Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6142
Revised: July 8, 2006
Accepted: July 22, 2006
Published online: October 14, 2006
AIM: To evaluate the therapeutic effect of Chunggan extract (CGX), a modified traditional Chinese hepatotherapeutic herbal, on the dimethylnitrosamine (DMN)-induced chronic liver injury model in rats.
METHODS: Liver injuries were induced in Wistar rats by injection of DMN (ip, 10 mg/mL per kg) for 3 consecutive days per week for 4 wk. The rats were administered with CGX (po, 100 or 200 mg/kg per day) or distilled water as a control daily for 4 wk starting from the 15th d of the DMN treatment. Biochemical parameters (serum albumin, bilirubin, ALP, AST and ALT), lipid peroxides, hydroxyproline, as well as histological changes in liver tissues were analyzed. In addition, gene expression of TNF-α, TGF-β, TIMP-1, TIMP-2, PDGF-β, and MMP-2, all of which are known to be associated with liver fibrosis, were analyzed using real-time PCR.
RESULTS: CGX administration restored the spleen weight to normal after having been increased by DMN treatment. Biochemical analysis of the serum demonstrated that CGX significantly decreased the serum level of ALP (P < 0.05), ALT (P < 0.01), and AST (P < 0.01) that had been elevated by DMN treatment. CGX administration moderately lowered lipid peroxide production and markedly lowered hydroxyproline generation caused by DMN treatment in accordance with histopathological examination. DMN treatment induced a highly up-regulated expression of TNF-α, TGF-β, TIMP-1, TIMP-2, PDGF-β, and MMP-2. Of these, the gene expression encoding PDGF-β and MMP-2 was still further enhanced 2 wk after secession of the 4-wk DMN treatment, and was remarkably ameliorated by CGX administration.
CONCLUSION: CGX exhibits hepatotherapeutic proper-ties against chronic hepatocellular destruction and consequential liver fibrosis.
- Citation: Shin JW, Son JY, Oh SM, Han SH, Wang JH, Cho JH, Cho CK, Yoo HS, Lee YW, Lee MM, Hu XP, Son CG. An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J Gastroenterol 2006; 12(38): 6142-6148
- URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6142.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6142
Several pathologic conditions, including chronic alcohol consumption, viral B or C hepatitis, and constant cholestasis, induce chronic injury to liver tissue. Moreover, these chronic liver disorders lead progressively to fibrosis or liver cirrhosis, which is an extreme form of chronic liver disease that reduces the quality of life and life span of the patients[1]. Therefore, a crucial step in treating chronic liver disease involves preventing the development of cirrhosis. One of the best therapeutic strategies involves removing the underlying causes of liver damage[2], and major findings on the epidemiological and pathogenic features that involve various cytokines or the transformation of stellate cells in the progression of chronic liver disease have recently been reported[3-5]. Currently, however, no universally effective therapeutics exist, and chronic liver disease remains a major concern for global public health.
Medical research on natural products for treating chronic liver disease has been increasing in recent years[6-8]. Chunggan extract (CGX, also called Qinggan extract, which means “cleaning liver”) is a modified herbal drug based on a traditional Chinese hepatotherapeutic formula. It has therapeutic properties particularly for hyperlipidemia, alcohol-induced liver damages in animal models, and other chronic liver diseases, including chronic viral B type hepatitis in clinical tests[9-11]. Nevertheless, further studies are needed to evaluate the pharmaceutical efficacy based on scientifically controlled experiments before an appropriate clinical application can be implemented for treating various liver diseases.
We herein investigated the therapeutic effect of this drug on a chronic liver injury model in rats caused by dimethylnitrosamine (DMN).
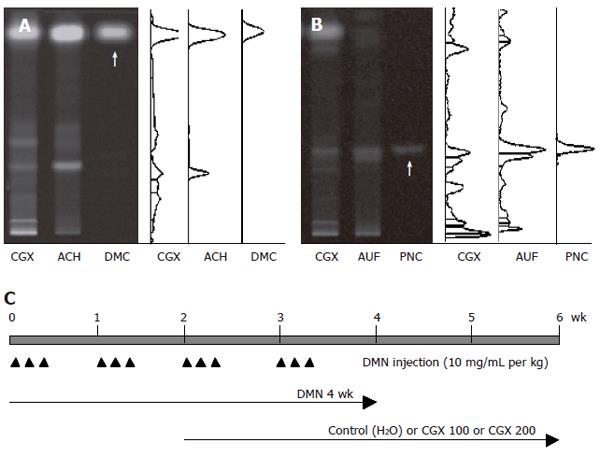
The ingredients of CGX include 5 g each of Artemisia capillaris Herba, Trionycis Carapax, Raphani Semen; 3 g each of Atractylodis Macrocephalae Rhizoma, Poria, Alismatis Rhizoma, Atractylodis Rhizoma, Salviae Miltiorrhizae Radix; 2 g each of Polyporus, Amomi Fructus, Aurantii Fructus, and 1 g of Glycyrrhizae Radix or Helenii Radix. The 10.71% (w/w) lyophilized water-extract was obtained from the initial dried mixture according to the Korean standard over-the-counter (OTC) monographs, and its high-performance thin layer chromatography (HPTLC)-based fingerprint was produced by the CAMAG application system (Muttenz, Switzerland) (Figure 1A and 1B). For the HPTLC analysis, water extracts of CGX, Artemisia capillaris Herba, Aurantii Fructus, and their standard components, 6, 7-dimethoxycoumarin (Sigma Chemical Co., St. Louis, MO, USA) and Poncirin (Fluka, St. Louis, MO, USA), were dissolved in HPLC-grade methanol and applied to pre-washed silica gel 60 F254 HPTLC plates (size 10 cm × 10 cm, thickness of the silica gel 0.2 mm; Merck, Darmstadt, Germany) with an automated applicator (Linomat IV; CAMAG). The samples were then separated (migration distance 75 mm) using HPLC-grade ethyl acetate/formic acid/acetic acid/water (15:1:1:2). The migrated components were visualized under UV radiation at 366 nm using Reprostar 3 with a digital camera (CAMAG).
Specific pathogen-free 4-wk-old male Wistar rats were purchased from a commercial animal breeder (Samtako, Osan, Korea). Forty rats were acclimated for 3 wk and housed in an environmentally controlled room at 22 ± 2°C, 55% ± 10% relative humidity, and 12-h light/dark cycles, and fed commercial pellets (Samtako) and tap water ad libitum. Animal experiments were conducted in accordance with the Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Dimethylnitrosamine (DMN, N-Nitrosodimethylamine) and other reagents, including hydroxyproline, p-dimethylaminobenzaldehyde, and chloramine-T were purchased from Sigma, and perchloric acid was obtained from GFS Chemical Co. (Columbus, OH, USA).
Forty Wistar rats with average body weight 210 ± 15 g were randomly divided into 5 groups (8 rats per group) as follows: naive (no treatment); DMN 4 wk (4-wk DMN treatment and sacrificed following the treatment period); control (4-wk DMN treatment and 4-wk water administration from the third week of DMN treatment); and CGX 100 or CGX 200 (4-wk DMN treatment and 4-wk CGX administration from the third week of DMN treatment). DMN diluted in saline was intraperitoneally injected at 10 mg/mL per kg for 3 consecutive days during each week for the DMN 4 wk, control, CGX 100, and CGX 200 groups. The DMN 4 wk group was sacrificed at the end of the 4-wk DMN treatment. The CGX 100, CGX 200, and control groups were administered CGX with (100 or 200 mg/kg per day) or distilled water as a control daily for 4 wk starting from the third week of DMN-treatment. This experimental model is presented in Figure 1C.
The changes in body weight for all animals were recorded weekly throughout the experimental period. The animals were sacrificed on the last day of experimentation or following the 4-wk induction for the DMN-4 wk group; the liver and spleen were removed and weighed to determine changes in the organ weights by treatment. The liver was removed and separately stored for histomorphologic examination, RNA expression analysis, as well as hydroxyproline and lipid peroxide determination.
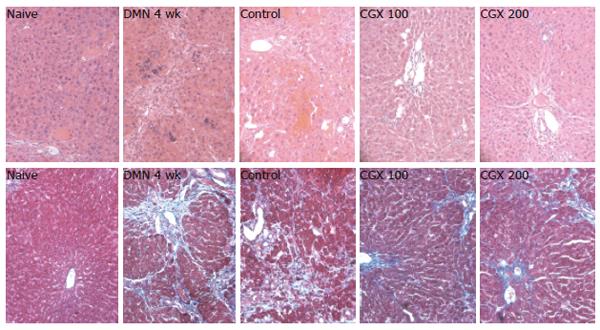
For the histomorphological evaluation, a portion of liver tissue was fixed in Bouin’s solution. The paraffin-embedded liver was sectioned (4-μm thickness), and Hematoxylin & eosin staining and Masson’s trichome staining were performed. The representative histopathological features, such as necrosis or inflammatory cell infiltration and fibrosis, were examined under microscopy.
The rats were bled via abdominal aorta after ether anesthesia on the final day of the 4-wk induction for the DMN 4 wk group or on the final day of full experimentation for others. Complete blood counts were performed by blood cell counter (HEMAVET; CDC Technologies Inc., Oxford, CT, USA) using small amounts of blood mixed in EDTA.
Once sera were prepared from the remaining blood, the levels of total protein, albumin, total bilirubin, alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT) were determined in the serum using an Auto Chemistry Analyzer (Chiron Ltd., Emeryville, CA, USA).
Lipid peroxidation in liver tissues was examined using the method of thiobarbitric acid reactive substances (TBARS) as previously described[12]. The concentration of TBARS was expressed as n moles of malondialdehyde (MDA) per milligram of tissue using 1.1.3.3-tetraethoxypropane (TEP) as a standard. Briefly, 0.2 g of liver tissue was homogenized in 2 mL of ice-cold 11.5 g/L KCl; then 0.13 mL of homogenate was mixed with 0.08 mL of 10 g/L phosphoric acid and 0.26 mL of 0.67% thiobarbituric acid. After heating the mixture for 45 min at 100°C, 1.03 mL of n-butanol was added into the mixture, followed by vigorous mixing and centrifugation at 3000 r/min for 15 min. The absorbance of the upper organic layer was measured at 535 and 520 nm with a spectrophotometer, and compared to the value from freshly prepared TEP as a standard.
Hydroxyproline assays were performed as previously described with a slight modification[13]. Briefly, liver tissues (0.2 g) stored at -70°C were homogenized in 2 mL of 6 mol/L HCl and incubated overnight at 110°C. After filtering the acid hydrolysates using filtering paper (Toyo Roshi Kaisha, Tokyo, Japan), 50 μL of samples and standard hydroxyproline in 6 mol/L HCl were incubated to dry out the HCl; 50 μL of methanol, 1.2 mL of 500 mL/L isopropanol, and 200 μL of chloramine T solution were sequentially added to the samples, followed by incubation at room temperature for 10 min. Next, 1.3 mL of Ehrlich’s solution was added to the mixtures and incubated further at 50°C for 90 min. At the end of incubation, absorbance of the reaction mixtures was read at 558 nm. A standard curve was constructed using 0-1.0 mg/50 μL of hydroxyproline solutions.
Total RNA was extracted from liver tissue samples with Trizol (Invitrogen, Carlsbad, CA, USA), RNAlater (Ambion, Inc., Austin, TX, USA), and RNA easy column (Qiagen, Inc., Valencia, CA, USA). Complementary DNA (cDNA) was synthesized using 10 pmol of oligo dT and 10 pmol/L of random hexamer (Bioneer, Daejeon, Korea). After cDNA synthesis, quantitative real-time PCR was performed using SYBR green supermix reagent (Bio-Rad, Hercules, CA, USA) with the primers (forward and reverse, respectively) for β-actin: 5’- GTGGGGCGCCCCAGGCACCA-3’ and 5’- CTCCTTAATGTCACGCACGATTTC-3’; tumor necrosis factor-alpha (TNF-α): 5’- CTCCCAGGTTCTCTTCAAGG-3’ and 5’- TGGAAGACTCCTCCCAGGTA; transforming growth factor-beta (TGF-β): 5’- TGAGTGGCTGTCTTTTGACG-3’ and 5’- TTCTCTGTGGAGCTGAAGCA; platelet-derived growth factor beta (PDGF-β): 5’- GAGTCGAGTCGGAAAGCTCA-3’ and 5’- CTGCTGCAGCCAGAGACC; matrix metalloproteinase-2 (MMP-2): 5’-GCGCTTTTCTCGAATCCAT-3’ and 5’- GGGTATCCATCTCCATGCTC-3’; tissue inhibitor of metalloproteinase-1 (TIMP-1): 5’-CGGACCTGGTTATAAGGGCT-3’ and 5’- ACTCTCCAGTTTGCAAGGGA; and tissue inhibitor of metalloproteinase-2 (TIMP-2): 5’-GCATCACCCAGAAGAAGAGC-3’ and 5’- GTTTCCAGGAAGGGATGTCA-3’. Reactions were performed with 12.5 μL of iQ SYBR green supermix, 1 μL of 10 pmol/L primer pairs, 10.5 μL of distilled water, and 1 μL of cDNA. Each PCR run was performed under the following conditions: initial denaturation at 95°C for 5 min, 40 amplification cycles including denaturation at 95°C for 1 min, annealing at 58°C for 40 s-, and elongation at 72°C for 40 s, followed by a single fluorescence measurement.
The results were expressed as the mean ± SD. Statistical analysis of the data was carried out using Student’s t test. P values less than 0.05 were considered statistically significant.
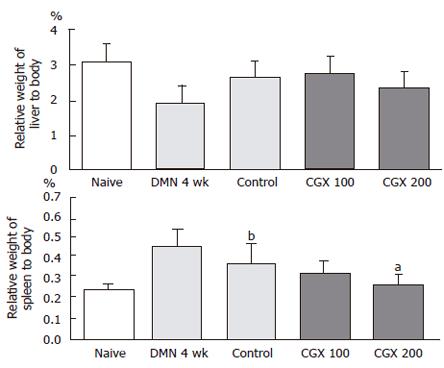
As shown in Figure 2, 4-wk DMN treatment decreased relative liver weights compared to the control group, while it increased relative spleen weight. A 2-wk cessation of DMN following the 4-wk DMN treatment slightly restored these changes as seen in the control groups. These restorations were augmented by CGX administration (200 mg/kg), especially the spleen weights, compared to the control group (P < 0.05; Figure 2).
DMN treatment for 4 wk decreased the level of erythrocytes, hemoglobin, hematocrit and platelets, and decreased the lymphocyte population in peripheral blood compared to the naive group (Table 1). Most of these hematological changes, however, had almost completely recovered to normal levels by cessation of the treatment for 2 wk (control group). These hematological improvements may, therefore, not have been due to the effect of CGX, with the exception of the decreased monocyte population of leukocytes in CGX 100 and CGX 200 groups (P < 0.05).
| Groups | Naive | DMN 4wk | Control | CGX 100 | CGX 200 |
| Erythrocyte (106/μL) | 8.0 ± 0.3 | 7.1 ± 0.9 | 7.5 ± 1.5 | 8.4 ± 0.4 | 8.2 ± 0.5 |
| Hemoglobin (g/dL) | 15.4 ± 0.8 | 13.5 ± 1.3 | 14.0 ± 2.3 | 14.9 ± 0.6 | 14.8 ± 0.4 |
| Hematocrit (%) | 41.9 ± 1.4 | 35.2 ± 2.9 | 40.0 ± 6.1 | 40.9 ± 1.8 | 41.2 ± 2.6 |
| Platelets (103/μL) | 596 ± 459 | 449 ± 170 | 848 ± 265 | 795 ± 357 | 502 ± 305 |
| Leukocytes (103/μL) | 4.8 ± 1.2 | 4.9 ± 2.0 | 4.2 ± 1.8 | 2.6 ± 1.0 | 3.3 ± 1.2 |
| Neutrophil (%) | 17.1 ± 3.7 | 50.5 ± 7.1 | 39.7 ± 18.5b | 32.2 ± 9.1 | 34.7 ± 20.2 |
| Lymphocyte (%) | 79.2 ± 3.7 | 44.2 ± 6.0 | 53.3 ± 18.4b | 63.9 ± 10.1 | 61.1 ± 19.2 |
| Monocytes (%) | 3.6 ± 0.8 | 4.7 ± 1.7 | 6.6 ± 1.5c | 3.6 ± 2.0a | 3.8 ± 1.3a |
| Eosinophil (%) | 0.1 ± 0.1 | 0.4 ± 0.3 | 0.4 ± 0.4 | 0.3 ± 0.2 | 0.3 ± 0.3 |
| Basophil (%) | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0.2 |
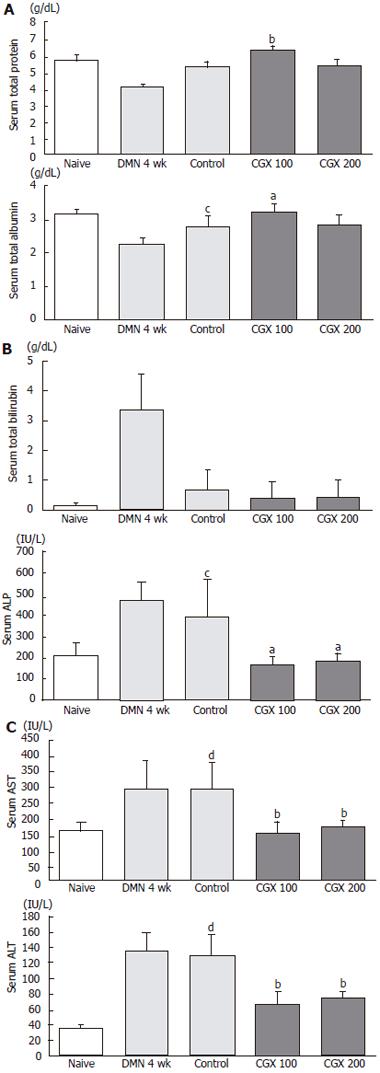
Four-week treatment with DMN induced liver dysfunction, including lowered levels of total protein and albumin along with elevated level of total bilirubin, ALP, AST, and ALT (Figure 3A-3C). Although these biochemical parameters were partially restored after the 2-wk cessation period in the control group, AST and ALT levels still differed significantly from their normal values.
CGX treatment (100 mg/kg) showed significant restoring effects on serum level of total proteins and albumin (P < 0.0l and P < 0.05, respectively; Figure 3A). However, bilirubin, which was rapidly reduced by cessation of DMN treatment for 2 wk, was not affected by CGX administration. The elevated ALP level significantly decreased with treatment by both concentrations of CGX (Figure 3B), and the CGX treatment significantly lowered the of AST back to nearly normal level and moderately reduced the level of ALT (P < 0.0l; Figure 3C), while no significant changes were observed after 2-wk cessation of DMN following the 4-wk DMN treatment without CGX.
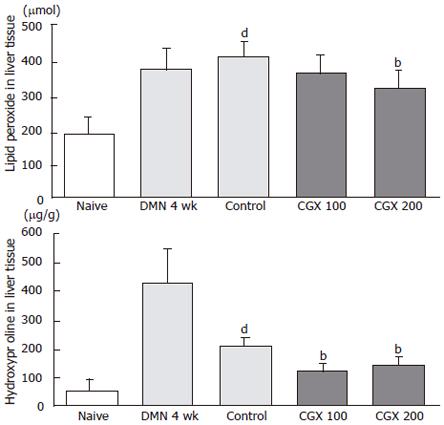
To examine the effects of CGX on lipid peroxidation and fibrosis in liver tissue, lipid peroxide and hydroxyproline were determined from frozen tissues. Prominent lipid peroxidation occurred during the 4-wk DMN treatment and progressed further during the 2-wk cessation period (control group), but 200 mg/kg CGX administration lowered it significantly (P < 0.01). Hydroxyproline, however, rapidly increased with DMN treatment but then dropped dramatically 2 wk later (control group), and CGX administration (100 and 200 mg/kg) significantly augmented its concentration (P < 0.01; Figure 4).
Four-week DMN treatment (DMN 4 wk) appeared to shrink the liver and cause it to become blunt and congested with blood, whereas the CGX treatment reversed these changes. These gross findings were supported by microscopic examination after hematoxylin & eosin and Masson’s trichrome staining. DMN treatment for 4 wk caused severe local necrosis, inflammatory cell infiltration, hemorrhagic regions, and serious septal fibrosis. Although these pathologic features partially recovered by stopping the DMN treatment for 2 wk, CGX administration led to notable recovery effects (Figure 5).
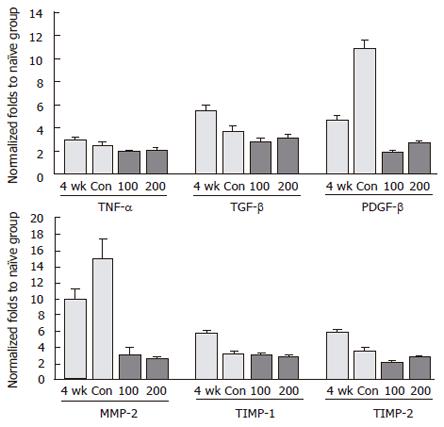
To inspect the changes in gene expression of cytokines linked to liver fibrosis and the anti-fibrotic effects of CGX, gene expressions of TNF-α, TGF-β, PDGF-β, MMP-2, TIMP-1, and TIMP-2 were analyzed using real-time PCR. As shown in Figure 6, DMN treatment induced high expression of all these genes compared to the naive group. The DMN-induced high expression of TNF-α, TGF-β, TIMP-1, and TIMP-2 were spontaneously reduced to basal levels by cessation of the DMN treatment. However, the expression of genes encoding PDGF-β and MMP-2 was further activated during the 2-wk cessation period (control group). These patterns of over-expressed genes prominently subsided following CGX administration.
Liver tissue is generally characterized by its vigorous ability to completely regenerate when damaged during short periods, whereas chronic liver injury can cause gradual progression of fibrotic or cirrhotic changes or sometimes cancerous transformation, resulting in dysfunction of the liver itself with whole-body consequences[14]. Disorders with persistent destruction of liver tissue, known as chronic liver diseases, include chronic types of viral or alcoholic hepatitis, autoimmune hepatitis, liver cirrhosis, and liver cancer, and are a leading cause of death worldwide[15]. The development of cirrhosis is a critical step impacting the clinical outcome or mortality of patients suffering from chronic liver diseases. Because cirrhotic changes consisting of fibrosis and nodulation are often a consequence of the wound-healing response to chronic liver injury, treating the underlying pathogenic conditions, such as chronic inflammation, before the onset of fibrogenesis is an important strategy for the management of these disorders[16,17].
Despite the lack of well-designed clinical evidence, herbal medicines are becoming popular among patients with liver disease, and are attractive as putative anti-fibrotics or hepatotherapeutics[18-22]. CGX has shown some hepatoprotective properties or anti-hyperlipidemic effects in previous studies[9-11], so we further examined whether this drug has therapeutic effects in a chronic liver damage model, including anti-fibrotic effects.
We constructed a chronic liver injury model using Wistar rats as in previous studies[23-25], showing marked liver dysfunctions, such as low serum albumin, high level of bilirubin, ALP, AST, and ALT, and increased lipid peroxidation and hydroxyproline accumulation in liver tissues. In our experiments, the values of relative organ weights, serum albumin and bilirubin, and hydroxyproline in liver tissues moderately recovered to normal levels after 2-wk cessation of DMN without any treatment, whereas the levels of ALP, AST, and ALT remained dangerously out of normal physiologic conditions.
CGX administration showed major reducing effects on the increased levels of ALP, AST, and ALT induced by DMN treatment, and slight restorative effects on organ weight, serum albumin and bilirubin, and hydroxyproline concentration in liver tissues. In contrast to other biomarkers, the production of lipid peroxidation, measured as malondialdehyde (MDA), accelerated continuously up to the endpoint of this experiment in spite of stopping DMN treatment in the control group. This was partially restored by the administration of CGX (Figure 4). Because oxidative stress in DMN-treated animal models is known to be a major cause of hepatocellular damage and fibrosis[26], it is likely that CGX has some hepatotherapeutic effects as a result of reducing DMN-induced oxidative stress.
As expected, DMN treatment for 4 wk resulted in the development of fibrosis, thus causing shrunken livers and the accumulation of hydroxyproline and extracellular matrix. Any chronic types of hepatocellular injury generally lead to hepatic fibrosis and cirrhosis, and investigation of anti-fibrotics and anti-cirrhotics have been a central issue due to their importance in the clinical outcome of patients. Thus far, no effective anti-fibrotic or anti-cirrhotic treatments are available for clinical use[2,27]. This experiment demonstrated some anti-fibrotic effects of CGX administration, as shown by reduced hydroxyproline concentration in tissues and histological examination (Figure 5).
In order to determine the molecular mechanisms of these effects, we examined the change in expression of several fibrosis-associated genes in liver tissues. It has already been established that stellate cells and various cytokines work as key factors in the pathogenic process of liver fibrosis[2]. Among these, TGF-β and PDGF are considered the prominent profibrogenic cytokines[28-31]. As shown in Figure 6, CGX administration moderately inhibited TGF-β expression compared to four- and five-fold over-expression in the DMN 4 wk and control groups, respectively. In particular, PDGF-β was obviously over-expressed by DMN treatment, whereas it was markedly inhibited by CGX administration. In addition, the unbalance between synthesis and degradation of the extracellular matrix is an important feature of liver fibrosis, and increased expression of several matrix metalloproteinases, including MMP-2 and tissue inhibitors of metalloproteinases (TIMPs), is well-known to occur during fibrogenesis[32,33]. CGX administration also noticeably decreased the expression of MMP-2 that had been highly over-expressed during or after DMN treatment, similar to previous reports[34].
Among the many drugs derived from herbal medicines, silymarin is the most clinically popular for patients with various liver disorders, and is known to have hepatotherapeutic and anti-fibrotic properties[35-37]. We also used silymarin (50 mg/kg) as a positive control in this experiment, and observed that CGX exhibited greater anti-fibrosis effects than silymarin (data not shown).
Based on these results, we conclude that CGX possesses hepatotherapeutic properties against chronic hepatocellular destruction and consequential liver fibrosis. The mechanism of these therapeutic effects of CGX on liver diseases, however, should be further clarified so that CGX can be used as effectively as possible as a means of treatment.
S- Editor Liu Y L- Editor Kumar M E- Editor Bai SH
| 1. | Sharif F, Mohebbi S, Tabatabaee HR, Saberi-Firoozi M, Gholamzadeh S. Effects of psycho-educational intervention on health-related quality of life (QOL) of patients with chronic liver disease referring to Shiraz University of Medical Sciences. Health Qual Life Outcomes. 2005;3:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S-131S. [PubMed] [Cited in This Article: ] |
| 4. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1695] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 5. | Kitamura Y, Ninomiya H. Smad expression of hepatic stellate cells in liver cirrhosis in vivo and hepatic stellate cell line in vitro. Pathol Int. 2003;53:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Gao M, Zhang J, Liu G. Effect of diphenyl dimethyl bicarboxylate on concanavalin A-induced liver injury in mice. Liver Int. 2005;25:904-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Levy C, Seeff LD, Lindor KD. Use of herbal supplements for chronic liver disease. Clin Gastroenterol Hepatol. 2004;2:947-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12:559-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Cho JH, Lee YY, Seo SH, Yoo HS, Choi WJ, Lee YW, Son CG, Cho CK. A clinical report about 57 patients with chronic liver disease. Korean J Orient Med. 2001;21:112-121. [Cited in This Article: ] |
| 10. | Son CG, Choi WJ, Shin JW, Han SH, Cho JH, Song KC, Cho CK. Effects of gamichunggantang on hyperlipidemia. Acta Pharmacol Sin. 2003;24:133-139. [PubMed] [Cited in This Article: ] |
| 11. | Son CG, Choi BL, Shin JW, Choi WJ, Cho JH, Cho CK. Effect of Gamichunggantang on alcoholic metabolism and alcoholic liver disease. Korean Journal of Oriental Medicine. 2001;2:89-98 Available from: http: //kref.naver.com/doc.naverdocid=4299015. [Cited in This Article: ] |
| 12. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3306] [Cited by in F6Publishing: 3286] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 13. | Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29:669-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Chen HX, Zhou GW, Yin L, Peng CH, Li HW. Liver regeneration after split liver transplantation. Hepatobiliary Pancreat Dis Int. 2005;4:519-523. [PubMed] [Cited in This Article: ] |
| 15. | Younossi ZM, Guyatt G. Quality-of-life assessments and chronic liver disease. Am J Gastroenterol. 1998;93:1037-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Chuang JH, Wang PW, Tai MH. An adipocentric view of liver fibrosis and cirrhosis. Chang Gung Med J. 2004;27:855-868. [PubMed] [Cited in This Article: ] |
| 17. | Riley TR 3rd, Bhatti AM. Preventive strategies in chronic liver disease: part II. Cirrhosis. Am Fam Physician. 2001;64:1735-1740. [PubMed] [Cited in This Article: ] |
| 18. | Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 266] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol. 2002;97:2391-2397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67-E70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yamashiki M, Nishimura A, Huang XX, Nobori T, Sakaguchi S, Suzuki H. Effects of the Japanese herbal medicine "Sho-saiko-to" (TJ-9) on interleukin-12 production in patients with HCV-positive liver cirrhosis. Dev Immunol. 1999;7:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | George J, Chandrakasan G. Biochemical abnormalities during the progression of hepatic fibrosis induced by dimethylnitrosamine. Clin Biochem. 2000;33:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Shih CC, Wu YW, Hsieh CC, Lin WC. Effect of Anoectochilus formosanus on fibrosis and regeneration of the liver in rats. Clin Exp Pharmacol Physiol. 2004;31:620-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, Inoue H, Honda H, Ito S. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004;74:897-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S, Yildirim B, Aytekin H, Ozbay G, Mert A, Senturk H. Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res. 2004;37:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | George J, Roulot D, Koteliansky VE, Bissell DM. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci USA. 1999;96:12719-12724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Geremias AT, Carvalho MA, Borojevic R, Monteiro AN. TGF beta1 and PDGF AA override collagen type I inhibition of proliferation in human liver connective tissue cells. BMC Gastroenterol. 2004;4:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Lou SM, Li YM, Wang KM, Cai WM, Weng HL. Expression of platelet-derived growth factor-BB in liver tissues of patients with chronic hepatitis B. World J Gastroenterol. 2004;10:385-388. [PubMed] [Cited in This Article: ] |
| 32. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] [Cited in This Article: ] |
| 33. | Préaux AM, Mallat A, Nhieu JT, D'Ortho MP, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Zhu YK, Wang BE, Shen FJ, Jia JD, Ma H. [Dynamic evolution of MMP-2 gene expression and its enzymatic activities in experimental liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2005;13:509-512. [PubMed] [Cited in This Article: ] |
| 35. | Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Jia JD, Bauer M, Cho JJ, Ruehl M, Milani S, Boigk G, Riecken EO, Schuppan D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |