Published online Nov 21, 2006. doi: 10.3748/wjg.v12.i43.7058
Revised: February 9, 2006
Accepted: February 18, 2006
Published online: November 21, 2006
Carcinoids involving the ampulla of Vater are rare lesions that may produce painless jaundice. The published data indicate that these tumors, in contrast to their midgut counterparts, metastasize in approximately half of cases irrespective of primary tumor size. Therefore, radical excision in the form of pancreaticoduodenectomy is recommended regardless of tumor size. As with other gastrointestinal carcinoid tumors, biological treatment with octreotide analogues can be applied to symptomatic patients. Tumor-targeted radioactive therapy is a newly emerging treatment option. We here report case of a carcinoid tumor of the ampulla of Vater presenting as painless jaundice in a 65-year old man and review the relevant literature, giving special attention to the morphologic features, clinical characteristics, and treatment modalities associated with this disease process.
- Citation: Poultsides GA, Frederick WA. Carcinoid of the ampulla of Vater: Morphologic features and clinical implications. World J Gastroenterol 2006; 12(43): 7058-7060
- URL: https://www.wjgnet.com/1007-9327/full/v12/i43/7058.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i43.7058
Carcinoid tumors belong to the family of neuroendocrine tumors, which usually grow slowly with distinct biological and clinical characteristics. The incidence of these tumors is approximately 2.5 in 100 000 people per year. The appendix is the most common location, accounting for roughly 60% of all cases, followed by the ileum, the rectum and the stomach. Approximately 2% of cases involve the duodenum, 1% the biliary tract, and 0.6% the pancreas[1]. Carcinoid tumors that involve the ampulla are an extremely rare clinical entity, with 93 reported cases[2-7]. We report another case of a carcinoid tumor of the ampulla of Vater presenting as painless jaundice. The relevant literature is reviewed, giving special attention to the morphologic features, clinical characteristics, and treatment modalities associated with this disease process.
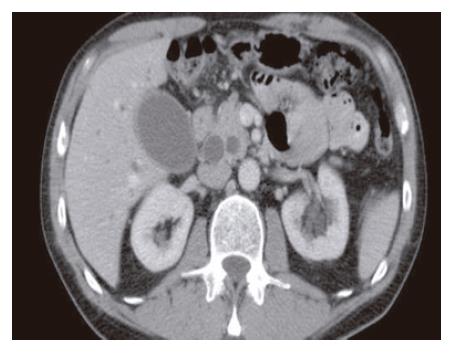
A 65-year old otherwise healthy man presented with a 2-wk history of intractable pruritus. Review of systems was notable for dark urine. He denied clay-colored stool, diarrhea, flushing or dyspnea. Physical examination was remarkable for mildly icteric sclerae. There were no palpable abdominal masses, adenopathy, or café-au-lait spots. Laboratory studies indicated a markedly elevated level of alkaline phosphatase (712 U/L). Total bilirubin was 29 mg/L. Abdominal computed tomography (CT) scan revealed significant dilatation of both the common bile duct (CBD) and the pancreatic duct, without any obvious periampullary mass, retroperitoneal adenopathy or liver lesions (Figure 1).
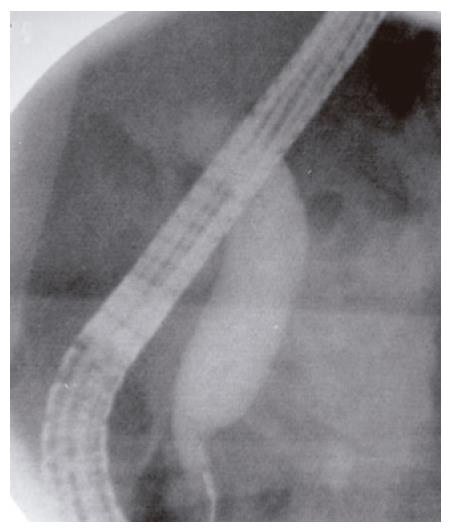
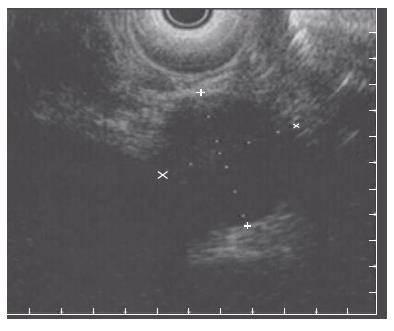
Endoscopic retrograde cholangiopancreatography (ERCP) showed a prominent major ampulla with normal overlying mucosa. The CBD measured 17 mm with an abrupt “shoulder” in the region of the ampulla (Figure 2). The ampullary obstruction was relieved with temporary biliary stent placement. Subsequent endoscopic ultrasonography (EUS) identified a 23 mm × 27 mm well circumscribed, round, hypoechoic mass in the region of the ampulla adjacent to the biliary stent, which was distinct from the pancreatic tissue and the duodenal wall (Figure 3). EUS-guided fine needle biopsy was suggestive of a carcinoid tumor. Positron emission tomography (PET) scan confirmed a lesion of increased metabolism in the vicinity of the pancreatic head, and excluded distant metastases. Urinary 5-hydroxyindoleacetic acid (5-HIAA), and serum carcinoembryonic antigen (CEA), CA19-9, serotonin, somatostatin and chromogranin A levels were all within normal limits.
A pylorus-preserving pancreaticoduodenectomy was performed. Pathology revealed a T3, N1 ampullary carcinoid extending into the peripancreatic fat, with 7/13 positive lymphnodes. Immunohistochemically, tumor cells expressed chromogranin A and synaptophysin, and focally serotonin and somatostatin, which were stained negative for insulin, glucagon, and gastrin. The patient recovered uneventfully and was discharged to home on the seventh postoperative day. The follow-up plan consists of structural studies and biochemical markers every 3 mo up to 1 year and every 6 mo thereafter. At 9 mo he remained asymptomatic with no radiographically or biochemically detectable disease.
In 1888 the first case of a carcinoid tumor was reported as a lesion of the ileum[8]. Oberndorfer coined the term karzinoide (“carcinoma-like”) in 1907 to distinguish these presumptively benign neoplasms from the malignant cecal adenocarcinomas[9]. Carcinoids have been detected in the entire gastrointestinal tract, from the esophagus to the rectum, as well as in extra-gastrointestinal locations, such as the bronchus, the testis, the ovary and the larynx. In contrast to the relative rarity of duodenal carcinoids, those of the ampulla of Vater represent a medical curiosity with only 93 cases reported in the world literature.
Indicative of their location, ampullary carcinoids cause jaundice as the leading symptom in approximately two-thirds of cases. Forty percent of patients present with abdominal pain and few with pancreatitis, weight loss, or malaise[2-7]. Every fourth patient has von Recklinghausen’s disease[2]. The etiology is not clear, but it is likely that mutation of the NF-1 tumor suppressor gene may predispose to the development of ampullary carcinoids. In contrast to midgut carcinoids, foregut carcinoids are very rarely associated with carcinoid syndrome. In the largest reported series of 73 ampullary carcinoid cases, a history of flushing, diarrhea, or asthma was found in only 2 patients who also had extensive liver metastases[2]. Walton et al[4] have described a “variant” form of carcinoid syndrome that may occur in association with gastric or other foregut carcinoid tumors, which may be hidden by the elaboration of other hormonally active substances, such as adrenocorticotropic hormone, parathyroid hormone, calcitonin, gastrin, vasoactive intestinal peptide, growth hormone, or insulin, but responds to treatment with antihistamines.
Immunohistochemistry is of principal importance in accurately diagnosing ampullary carcinoids. Makhlouf et al[7] compared the immunohistochemical features of 12 ampullary and 53 duodenal carcinoids and demonstrated that the former group always expresses chromogranin A, but almost never expresses gastrin which has been identified in 56% of cases of duodenal carcinoids. Apart from its role in immunohistochemical diagnosis, chromogranin A has proven particularly useful as a tumor marker for monitoring disease response and progression in patients with gastrointestinal carcinoids. Analyzing data from 301 such patients, Janson et al[10] found that serum chromogranin A level > 5000 μg/L (normal value: 0-76 μg/L) is an independent predictor of poor survival.
Surprisingly, in the case of ampullary carcinoids, tumor size does not correlate with metastatic potential. In the case review by Hatzitheoklitos et al[2], metastasis is present in 46% of ampullary carcinoids > 2 cm, in 50% of tumors between 1-2 cm, and in 66% of tumors < 1 cm. Makhlouf et al[7] have reported two tumors measuring less than 2 cm demonstrating metastases, as well as a 5 cm tumor without any evidence of metastatic disease. These data indicate that carcinoids involving the ampulla of Vater metastasize in approximately half of the cases regardless of tumor size. This is opposed to the classic teaching regarding midgut and hindgut carcinoids, in which the incidence of metastasis is felt to be a function of tumor size and is significantly higher with larger tumors.
Accordingly, the size of ampullary carcinoids cannot predict node-positive status, and therefore cannot determine the extent of operation. Should every patient with this tumor undergo a pancreaticoduodenectomy The small number of cases and reported follow-up in the literature are not sufficient to answer this question definitively. Instead, each case should be individualized realizing that the goal is complete tumor removal. Given the propensity of ampullary carcinoids smaller than 2 cm to show nodal involvement, and considering the safety of pancreaticoduodenectomy in experienced hands, radical excision should be the treatment of choice to completely extirpate the tumor-bearing tissue. It should be observed that in multiple reported cases, long-term survival has been achieved by local excision of the ampulla[2]. These recommendations, however, were made during a period of surgical history when the operative mortality rate for pancreaticoduodenectomy was high, and therefore are not applied currently.
In cases of liver metastases, surgical resection or other cytoreductive techniques, such as radiofrequency ablation and chemoembolization, have been shown to improve hormone-mediated symptoms, quality of life and survival in certain groups of patients[11]. Patients with slowly growing carcinoid tumors do not generally benefit from cytotoxic chemotherapy. Somatostatin analogues can induce a symptomatic and biochemical response, but more recent studies have also indicated a cytostatic effect[11]. Tumor-targeted radioactive treatment with 90yttrium and 177lutetium coupled to a somatostatin analogue is currently under clinical evaluation[12]. Preliminary data indicate interesting clinical potentials.
In summary, carcinoids of the ampulla of Vater are rare tumors. They can cause symptoms mainly secondary to their periampullary location. Up to 25% of patients have von Recklinghausen’s disease. Carcinoid syndrome is uncommon, unless hepatic metastasis is present. Chromogranin A is an important tumor marker. Determination of histopathology is of utmost importance and involves specific immunohistochemical staining. Aggressive operative extirpation is the cornerstone of treatment and provides the only chance for cure. Biological treatment with somatostatin analogues can be applied in symptomatic patients with slowly growing neoplasms. Tumor-targeted radiotherapy has been introduced recently with promising results. Future therapy will be based on specific tumor biology and treatment will be customized for each individual patient.
S- Editor Wang GP L- Editor Wang XL E- Editor Ma WH
| 1. | Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: clarifying these clinical conundrums. World J Surg. 2005;29:92-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Hatzitheoklitos E, Büchler MW, Friess H, Poch B, Ebert M, Mohr W, Imaizumi T, Beger HG. Carcinoid of the ampulla of Vater. Clinical characteristics and morphologic features. Cancer. 1994;73:1580-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 3. | Ricci JL. Carcinoid of the ampulla of Vater. Local resection or pancreaticoduodenectomy. Cancer. 1993;71:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 4. | Walton GF, Gibbs ER, Spencer GO, Laws HL. Carcinoid tumors of the ampulla of Vater. Am Surg. 1997;63:302-304. [PubMed] [Cited in This Article: ] |
| 5. | Clements WM, Martin SP, Stemmerman G, Lowy AM. Ampullary carcinoid tumors: rationale for an aggressive surgical approach. J Gastrointest Surg. 2003;7:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Baskin LR, Welling RE, Manegold MA. Neurofibromatosis type 1 with a case of carcinoid of the ampulla of Vater. Contemp Surg. 2000;56:76-78. [Cited in This Article: ] |
| 7. | Makhlouf HR, Burke AP, Sobin LH. Carcinoid tumors of the ampulla of Vater: a comparison with duodenal carcinoid tumors. Cancer. 1999;85:1241-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 8. | Lubarsch O. Uber den primaren Krebs des Ileuma, nebst Bemerkungen uber das gleichzeitige Vorkommen von Krebs und Tuberkulose. Virchow Arch Path Anat. 1888;3:280-284. [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 149] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Oberndorfer S. Uber die kleinen Dunndarmkarzinome. Verhandl Deutsch Path Gessellsch. 1907;11:113. [Cited in This Article: ] |
| 10. | Janson ET, Holmberg L, Stridsberg M, Eriksson B, Theodorsson E, Wilander E, Oberg K. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 311] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Oberg K. Management of neuroendocrine tumours. Ann Oncol. 2004;15 Suppl 4:iv293-iv298. [PubMed] [Cited in This Article: ] |
| 12. | Oberg K, Eriksson B. Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:265-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |