Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6874
Revised: September 20, 2006
Accepted: September 29, 2006
Published online: November 14, 2006
AIM: To detect distribution and relative frequency of endocrine cells in gastrointestinal tract of flower fish (Pseudophoxinus antalyae).
METHODS: The intestinal tract of flower fish was divided into four portions from proximal to distal; the enlarged area after oesophagus and anterior, middle and posterior intestine. Immunohistochemical method using the peroxidase anti-peroxidase complex was employed. All antisera between four portions of flower fish were compared using ANOVA.
RESULTS: Eleven types of gut endocrine cells were determined; they were immunoreactive for calcitonin gene related peptide, substance P, vasoactive intestinal peptide, bombesin, somatostatin-14, secretin, TrkA, TrkB, TrkC, neurotensin, neuropeptide Y, which were found in almost all portions of the gastrointestinal tract.
CONCLUSION: The regional distribution and relative frequency of immunoreactive cells in the flower fish, Pseudophoxinus antalyae, are essentially similar to those of other fish.
- Citation: Çınar K, Şenol N, Özen MR. Immunohistochemical study on distribution of endocrine cells in gastrointestinal tract of flower fish (Pseudophoxinus antalyae). World J Gastroenterol 2006; 12(42): 6874-6878
- URL: https://www.wjgnet.com/1007-9327/full/v12/i42/6874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i42.6874
Gastrointestinal hormones are secreted by endocrine cells which are distributed throughout the mucosa of the gastrointestinal tract. They play important roles in the overall regulation of digestive processes such as nutrient absorption, gut motility and intestinal blood flow[1]. Fish digestive tract shows remarkable differences in function and morphology. Despite some differences in species basis, some basic histological structures are somewhat common. Differences observed at specific level should be related with food, feeding habits, body weight, shape and sex[2-4] . Furthermore, presence of a relation between feeding behaviour and basic histological structure[2] or improbability of this[3,5] have been argued.
Almost all the activities involved in the physiological control of vertebrate gut function during fasting or feeding are mediated by the neuroendocrine system. In the vertebrate gut, epithelial cells belonging to the diffuse endocrine system (DES) interact with components of the enteric nervous system (ENS) in regulating digestive functions, such as enzyme secretion, nutrient uptake and the progression of food through the alimentary canal. Concerning fish, several peptides are produced by the components of the neuroendocrine system and are involved in the communication between DES and ENS. Some of these peptides are associated with the modulation of fish alimentary behaviour[6].
Although they are one of the most common endemic species, the regional distribution and relative frequency of endocrine cells in the flower fish, Pseudophoxinus antalyae, have not been studied. Therefore, in the present study, the regional distribution and relative frequency of endocrine cells in the gastrointestinal tract was investigated by immunohistochemistry using 11 types of specific antisera, calcitonin gene related peptide (CGRP), substance P, vasoactive intestinal peptide (VIP), bombesin, somatostatin-14, secretin, neurotensin, neuropeptide Y (NPY), TrkA, TrkB, TrkC raised against mammalian regulatory peptides.
Ten adult (length 15-20 cm, weight 40-50 g) flower fish (Pseudophoxinus antalyae) were used in this study without sexual distinction. After being anaesthetized with ethyl ether, the intestinal tract of flower fish was divided into four portions from proximal to distal; the enlarged area after oesophagus and anterior, middle and posterior intestine. All samples were fixed for 12 h in Bouin’s solution and embedded in paraffin. Serial, transverse 6-7 μm sections of these portions were cut. Each representative section was deparaffinized, rehydrated and immunostained using the peroxidase anti-peroxidase (PAP) method[7]. Sections were treated with methanol containing 0.3% H2O2 for 30 min to block any endogenous peroxidase. Subsequently, the sections were incubated for 1 h at room temperature in normal goat serum (1:100), then stained immunohistochemically to identify specific endocrine cells using PAP. The sections were incubated with specific antisera for individual peptides for 12 h at 4°C. Details of primary antisera used in this study are listed in Table 1. After rinsing with phosphate buffered saline (PBS, 0.01 mol/L, pH 7.4), the sections were incubated in secondary antiserum (ant-rabbit IgG serum raised in goats; 1:200) for 1 h at room temperature. Sections were then washed with PBS buffer and incubated with PAP complex (1:400) for 1 h at room temperature. The peroxidase reaction was carried out in 0.02% 3, 3-diaminobenzidine tetrahydrochloride solution containing 0.01% H2O2 in Tris-HCl buffer (0.05 mol/L, pH 7.6). After immunostaining, the sections were lightly counterstained with Mayer’s haematoxylin and immunoreactive (IR) cells were observed with a light microscope.
| Antiserum | Code | Dilution | Source |
| CGRP | sc-28920 | 1:200 | Santa Cruz Biotech Inc. |
| Substance P | sc-9758 | 1:200 | Santa Cruz Biotech Inc. |
| VIP | NCL-VIPp | 1:200 | Nova Castra Lab, UK |
| Bombesin | NCL-BOMp | 1:200 | Nova Castra Lab, UK |
| Somatostatin | 14CL-SOMATOp | 1:200 | Nova Castra Lab, UK |
| Secretin | sc-20938 | 1:200 | Santa Cruz Biotech Inc. |
| Neurotensin | sc-20806 | 1:200 | Santa Cruz Biotech Inc. |
| NPY | sc-28943 | 1:200 | Santa Cruz Biotech Inc. |
| TrkA | sc-118 | 1:200 | Santa Cruz Biotech Inc. |
| TrkB | sc-12 | 1:200 | Santa Cruz Biotech Inc. |
| TrkC | sc-117 | 1:200 | Santa Cruz Biotech Inc. |
The mean number of endocrine cells per intestinal fold that were immunoreactive to CGRP, substance P, VIP, bombesin, somatostatin-14, secretin, neurotensin, NPY, TrkA, TrkB, TrkC antisera between four portions of flower fish were compared using ANOVA. The level of significance was set at 0.001.
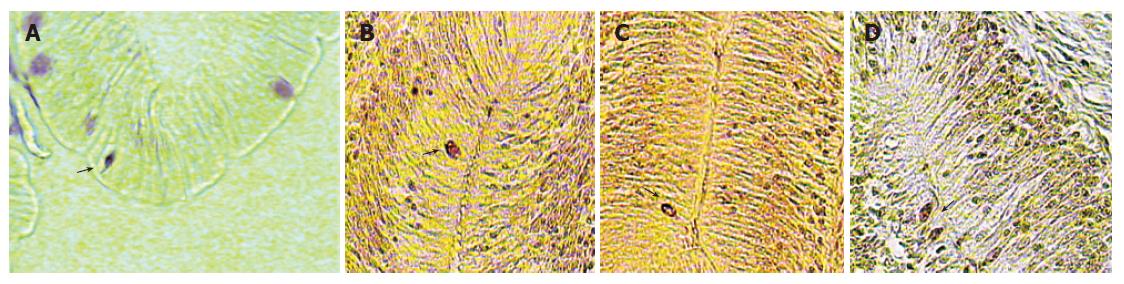
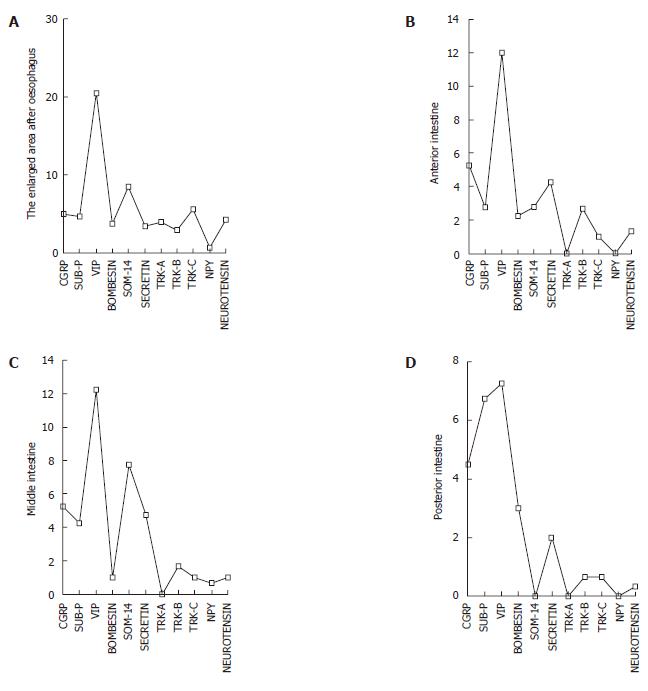
Eleven kinds of immunoreactive cells were detected using the antisera against CGRP, substance P, VIP, bombesin, somatostatin-14, secretin, neurotensin, NPY, TrkA, TrkB, TrkC in the intestinal tract. According to the location of the gut and situation in those regions, different regional distribution and relative frequencies of these immunoreactive cells were observed (Figure 1). These differences are shown in Table 2 and Figure 2.
