2 | 1.60 ± 0.2 | 1.67 ± 0.3 | 1.70 ± 0.1 | 1.50 ± 0.1 | 1.70 ± 0.1 | 1.57 ± 0.1 |
| C.phosphokinase (IU/L) | 133 ± 15 | 130 ± 39 | 171 ± 89 | 157 ± 48 | 199 ± 30 | 116 ± 24 | 155 ± 37 | 144 ± 23 |
| Triglyceride (mg/dL) | 25.0 ± 3.0 | 35.6 ± 24.8 | 30.0 ± 3.6 | 287 ± 7.2 | 36.0 ± 16.6 | 28.4 ± 9.5 | 36.0 ± 5.2 | 47.3 ± 17.1 |
| Calcium (mg/dL) | 14.8 ± 0.5 | 14.5 ± 0.5 | 14.6 ± 0.3 | 14.4 ± 0.2 | 14.5 ± 0.5 | 14.7 ± 0.9 | 14.1 ± 0.4 | 14.6 ± 0.7 |
| Inorganic pho. (mg/dL) | 3.33 ± 1.1 | 4.43 ± 0.3 | 4.23 ± 0.3 | 4.43 ± 0.6 | 3.80 ± 0.5 | 3.90 ± 0.6 | 4.03 ± 0.8 | 3.63 ± 0.3 |
| LDH (IU/L) | 105 ± 45.7 | 132 ± 73.5 | 91.7 ± 32.8 | 106 ± 60.3 | 80.0 ± 11.5 | 68.3 ± 9.7 | 146 ± 60.7 | 146 ± 126.1 |
| GGT (IU/L) | 4.63 ± 0.3 | 3.93 ± 1.3 | 4.03 ± 0.4 | 4.37 ± 1.3 | 4.93 ± 1.0 | 5.13 ± 0.4 | 4.57 ± 1.0 | 5.53 ± 0.5 |
| Sodium (mmol/dL) | 148 ± 2.0 | 146 ± 1.1 | 144 ± 2.1 | 144 ± 0.6 | 146 ± 1.2 | 148 ± 1.00 | 147 ± 2.0 | 147 ± 0.4 |
| Potassium (mmol/dL) | 4.21 ± 0.1 | 4.32 ± 0.1 | 4.31 ± 0.2 | 4.18 ± 0.2 | 4.08 ± 0.23 | 3.95 ± 0.2 | 4.24 ± 0.3 | 4.42 ± 0.4 |
| Chloride (mmol/dL) | 116 ± 1.1 | 115 ± 2.5 | 115 ± 2.3 | 116 ± 0.6 | 117 ± 1.5 | 114 ± 1.5 | 114 ± 3.2 | 114 ± 1.5 |
Among the variety of complementary and alternative medicines, Asian doctors have prescribed herbal mixtures to treat various diseases according to traditional Oriental pharmacology. Since this traditional treatment is based on extensive knowledge gathered from applications of natural resources to humans, people have usually assumed that the treatment is safe. However, as Oriental remedies have been rapidly growing in popularity worldwide, researchers are paying as much attention to safety issues as to their therapeutic efficacy[17-21]. Many studies have therefore been conducted to evaluate the safety of Oriental herbal medicines or the associated risk of adverse effects[22-25].
This study applied a systemic acute and subacute toxicological examination using beagle dogs to investigate the possibility of adverse effects associated with Chunggan extract, a modified-traditional hepatotherapeutic herbal drug.
In clinical application, the therapeutic dosage of CGX is 100 mg/kg per day, usually divided into three daily doses. We treated dogs with 50 times the clinically recommended dosage of CGX, but observed no clinical signs of adverse effects except slightly decreased activity for 1 h in every group including the control. The decrease in activity might have resulted from the forced administration of a high volume of CGX (or water in the control group) via a catheter. Typical adverse signs of any drug include changes in appetite or faeces; even a 50-fold increase in CGX did not affect appetite or faeces in any group, nor did it affect body weight changes in any group (Figure 2A). A similar pattern was exhibited over repeated CGX administration for 4 wk with dosages ranging from 100 to 400 mg/kg (Table 1). However, no dog in any group (male or female) gained body weight during the 4 wk, which may have resulted from the daily repeated stress of drug or water administration. The variations in body weight on the final day corresponded to the differences in initial body weight at the starting point (Figure 2C and D).
During the subacute toxicological study, no groups exhibited changes in clinical signs. Ophthalmologic examination, analysis of organ weights, and autopsy findings on the final day revealed dogs were free of pathologic states. Because the microscopic examination revealed that every group including the control exhibited focally pathological findings in livers, kidneys, lungs, stomachs, and spleens (Table 1, Figure 3), they were not caused by CGX administration. Furthermore, the positive results for H pylori in all groups might be related to statistics showing that this disease has infected 78% of dogs in Korea[26].
Haematology and chemistry analysis revealed that groups treated with CGX showed no significantly changed values except MCHC in males treated with 100 mg (41.7 ± 0.6) and BUN in females treated with 400 mg (24.4 ± 2.8), shown in Tables 2 and 3 respectively. However, the significance of these changes is doubtful since the values were within the normal range for dogs and the results did not exhibit a dose-dependent or histopathological correlation. It is important to examine potential hepatotoxicity in herbal products because therapeutic and toxic properties apply similar biological processes, especially in the liver[27]. Serum values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin are among the most sensitive markers of liver damage[28]. As expected, treatment with CGX during this study resulted in normal values identical to the control group.
In conclusion, this study verifies that CGX could be safely used in a clinical application with a very large therapeutic index.
S- Editor Liu Y L- Editor Lqbal A E- Editor Bi L
| 1. | Duke K. A century of CAM in New Zealand: a struggle for recognition. Complement Ther Clin Pract. 2005;11:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gavin JA, Boon H. CAM in Canada: places, practices, research. Complement Ther Clin Pract. 2005;11:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Sinclair S. Chinese herbs: a clinical review of Astragalus, Ligusticum, and Schizandrae. Altern Med Rev. 1998;3:338-344. [PubMed] [Cited in This Article: ] |
| 4. | Ye YN, Liu ES, Li Y, So HL, Cho CC, Sheng HP, Lee SS, Cho CH. Protective effect of polysaccharides-enriched fraction from Angelica sinensis on hepatic injury. Life Sci. 2001;69:637-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Dhuley JN. Hepatoprotective effect of rhinax on antitubercular drug-induced hepato-toxicity in rats. Hindustan Antibiot Bull. 2002;44:53-59. [PubMed] [Cited in This Article: ] |
| 6. | Malik IA, Gopalan S. Use of CAM results in delay in seeking medical advice for breast cancer. Eur J Epidemiol. 2003;18:817-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Shekelle PG, Morton SC, Suttorp MJ, Buscemi N, Friesen C. Challenges in systematic reviews of complementary and alternative medicine topics. Ann Intern Med. 2005;142:1042-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Stickel F, Seitz HK, Hahn EG, Schuppan D. [Liver toxicity of drugs of plant origin]. Z Gastroenterol. 2001;39:225-232, 234-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Williams GM, Iatropoulos MJ. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 2002;30:41-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Ahn BM. [Herbal preparation-induced liver injury]. Korean J Gastroenterol. 2004;44:113-125. [PubMed] [Cited in This Article: ] |
| 11. | Cho JH, Lee YY, Seo SH, Yoo HS, Choi WJ, Lee YW, Son CG, Cho CK. A clinical report about 57 patients with chronic liver disease. Korean J Oriental Med. 2001;21:112-121. [Cited in This Article: ] |
| 12. | Son CG, Choi BL, Shin JW, Choi WJ, Cho JH, Cho CK. Effect of gamichunggantang on alcoholic metabolism and alcoholic liver disease. Korean J Oriental Med. 2001;2:89-98. [Cited in This Article: ] |
| 13. | Son CG, Choi WJ, Shin JW, Han SH, Cho JH, Song KC, Cho CK. Effects of gamichunggantang on hyperlipidemia. Acta Pharmacol Sin. 2003;24:133-139. [PubMed] [Cited in This Article: ] |
| 14. | Shin JW, Son JY, Oh SM, Han SH, Wang JH, Cho JH, Cho CK, Yoo HS, Lee YW, Lee MM. An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J Gastroenterol. 2006;12:6142-6148. [PubMed] [Cited in This Article: ] |
| 15. | Dunnett CW. Multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096-1121. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3083] [Cited by in F6Publishing: 2121] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 16. | Dunnett CW. New tables for multiple comparison with a control. Biometrics. 1964;20:482-492. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2300] [Cited by in F6Publishing: 2297] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 17. | Cupp MJ. Herbal remedies: adverse effects and drug interactions. Am Fam Physician. 1999;59:1239-1245. [PubMed] [Cited in This Article: ] |
| 18. | Pinn G. Adverse effects associated with herbal medicine. Aust Fam Physician. 2001;30:1070-1075. [PubMed] [Cited in This Article: ] |
| 19. | Kelly J. Toxicity and adverse effects of herbal complementary therapy. Prof Nurse. 2002;17:562-565. [PubMed] [Cited in This Article: ] |
| 20. | Ernst E. Serious psychiatric and neurological adverse effects of herbal medicines -- a systematic review. Acta Psychiatr Scand. 2003;108:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Rousseaux CG, Schachter H. Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Res B Dev Reprod Toxicol. 2003;68:505-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Kobayashi H, Mizuno N, Teramae H, Kutsuna H, Ueoku S, Onoyama J, Yamanaka K, Fujita N, Ishii M. Diet and Japanese herbal medicine for recalcitrant atopic dermatitis: efficacy and safety. Drugs Exp Clin Res. 2004;30:197-202. [PubMed] [Cited in This Article: ] |
| 23. | Cho KH, Kang HS, Jung WS, Park SU, Moon SK. Efficacy and safety of chunghyul-dan (qingwie-dan) in patients with hypercholesterolemia. Am J Chin Med. 2005;33:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | MacPherson H, Liu B. The safety of Chinese herbal medicine: a pilot study for a national survey. J Altern Complement Med. 2005;11:617-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Woodward KN. The potential impact of the use of homeopathic and herbal remedies on monitoring the safety of prescription products. Hum Exp Toxicol. 2005;24:219-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Hwang CY, Han HR, Youn HY. Prevalence and clinical characterization of gastric Helicobacter species infection of dogs and cats in Korea. J Vet Sci. 2002;3:123-133. [PubMed] [Cited in This Article: ] |
| 27. | Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, Ruhren R. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health. 1986;18:161-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 191] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Pari L, Kumar NA. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J Med Food. 2002;5:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |