INTRODUCTION
The spindle is a vital apparatus of mitotic cells. The mechanism of its formation has attracted great research attention. In centrosome-containing cells, centrosomes are thought to be instrumental for organization of the spindle poles and determination of both the microtubule polarity and the spindle axis[1]. The spindle poles often contain widely different amounts of known spindle-pole components, such as γ-tubulin. At the beginning of mitosis, γ-tubulin is recruited to the poles and forms a γ-tubulin ring complex (γTuRC), which can collect both α and β tubulins to form spindle fibers[2,3]. At the same time, accessory proteins also join the process. One of these types is microtubule-associated proteins (MAPs). Three major neural MAPs (MAP1, MAP2 and tau) and one non-neural MAP (MAP4) have been identified and well characterized at the biochemical level[4,5]. These MAPs possess the ability to stimulate tubulin polymerization and to bind to microtubules in vitro and are believed to play an important part in the regulation of microtubule formation and stabilization in vivo[6].
Vasodilator-stimulated phosphoprotein (VASP) is a proline-rich founding member of the Ena (drosophila enabled)/VASP family, a group of multifunctional proteins associated with regulation of actin. VASP was first found in platelets and is known to be a substrate for cAMP dependent protein kinase (PKA) and cGMP dependent protein kinase (PKG) in a variety of cells, such as vascular smooth muscle cells and endothelial cells[7,8]. It is also known to be actin cytoskeletal regulatory proteins. In a variety of cells like vascular endothelial cells, platelets, smooth muscle cells, and fibroblasts, VASP has been found to be highly expressed where it can be found in focal adhesions, along stress fibers, and in the areas with highly dynamic membrane activity, such as extending lamellipodia and filopodia[9-12]. In vivo evidence indicates that VASP is a crucial factor in the formation of actin filaments and the integration of signals transmitted between cytoskeleton, cytoskeleton-membrane interface and the two cyclic nucleotide signal transduction pathways[9]. Even though increasing data address the importance of VASP, the biological role of this protein still remains largely unknown. We have recently found that phosphorylated VASP (p-VASP) is co-located with α-tubulin on the spindles of mitotic cells, indicating that this cytoskeletal organizing protein may also have a role in assembling and stabilizing the mitotic spindles.
MATERIALS AND METHODS
Cell line
Human gastric epithelial cell line SGC-7901 was provided by Institute of Cell Biology of China (Shanghai, China).
Reagents
Dulbeccos’s modified Eagle’s medium (DMEM) was from GiBco (Grand Island, NY). Newborn calf serum (NBCS) was from Minhai Bio-engineering Co., LTD (Lanzhou, China). Antibodies against VASP (Cat. No. sc-46668) and phosphorylated VASP (Cat. No. sc-23506-R) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against α-tubulin (BM1452) was from Wuhan Boster Biological Technology Ltd (Wuhan, China). FITC- and TRITC- conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Protein kinase A inhibitor H89 (Cat. No. 371963) was from Calbiochem (San Diego, CA). Nuclear fluorochrome Hoechst 33342 was from Sigma (St. Louis, Missouri).
Immunofluorescent microscopy
The cells grown on cover slips were incubated to reveal nuclei with 0.2 µmol/L Hoechst 33 342 for 10 min. Then, the cells were fixed with freshly prepared 40 g/L paraformaldehyde in PBS at 4°C overnight (o/n). After being penetrated with 30 mL/L Triton × 100 and blocked with 30 g/L BSA, the cells were incubated with primary antibodies at 4°C o/n, and then with FITC- or TRITC- conjugated secondary antibodies for 1 h at room temperature (RT), and washed three times after each time of incubation. For immunofluorescence co-staining of α-tubulin and p-VASP, the cells on cover slips were subsequently incubated with mouse monoclonal antibody against α-tubulin at 4°C o/n, rabbit polyclonal antibody against p-VASP for 2 h at RT, goat anti-mouse IgG conjugated with FITC for 1 h at RT, and finally TRITC-conjugated goat anti-rabbit IgG for 1 h at RT, and washed three times after each time of incubation. The cover slips were mounted in 900 mL/L glycerol and 100 mL/L Tris-HCl (25 mmol/L, pH 7.5). The fluorescence of FITC (green), TRITC (red), and Hoechst 33 342 (blue) was observed under a fluorescence microscope with a CCD camera (Leica).
RESULTS
Distribution of p-VASP and α-tubulin in interphase of SGC-7901 cells
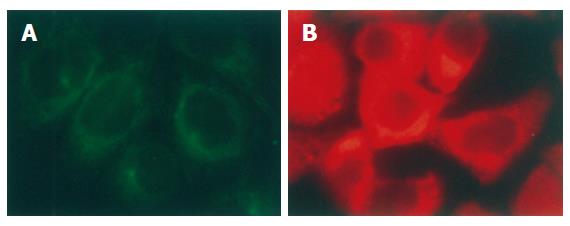
Immunofluorescence staining showed that both p-VASP and α-tubulin were mainly distributed in the cytoplasm of the cells in interphase (Figure 1A and B)
Figure 1 Distribution of α-tubulin (A) and p-VASP (B) in interphase of SGC-7901 cells (immunofluorescent staining × 200).
α-tubulin was stained with monoclonal antibody against the protein. p-VASP was stained with polyclonal antibody specifically against VASP phosphorylated at Ser157. Both α-tubulin and p-VASP were located in cytoplasm of the cells.
p-VASP but not VASP was located with α-tubulin on the spindle of mitotic cells
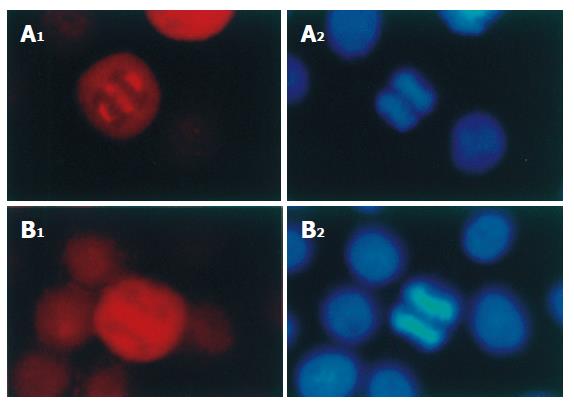
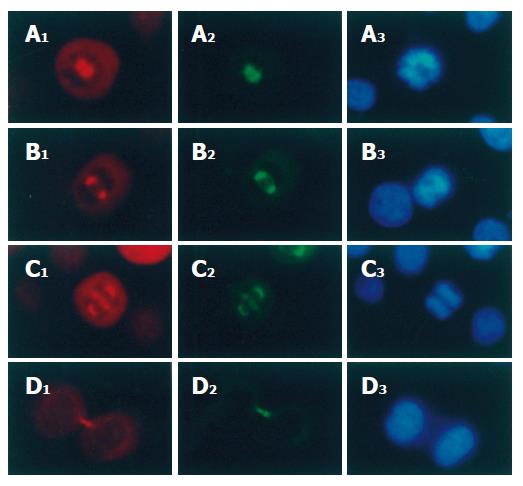
Immunofluorescence staining showed that p-VASP but not VASP was localized both on the spindle poles and on the spindle fibers of mitotic cells (Figure 2A and B). In prophase, the chromosomes became condensed with formation of p-VASP-containing spindle poles (Figure 3A). In metaphase, the chromosomes converged at the metaphase plate (Figure 3B) and the p-VASP-containing spindle poles were located at the opposite sides of chromosomes and the p-VASP-containing spindle fibers irradiated to the chromosomes (Figure 3B). In anaphase, daughter chromosomes were separated and moved toward the opposite p-VASP-containing spindle poles (Figure 3C). Both p-VASP-containing spindle poles and fibers could be seen in this phase (Figure 3C). In telophase, p-VASP-containing spindle disappeared and new daughter nuclei formed (Figure 3D). However, p-VASP could be detected at the cleavage furrow between two separating cells (Figure 3D). Immunofluorescence co-staining showed that α-tubulin was co-localized with p-VASP throughout all phases of mitosis (Figure 3A-D).
Figure 2 Distribution of p-VASP (A) and VASP (B) in mitotic SGC-7901 cells (× 200).
p-VASP and VASP in mitotic SGC-7901 cells were immunofluorescently stained with polyclonal and monoclonal antibodies respectively. The chromosomes were stained with Hoechst 33342.
Figure 3 p-VASP co-localization with α-tubulin and chromosomes on spindles of mitotic SGC-7901 cells (× 200).
p-VASP and α-tubulin were stained with polyclonal and monoclonal antibodies respectively. A1-D1: p-VASP localization in prophase, metaphase, anaphase and telophase respectively; A2-D2: α-tubulin localization in the same phases; A3-D3: chromosomes of the cells were stained with Hoechst 33342.
PKA and PKG inhibitor had no effect on the distribution of p-VASP in mitotic spindle
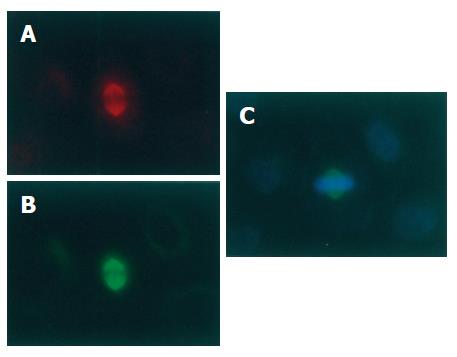
H89, an inhibitor of PKA and PKG, had no obvious effect on the localization of p-VASP on the mitotic spindle. Immunofluorescent staining of p-VASP could still be seen on the spindles of mitotic cells treated with H89 (10 μmol/L) for different periods of time (Figure 4).
Figure 4 No effect of PKA and PKG inhibitor H89 on location of p-VASP on spindles of mitotic SGC-7901 cells (× 200).
SGC-7901 cells were treated with H89 (10 μmol/L) for 6 h and immunofluorescently stained for p-VASP (A) and α-tubulin (B). The chromosomes were stained with Hoechst 33342 (C).
DISCUSSION
Mitosis is an important event of the cell cycle. During this process, duplicated DNA genetic material is condensed to chromosomes moving into two daughter cells with high fidelity. In eukaryotic cells, the accurate segregation of chromosomes during cell division occurs on the spindle. The formation of the spindle is a complicated process that requires the participation of several types of proteins such as tubulins, spindle-microtubule motor proteins, and microtubule-associated proteins (MAPs)[13,14]. Even though accumulating data address the dynamic process of spindle formation, the exact mechanism and process still need to be further clarified.
In this experiment, we showed for the first time that p-VASP was localized both on spindle poles and on spindle fibers of the mitotic cells. The localization of p-VASP exerted a dynamic change associated with stages of the cell cycle. In interphase, p-VASP was detected in the cytoplasm. In mitotic cells, p-VASP was localized on the mitotic spindle from prophase to anaphase. In prophase, p-VASP was localized on the poles of the spindle. p-VASP could be detected on both the poles and fibers of the spindles from metaphase to anaphase. The localization of p-VASP in mitotic cells was exactly coincident with the localization of alpha tubulin, suggesting that p-VASP might play a role in the formation of mitotic spindles, which is associated with tubulin.
Microtubule-associated proteins (MAPs) play an important role in the formation of spindles, and can bind to tubulin and assist the assembling and stabilizing of spindles. The microtubule-binding domains of MAPs are divided into three sub-domains: a proline-rich region, an AP sequence region and a short hydrophobic tail[4]. The importance of the proline-rich region in in vitro MAP-induced microtubule assembly has been widely recognized[15-18]. Tokuraku et al[6] showed that this proline-rich region plays a new role as the protofilament linker in in vitro MAP-induced microtubule assembly. Since microtubules consist of tubulin as its major component, the proline-rich region of MAPs may be one of the structural features that permit the protein be able to bind to tubulin. Interestingly, VASP also has a proline-rich region in its structure. This protein is organized in three domains, an Ena-VASP-homology 1 (EVH1) NH2-terminal domain, a proline-rich central domain, and an EVH2 COOH-terminal domain. The proline-rich region comprises a (GP5)3 sequence presenting as a single or repeated motif in different proteins. It has been confirmed that profilin binds to VASP in vitro and cell extracts probably via the (GP5)3 repeats[19]. Considering this structural feature of VASP, it is reasonable to speculate that p-VASP may be able to bind to tubulin and the possible function of the p-VASP-tubulin binding is to stimulate the polymerization of tubulin on spindle fibers and increase the stability of microtubules of the spindles, a role similar to that of MAPs.
Research data indicate that phosphorylation is necessary for the accessory proteins to exert their role in forming and stabilizing the spindles. For example, mutations that cause loss of function of the protein kinase aurora prevent centrosome separation in Drosophila[20], while phosphorylation of Eg5 has been shown to be necessary for its localization on spindle poles[21]. Since our results showed that p-VASP was a form of this protein detected on spindles, it is possible that phosphorylation is the precondition for VASP to exert its role in spindle formation and stabilization. VASP has a peptide chain containing 380 amino acid residues. Three of them are known as phosphorylation sites, of which two serine residues are Ser157 and Ser239 respectively and the threonine residue is Thr278. Phosphorylation of VASP at these sites is directly regulated by PKA and PKG, and VASP has also been shown to be directly phosphorylated on Ser157 by protein kinase C (PKC)[22]. It has been confirmed that VASP phosphorylation is a modulatory event for VASP-dependent regulation of the actin cytoskeleton. The phosphorylation can affect the cytoskeleton structure and may induce morphological changes of cells[23-25]. Research data also indicate that phosphorylation of VASP on different amino acid residues has different functions. For example, serum-induced VASP phosphorylation at serine157 by both PKA and PKC promotes smooth muscle cell (SMC) proliferation, whereas VASP phosphorylation at serine239 induced by nitric oxide (NO)/PKG is required to induce the inhibitory effects of NO on SMC proliferation[26]. One interesting phenomenon observed in our experiment was that PKA (also PKG) inhibitor H89 could not prevent the localization of p-VASP on the mitotic spindles, suggesting that VASP on the spindle is not the substrate of PKA and PKG.
In conclusion, p-VASP might play a role in mitotic spindle formation and stabilization, which implies a potential target for controlling the division and proliferation of gastric cancer cells. The exact mechanism and which protein kinase is responsible for the phosphorylation of VASP on the mitotic spindle need to be further investigated.