| Region | Calcitonin gene related peptide | Substance-P | Vasoactive intestinal polypeptide | Bombesin | Somatostatin-14 | Secretin | TrkA | TrkB | TrkC | Neuropeptide Y | Neurotensin | Anova(F) | P |
| The enlarged area after oesophagus | 5.00 ± 1.550 | 4.75 ± 2.217 | 20.50 ± 9.883 | 3.75 ± 2.500 | 8.50 ± 9.037 | 3.50 ± 1.291 | 4.00 ± 4.359 | 3.00 ± 100 | 5.67 ± 3.215 | 0.67 ± 0.577 | 4.33 ± 3.215 | 4.456 | 0.001 |
| Anterior intestine | 5.25 ± 2.500 | 2.75 ± 0.957 | 12.00 ± 6.481 | 2.25 ± 1.258 | 2.75 ± 1.500 | 4.25 ± 2.062 | 0.00 ± 0.000 | 2.67 ± 2.887 | 1.00 ± 1.000 | 0.00 ± 0.000 | 1.33 ± 0.577 | 6.186 | 0.000 |
| Middle intestine | 5.25 ± 2.217 | 4.25 ± 0.957 | 12.25 ± 6.702 | 1.00 ± 0.816 | 7.75 ± 1.708 | 4.75 ± 1.708 | 0.00 ± 0.000 | 1.67 ± 1.528 | 1.00 ± 1.000 | 0.67 ± 0.577 | 1.00 ± 1.000 | 8.115 | 0.000 |
| Posterior intestine | 4.50 ± 2.380 | 6.75 ± 2.217 | 7.25 ± 2.217 | 3.00 ± 0.816 | 0.00 ± 0.000 | 2.00 ± 1.44 | 0.00 ± 0.000 | 0.67 ± 1.155 | 0.67 ± 1.155 | 0.00 ± 0.000 | 0.33 ± 0.577 | 12.829 | 0.000 |
CGRP-IR cells were found throughout the gastrointes-tinal tract at various relative frequencies. Numerous CGRP-IR cells were detected in the epithelial mucosa. These cells were moderate in number in the enlarged area after oesophagus and anterior, middle intestine and then decreased slightly in the posterior intestine. Substance P-IR cells were found in all portions of gastrointestinal tract. These cells were moderate in number in the enlarged area after oesophagus and middle intestine. The number of cells was decreased in middle intestine. Cells immunoreactive for VIP were numerous in posterior intestine. VIP-IR cells, which were at highest frequency in the enlarged area after oesophagus, were found in the epithelia throughout the tract at various frequencies. These cells increased in number from the posterior intestine to the enlarged area after oesophagus.
Bombesin-IR cells varied in number from moderate to numerous in the tract. They then decreased gradually from moderate numbers in the anterior intestine to only a few in the middle intestine. Cells that were immunoreactive for somatostatin-14 were found throughout the gastrointestinal tract except for the posterior intestine. Somatostatin-14 IR cells were demonstrated in both the enlarged area after oesophagus and middle intestine but were more numerous in the former. No somatostatin-IR cells were observed in the posterior intestine. The secretin-IR cells were restricted to the posterior intestine with a rare frequency. They were most numerous in the other portions.
In the enlarged area after oesophagus the presence of endocrine cells which were TrkA immunoreactive, was of a moderate frequency. No cells were found in other regions. TrkB and TrkC IR cells were found at a very low frequency, with the exception of the enlarged area after oesophagus, where their frequency was very low.
NPY-IR cells were demonstrated in both the enlarged area after oesophagus and middle intestine, but they were very rare. No cells were determined in the anterior and posterior intestine. Neurotensin-immunoreactive cells, which were at highest frequency in the enlarged area after oesophagus, were found in all portions at various frequencies. These cells declined in number from the anterior intestine to the middle intestine. These immunoreactive cells were found in the posterior intestine at very low frequencies.
Endocrine cells of the gastrointestinal tract are different in the relative frequency in regional distribution and cell types, between animal species[8]. In the present study, CGRP, substance P, VIP, bombesin, somatostatin-14, secretin, TrkA, TrkB, TrkC, NPY, neurotensin-IR cells were identified in the gastrointestinal tract of the flower fish, Pseudophoxinus antalyae.
The appearance of CGRP-IR cells was well demonstrated in the gastrointestinal tract of the Anguilla anguilla[9] , the Salmo trutta[10]. Dezfuli et al[10] reported that CGRP-IR cells localized in only nerve cell bodies of the myenteric plexus, but in this study these cells were observed in surface epithelia and crypt.
It is well known substance P exerts immunostimulatory and pro-inflammatory effects[11]. Our present results on the GI tract of flower fish agree with previous reports[6,9,10,12-15].
VIP is a peptide consisting of 28 amino acids. Experiments carried out in fishes demonstrated that this peptide is found in nerves and acts as an exciting or inhibiting agent[1]. In the present study, numerous VIP immunoreactive cells were determined in all regions of the gastrointestinal tract. Similar results were reported by Dezfuli et al[10] who also found that VIP IR cells were sparsely distributed in the Salmo trutta digestive tract. VIP-immunoreactivity in the intestinal nerve fibres of myenteric plexus and ganglion cells and intestinal muscle layer has also been reported in Anguilla anguilla[9], Scyliorhinus stellaris and Brachydanio rerio[16], Barbus conchonius[12].
Bombesin is a tetradecapeptide originally isolated from the skin of the amphibian Bombina bombin. The presence of bombesin immunoreactivity has been reported in ECs of the gastrointestinal tract in representatives of all the major vertebrate groups except mammals, and in the enteric nerves of mammals, amphibians and fish[6]. Bombesin-like peptide may play an endocrine role in control of gastric functions such as regulation of acid secretion and gastric motility[17]. These cell types were observed in the gut of Amia calva[17], Oncorhynchus mykiss[18], Salmo trutta[6]. Smilar results were obtained in this study.
Somatostatin, which consists of 14 amino acids, was isolated from the hypothalamus of sheep for the first time and it could be subdivided into straight form and cyclic form[19]. As in the present study, somatostatin-14 immunoreactive cells have been determined in the intestine of many fish species, such as Oncorhynchus mykiss[18], species of the Osteoglossomorpha[20], Pelteobagrus fuluidraca, Monopterus albus, Siniperca chuatsi[15], Zacco platypus[19], Coreoperca herzi[9].
Secretin is a 27-amino acid polypeptide and belongs to the structurally-related peptides of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily[21]. In the present study, secretin-IR cells were found in the gastrointestinal tract. These findings agree well with those of previous reports[6,22] , but differ somewhat from results in the Zacco platypus[19] and Coreoperca herzi[9] where no secretin immunoreactive cells were observed.
Lucini et al[23] showed that Trk-like proteins (TrkA, TrkB, Trkc) IR cells were present throughout the intestine of teleost species. They also stated that this immunoreactivity was present in the myenteric plexus of whole intestinal regions containing nerve fibers in the muscle layer. In addition, Radaelli et al[24] reported that these cells were present in the gut of both Pagrus major and Dentex dentex. In the present study, TrkA immunoreactive cells were found only in the enlarged area after oesophagus. In the other portions of gastrointestinal tract, no cells were detected. TrkB and TrkC immunoreactive cells were scattered amongst the epithelial cells throughout the intestine, similar to that reported by Radaelli et al[24] and Lucini et al[23].
NPY is a 36-amino acid peptide that is widely and abundantly expressed in the central nervous system of all vertebrates investigated[25]. In the present study, NPY-IR cells were found in the enlarged area after oesophagus and middle intestine. These findings are consistent with those of previous reports[9,20]. Rombout and Reinecke[12] showed that neurotensin IR cells were present in the gut of Barbus conchonius. They also stated that this immunoreactivity was also present in the myenteric plexus of whole intestinal regions containing nerve fibres in the muscle layer. We found that, a sparse distribution of neurotensin IR cells were observed in all regions of the digestive tract.
In conclusion, the regional distribution and relative frequency of immunoreactive cells in the flower fish, Pseudophoxinus antalyae, are essentially similar to those of other fish[9,10,19,20,25]. However, some characteristic differences are observed in this species, which may be due to differences in the antisera tested, the methods used and/or the species investigated in the various studies.
S- Editor Wang GP L- Editor Zhu LH E- Editor Bai SH
| 1. | Çınar K, Diler A. Immunohistochemical localization of glucagon, sunstance-P, and vasoactive intestinal peptide in gastrointestinal tract mucosa of zander. J Fish Biol. 2002;60:319-327. [Cited in This Article: ] |
| 2. | Grosh A, Das KM. Morphohistology of the digestive tract of a mullet, Liza parsia (Ham) in relatios to its food habits. J Indian Soc Coast Agric Res. 1987;5:437-444. [Cited in This Article: ] |
| 3. | Boglione B, Bertolini B, Russiello M, Cataduella S. Embriyonic and larval development of Thick-Lipped Mullet (Chelon labrosus) under controlled reproduction conditions. Aquaculture. 1992;101:349-359. [DOI] [Cited in This Article: ] |
| 4. | Murray HM, Wright GM, Goff GP. A comparative histological and histochemical study of the stomach from three species of Pleuronectid, the Atlantic halibut, Hippoglossus hippoglossus, the yellowtail flounder, Pleuronectes ferruginea, and the winter flounder, Pleuronectes americanus. Can J Zool. 1994;72:1199-1210. [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Martin TJ, Blaber SJM. Morphology and histology of the alimentary tract of Ambassidae (Teleostei) in relation to feeding. J Morphol. 1984;182:295-305. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Dezfuli BS, Giari L, Arrighi S, Domeneghini C, Bosi G. Influence of enteric helminths on the distribution of intestinal endocrine cells belonging to the diffuse endocrine system in brown trout, Salmo trutta L. J Fish Dis. 2003;26:155-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Sternberger LA. The unlabeled antibody peroxidase-antiperoxidase (PAP) method. Sternberger LA ed. New York: John Wiley & Sons 1987; 104-169. [Cited in This Article: ] |
| 8. | Lee JH, Ku SK, Park KD, Lee HS. Immunohistochemical study of the gastrointestinal endocrine cells in the Korean aucha perch. J Fish Biol. 2004;65:170-181. [DOI] [Cited in This Article: ] |
| 9. | Domeneghini C, Radaelli G, Arrighi S, Mascarello F, Veggetti A. Neurotransmitters and putative neuromodulators in the gut of Anguilla anguilla (L.). Localizations in the enteric nervous and endocrine systems. Eur J Histochem. 2000;44:295-306. [PubMed] [Cited in This Article: ] |
| 10. | Dezfuli BS, Arrighi S, Domeneghini C, Bosi G. Immuno-histochemical Detection of Neuromodulators in the Intestine of Salmo trutta L. Naturally Infected with Cyathocephalus truncatus Pallas (Cestoda). J Fish Dis. 2000;23:265-273. [DOI] [Cited in This Article: ] |
| 11. | Collins SM, Van Assche G, Hogaboam C. Alterations in enteric nerve and smooth-muscle function in inflammatory bowel diseases. Inflamm Bowel Dis. 1997;3:38-48. [PubMed] [Cited in This Article: ] |
| 12. | Rombout JH, Reinecke M. Immunohistochemical localization of (neuro)peptide hormones in endocrine cells and nerves of the gut of a stomachless teleost fish, Barbus conchonius (Cyprinidae). Cell Tissue Res. 1984;237:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Abad ME, Binkhorst FM, Elbal MT, Rombout JH. A comparative immunocytochemical study of the gastro-entero-pancreatic (GEP) endocrine system in a stomachless and a stomach-containing teleost. Gen Comp Endocrinol. 1987;66:123-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Maake C, Kaufmann C, Reinecke M. Ontogeny of neurohormonal peptides, serotonin, and nitric oxide synthase in the gastrointestinal neuroendocrine system of the axolotl (Ambystoma mexicanum): an immunohistochemical analysis. Gen Comp Endocrinol. 2001;121:74-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Pan QS, Fang ZP, Huang FJ. Identification, localization and morphology of APUD cells in gastroenteropancreatic system of stomach-containing teleosts. World J Gastroenterol. 2000;6:842-847. [PubMed] [Cited in This Article: ] |
| 16. | Tagliafierro G, Faraldi G, Morescalchi A. The GEP neuroendocrine system in fishes: distribution and ontogeny. Regulatory Peptides. 1996;64:1-3. [Cited in This Article: ] |
| 17. | Rajjo IM, Vigna SR, Crim JW. Cholecystokinin immunoreactivity in the digestive tract of bowfin (Amia calva), bluegill (Lepomis macrochirus), and bullfrog (Rana catesbeiana). Gen Comp Endocrinol. 1988;70:133-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Barrenechea MA, López J, Martínez A. Regulatory peptides in gastric endocrine cells of the rainbow trout Oncorhynchus mykiss: general distribution and colocalizations. Tissue Cell. 1994;26:309-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ku SK, Lee JH, Lee HS. Immunohistochemical study on the endocrine cells in gut of the stomachless teleost, Zacco platypus (Cyprinidae). Anat Histol Embryol. 2004;33:212-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | al-Mahrouki AA, Youson JH. Immunohistochemical studies of the endocrine cells within the gastro-entero-pancreatic system of Osteoglossomorpha, an ancient teleostean group. Gen Comp Endocrinol. 1998;110:125-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Bosi G, Shinn AP, Giari L, Simoni E, Pironi F, Dezfuli BS. Changes in the neuromodulators of the diffuse endocrine system of the alimentary canal of farmed rainbow trout, Oncorhynchus mykiss (Walbaum), naturally infected with Eubothrium crassum (Cestoda). J Fish Dis. 2005;28:703-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lucini C, de Girolamo P, Maruccio L, Lamanna C, Castaldo L, Vega JA. Trk-neurotrophin receptor-like immunoreactivity in the gut of teleost species. Cell Tissue Res. 1999;296:323-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Radaelli G, Domeneghini C, Arrighi S, Castaldo L, Lucini C, Mascarello F. Neurotransmitters, neuromodulators, and neurotrophin receptors in the gut of pantex, a hybrid sparid fish (Pagrus major x Dentex dentex). Localizations in the enteric nervous and endocrine systems. Histol Histopathol. 2001;16:845-853. [PubMed] [Cited in This Article: ] |
| 25. | Cerdá-Reverter JM, Martínez-Rodríguez G, Zanuy S, Carrillo M, Larhammar D. Molecular evolution of the neuropeptide Y (NPY) family of peptides: cloning of three NPY-related peptides from the sea bass (Dicentrarchus labrax). Regul Pept. 2000;95:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |