Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5447
Revised: April 28, 2006
Accepted: June 14, 2006
Published online: September 14, 2006
Several hundred species of bacteria inhabit the gut, and affect its cell biology, morphology and homeostasis. Many bacteria are however potential pathogens, especially if the integrity of the epithelial barrier is physically or functionally breached. Conversely, the interaction between host and commensal microbes can confer important health benefits. This has led to commercial and public interest in 'probiotics', live microbes principally taken as food supplements. Might probiotics also be used in disease therapy Experimental evidence that probiotics modulate gut physiology, particularly barrier integrity and immunological function, underpins exciting new gastroenterological research. We discuss below the scientific basis for probiotic effects and present a critical perspective for their use in relation to gastrointestinal disease.
- Citation: Limdi JK, O’Neill C, McLaughlin J. Do probiotics have a therapeutic role in gastroenterology? World J Gastroenterol 2006; 12(34): 5447-5457
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5447
The concept of probiotics probably dates back to 1908, when Nobel Prize winner Eli Metchnikoff suggested that the long life of Bulgarian peasants resulted from their consumption of fermented milk products[1]. In 1965 Lilly and Stillwell first used the term ‘probiotic’ when describing ‘substances secreted by one organism which stimulate the growth of another’[2]. Parker[3] described probiotics as ‘organisms and substances which contribute to intestinal microbial balance’ and Fuller proposed in 1989 that probiotics were ‘a live microbial supplement which beneficially affects the host animal by improving its microbial balance[4]. Salminen et al[5] defined them as ‘foods containing live bacteria which are beneficial to health’, whilst Marteau et al[6] define them as ‘microbial preparations or components of microbial cells that have a beneficial effect to health and well being’. Such definitions underpin the current popular commercial usage of various ‘friendly bacteria’ to secure non-specific benefits to health.
With improved understanding of the physiology and therapeutic role of probiotics, definitions have evolved bolder claims, which now enter medical territory. Charteris et al[7] defined probiotics as ‘micro-organisms, which when ingested, may have a positive effect in the prevention and treatment of a specific pathological condition’.
Two related terms are prebiotics and synbiotics: prebiotics are defined as “non digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon that can improve host health”[8]. When prebiotics and probiotics are administered together, this is referred to as a synbiotic.
The colon contains tenfold more bacteria than the total number of mammalian cells constituting the host. The relationship is symbiotic: for example, bacterial vitamin K synthesis contributes to haemostasis, whilst short chain fatty acids generated by colonic bacteria salvage additional energy from otherwise ‘wasted’ dietary fibre.
Colonisation of the gastrointestinal tract starts immediately after birth, initially with maternal vaginal and intestinal flora. Other sources are diet (breast or formula based feeds)[9-12] and environment, as reflected by the different gut flora in infants born in developing and developed countries[13-15]. Infants who are breast fed predominantly harbour Bifidobacteria whilst formula fed infants have a more complex bacterial profile comprising Enterobacteria, Bacteroides, Clostridia, Lactobacilli, Bifidobacteria and Streptococci[11].
These ‘pioneer’ bacteria are important because they modulate epithelial cell gene expression, creating a favourable habitat for themselves by inhibiting the growth of bacteria introduced later[16]. This renders initial colonisation causally determinant to the final composition of bacterial flora in adults[17].
During development, the gut flora changes. The mouth harbours mainly facultative and strict anaerobes including Streptococci, Bacteroides and yeasts. The oesophagus has no significant resident microbial colonisation but is constantly transited by swallowed organisms. The widely held idea that the upper gut is largely sterile is not valid. The stomach and duodenum harbour up to 104 colony forming units (CFU) per gm of Candida albicans, Bacteroides, Lactobacillus and Streptococcus. H pylori are specifically adapted for gastric residence. The jejunum again harbours Bacteroides, Candida albicans, Lactobacillus and Streptococcus but with a content of 105-107 CFU/g. Scepticism that probiotics will not survive the passage through the acidic stomach is therefore unfounded. From the ileum onwards bacterial colonisation increases from 107-108 CFU/g in the ileum to 1010-1011 CFU/g in the colon, with a predominance of Bacteroides, Bacillus, Clostridium, Enterococcus, Peptostreptococcus, and Streptococcus species[18].
Recent research has demonstrated that a dynamic and reciprocal interplay exists between the gut microflora and the host. In particular, there is growing evidence that bacteria play an important role in directing epithelial differentiation and reinforcement of the gut barrier, through complex host-bacterial cross talk which occurs at a molecular level in interacting cells. This new biology underpins the putative effects of probiotics, since host-bacterial interactions can be re-engineered by purposeful manipulation of luminal ecology.
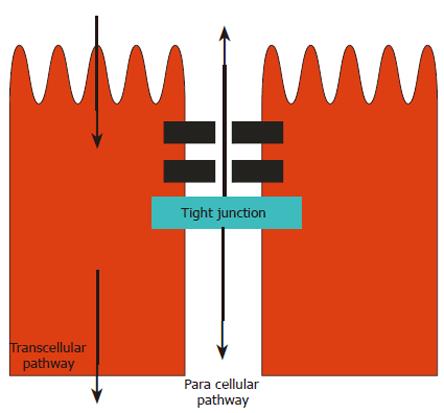
The intestinal epithelium constitutes an anatomical and functional barrier, effectively a bipolar monocellular obstacle between luminal microbes and the cells of the lamina propria. Barrier function is normally maintained by a complex interplay of numerous proteins, assembled to form the tight junction complexes (TJs)[19,20] in the juxta-apical region of the cell membrane (Figure 1). Commensal organisms contribute uncharacterised constitutive signals supporting epithelial integrity. Disruption of TJs may be elicited by pathogenic bacteria, stress and injury, via pro-inflammatory cytokines. Disruption of TJ integrity results in increased paracellular permeability, which is measurable in a variety of experimental models, and which may initiate, exacerbate or perpetuate intestinal inflammation in disease[21]. Altered intestinal permeability has been demonstrated in Crohn’s disease, celiac disease, intestinal infections and NSAID-induced enteropathy[22]. In order to counteract the harmful effects of luminal pathogens and toxins, and to protect barrier homeostasis, intestinal epithelial cells exhibit several additional defensive features, which include production of defence peptides and mucins[23]. In addition, a class of bacterial-sensing immunocytes, the dendritic cells, are able to project sensory dendrites into the lumen between adjacent enterocytes via TJ regions. Dendritic cells express receptors evolved to sense bacterial components, through which highly patterned host immune responses are evoked appropriately and constantly. Supporting experiments in rats have shown that colonisation of an excluded colonic loop with E. coli increased paracellular permeability, but this was partially reversed by colonisation with a putative probiotic, Lactobacillus brevis[24]. In addition, the chronic colitis occurring in Interleukin-10 (IL-10) deficient mice[25] is associated with increased colonic permeability, which is reversed in mice pre-treated for 4 wk. with a probiotic product, VSL#3 consisting of Bifidobacterium, Streptococcus and Lactobacillus species.
The potential cellular basis has also been addressed in human cell models[26]. The influence of whole probiotic bacteria E. coli Nissle 1917 (EcN) or VSL#3, bacterial cell lysates or conditioned medium from bacterial cultures were assessed against a variety of barrier and defensive parameters in human intestinal epithelial cell lines. These included a measure of TJ function (transepithelial resistance, TER) and tight junction protein abundance, IL-8 secretion and mucin gene expression. In addition, protective effects on pathogen (Salmonella dublin) induced alterations were analyzed. The probiotic mixture and soluble protein released from it, increased basal TER, prevented pathogen-induced decrease in TER, and stabilized TJs. This suggested that the organisms, or their secretory products, functionally modulate the intestinal epithelium of the host. These include competition of organisms for contact with the epithelial surface, stabilization of the cytoskeletal and barrier function, and the induction of mucin gene expression. Gram-negative and gram-positive organisms differed in the cellular mechanisms activated: perhaps a combination of organisms might be more effective than the application of a single strain[26]. The stumbling block inevitably lies in translating observations in these reductionist models to a proven clinical effect.
Recent interest has centred on regulation of the barrier via epithelial sensing of microbial components. The Toll-like receptors (TLRs) are a class of pattern-recognition receptors that specifically discriminate between self and microbial non-self based on the recognition of molecular patterns[27]. TLRs thereby play an important role in immune responses and the induction of antimicrobial effector pathways, leading to elimination and exclusion of host-threatening pathogens. It has been shown that the intestinal epithelium expresses several TLRs, which include TLR2, TLR3, TLR4 and TLR5[24-34]. It is possible that certain probiotic agents contain TLR-specific immunostimulatory features, leading to the amelioration of colitis by restoring intestinal epithelial barrier to protect the host[35].
The gastrointestinal mucosa is the primary interface between the external environment and the immune system. In the complete absence of intestinal microflora antigen transport is increased, indicating that the normal gut microflora maintain gut defences[25]. The microflora affect the development of gut associated lymphoid tissue at an early age by directing the regulation of systemic and local immune responsiveness, including promoting tolerance via hypo-responsiveness to antigens from micro-organisms and food[36]. Probiotic organisms have been shown to modulate immunoglobulin production. Secretory IgA plays an important role in mucosal immunity, contributing functionally to the barrier against pathogenic bacteria and viruses[37-40]. An enhanced IgA immune response has been shown in children with Crohn’s disease treated with Lactobacillus GG[41]. Interestingly, Madsen et al[42] demonstrated that IL10 deficient mice displayed significantly higher basal numbers of adherent bacteria compared with healthy control mice. When the colon was repopulated with Lactobacillus reuteri enemas the proportion of adherent and translocated bacteria, and the development of colitis, was significantly decreased. Further, Schultz[43] demonstrated that feeding Lactobacillus plantarum attenuated established colitis in IL-10 knockout mice. In rats, the effects of Lactobacilli and fibre (oatbase) were studied in Methotrexate-induced enterocolitis. Rats received an intragastric infusion of an elemental diet, with or without supplementation of oatbase, Lactobacillus reuteri R2LC and Lactobacillus plantarum DSM9843[44]. Methotrexate was injected intraperitoneally on d 3. By d 6 Lactobacillus decreased intestinal inflammation, re-established intestinal microecology and reduced bacterial translocation to extra-intestinal sites.
Data from human studies support a role for the gut microflora in the development of several gut associated inflammatory conditions, most likely triggered by immune response to their antigenic structures[45]. Thus probiotics may exert clinical effects by altering the intestinal inflammatory response to the luminal microflora.
Probiotic organisms exert a potentially positive effect on gut function through a trophic action on gut mucosa. In experimental models, crypt cell turnover is reduced in the colon of rats bred in germ free environments (gnotobiotic animals). Germ free crypts also contain fewer cells than those of colonised rats[46]. Butyrate is an important source of energy for colonocytes[47], whilst acetate and propionate are found in portal blood and are eventually metabolised in the liver or peripheral tissues, in particular muscle[47,48]. The most important role of SCFA is probably their trophic effect on colonic epithelium. Short chain fatty acids stimulate epithelial cell proliferation and differentiation in large and small bowel in vivo[49]. Butyrate however inhibits cell proliferation in epithelial cell lines of neoplastic origin[50]. Further, butyrate is pro-apoptotic and promotes reversion of cells from neoplastic to non-neoplastic phenotypes[51]. SCFA generation can clearly be altered by manipulating colonic micro-ecology, so probiotics and prebiotics may find a role in the prevention of colonic neoplasia and in the therapy of inflammatory bowel diseases.
Colonic micro-organisms play an important role in vitamin synthesis[52,53] and in the absorption of calcium, magnesium and iron[54-56]. Ion absorption in the caecum is improved by carbohydrate, and production of short chain fatty acids particularly acetate, propionate, and butyrate.
A mucus gel covers the gut epithelium acting as a protective barrier against pathogens and reducing physical trauma. Change in the mucus content or structure compromise barrier function. Interactions occur between bacteria and mucus, including the probiotic bacteria that bind to intestinal mucus[57]. This is potentially advantageous since it inhibits adhesion of enteropathogenic bacteria to mucus. For example Enterococcus faecium inhibits the adhesion of enterotoxigenic E. coli K88 to porcine small intestine mucus[58].
The experimental data discussed above demonstrate that several ‘probiotic’ organisms exert biological effects which might translate into clinical benefits. Current clinical evidence is limited and non-uniform. Key observations in relevant conditions are discussed below.
Probiotics have an emerging role in the treatment of gastrointestinal infections. Probably the best described is in acute infantile diarrhoea. Lactobacillus strain GG in fermented milk or freeze- dried powder was shown to reduce the duration of diarrhoea in acute rotavirus infection compared to a placebo group given pasteurised yoghurt[59]. Other studies have confirmed these results[60,61]. Suggested mechanisms of action are stabilisation of indigenous microflora[62], reduction in the increased gut permeability caused by rotavirus infection[63] and reduction in the duration of virus shedding[64]. This may be cause-specific. In a multicentre European trial of probiotics in acute childhood diarrhoea caused by rotavirus and other pathogens[65], probiotics (Lactobacillus GG) shortened the duration of diarrhoea in rotavirus diarrhoea, but showed no such effect with other pathogens.
Probiotics may also have a role in the prevention of acute infantile diarrhoea. In a double blind, placebo controlled trial hospitalised infants were randomised to receive a standard infant formula alone or supplemented with Bifidobacterium bifidum and Streptococcus thermophilus. After a 17-mo follow up period, 7% of those receiving a probiotic had experienced diarrhoea compared to 31% given the standard formula[66]. Viral shedding was lower in the probiotic supplemented group. Prophylactic use of Lactobacillus GG in 204 undernourished children followed up for 15 mo also decreased the incidence of acute diarrhoea[67]. This effect was confined to non-breast fed infants. In a more recently reported double blind randomised placebo controlled trial, 81 children between 1-3 years of age, hospitalised for reasons other than diarrhoea, and were given Lactobacillus GG or placebo for the duration of their admission. The incidence of nosocomial diarrhoea was lower in the probiotic group (7% as compared to placebo, 33%)[68]. Furthermore, although the prevalence of rotavirus infection was similar in both groups, the risk of rotavirus gastroenteritis was lower in the probiotic group. Finally, the benefits of Lactobacillus GG in rotavirus associated diarrhoea mainly comprise a reduction in duration of diarrhoea by 1-2 d compared to a median of 3 d.
The positive results for Lactobacillus GG in acute viral diarrhoea cannot necessarily be extrapolated to other probiotic strains, nor to other causes of acute diarrhoea. The data imply species and disease specificity, For example, in a study comparing various probiotic strains, Lactobacillus GG was found to have a beneficial effect in rotavirus gastroenteritis but this was not shared by L. rhamnosus, L. delbrueckii or Streptococcus thermophilus[60]. Saccharomyces boulardii has also displayed beneficial effects in acute diarrhoea in children and adults[60,70]. Enterococcus SF68 in adults with acute diarrhoea has however shown inconsistent results[71-73]. It is unclear whether the modest benefits suggested by these studies justify routine use of probiotics in diarrheal illnesses since most acute diarrhoeal diseases are self limited. The benefit may be greatest in situations where patients are at risk for complications such as in children with malnutrition in developing countries.
Diarrhoea can occur as an adverse effect of antibiotic therapy, in up to 39% of antibiotic treated hospitalised patients[74]. Broad spectrum antibiotics are more commonly implicated, possibly because of more profound alteration in colonic flora[75]. Several placebo-controlled studies have shown a decrease in the incidence of diarrhoea or change in stool consistency when patients were treated with probiotics in addition to antibiotics[76-82]. The probiotic organisms studied were Lactobacillus spp., Enterococcus and Saccharomyces boulardii. Not all studies support this possibility. A recent study of 267 patients on antibiotics randomised to Lactobacillus GG or placebo failed to show any decrease in incidence of diarrhoea, with 29% in both groups developing symptoms[83]. Three other smaller studies were also negative[78,84,85]. Although most studies looking at probiotics in antibiotic-associated diarrhoea are placebo controlled and conducted on a reasonable number of subjects, different antibiotics were used in these studies contributing to their heterogeneity. Two recent meta-analyses (of nine and seven randomised placebo controlled double blind trials) have looked at the effect of probiotics in prevention of antibiotic-associated diarrhoea[86,87]. The meta- analysis by D’Souza et al[87]reviewed nine randomised, double blind, placebo controlled trials. Four trials used yeast (Saccharomyces boulardii); four used Lactobacilli and another used a strain of enterococcus that produced lactic acid. Three trials used a combination of probiotic bacteria. Antibiotics were given with probiotics (or with placebo, in the control group) in all nine trials. The combined odds ratio in favour of active treatment over placebo in preventing diarrhoea associated with antibiotics was 0.37 (95% confidence interval, 0.26 to 0.53; P < 0.001). In the meta- analysis by Cremonini et al[86], twenty two studies using Lactobacillus and Saccharomyces species, matched the inclusion criteria of which seven studies were homogenous. The combined relative risk was 0.399 (95% confidence interval, 0.27-0.57) suggesting a strong benefit of probiotic administration on antibiotic associated diarrhoea. The pooled results suggested that probiotic administration had an overall benefit. However, the published data is discordant in that it is unclear what the optimal dose and timing of supplementation should be.
Clostridium difficile is a Gram positive bacterium that can cause colitis, mediated by two enterotoxins, enterotoxin A and B. The pathophysiology is not fully clear but risk factors include intercurrent or continued antibiotic therapy, elderly age, renal disease and female sex[88,89]. Probiotics have been partially evaluated in the prevention of CDAD. Early uncontrolled trials using Lactobacillus GG[90-92] and a preliminary report of a controlled trial using Lactobacillus GG suggested benefit in recurrent CDAD[93]. Similarly uncontrolled or open label studies[94,95] and subsequently two controlled trials[96,97] have suggested efficacy of Saccharomyces boulardii in recurrent CDAD. In the study by McFarland et al[94], 124 patients were studied, including 64 patients with an initial episode of CDAD and 60 who had at least one previous episode of CDAD. Subjects received oral Saccharomyces boulardii (1 g/d for 4 wk or placebo) and were followed up for an additional 4 wk after therapy. Multivariate analysis showed that patients treated with S. boulardii and standard antibiotics had a significantly lower risk of CDAD (relative risk 0.43; 95% confidence interval, 0.20-0.97) compared with placebo. In their subsequent study, Surawicz et al[97] tested patients receiving a standard antibiotic for 10 d and then added S. boulardii (1 g/d for 28 d) or placebo. A significant decrease in recurrence of CDAD (16.7%) was observed in patients treated with high-dose Vancomycin (2 g/d) compared with those receiving Vancomycin and placebo (50%; P < 0.05). Most studies were small and were mostly not placebo controlled.
A recent systematic review looking at studies in which probiotic therapy was used for prevention and treatment of C. difficile- associated diarrhoea concluded that the evidence for routine clinical use of probiotics in this setting was insufficient[98]. Again, although probiotics are generally considered safe, case reports have described Saccharomyces cerevisiae fungaemia and deaths in immunocompromised and critically ill patients who received a commercial preparation of S. boulardii (genomically identical to S. cerevisiae)[99]. Their routine use can therefore not be recommended.
Although the etiology of inflammatory bowel diseases is not entirely clear, dysfunctions in both innate and acquired immunity are implicated. There has been an increased interest in pathogenic and endogenous intestinal flora, with supportive data derived from several animal models. As noted above spontaneous colitis may develop in mice deficient in the immunoregulatory cytokine IL-10 but IL-10 deficient germ free mice remain disease free[100-102].
Clinical studies suggest a significant role for bacteria in the pathogenesis of human IBD. Crohn’s disease activity has been shown to improve with antimicrobial therapy, faecal diversion[103,104], bowel rest and intestinal lavage[105]. Furthermore, antibiotics may reduce postoperative relapse[106], postoperative pouchitis[107] and fistula related complications[108]. There has been particular interest recently in polymorphisms in the CARD15/NOD2 gene, an intracellular bacterial pattern recognition receptor, as a risk factor for the development of Crohn’s disease[109]. Probiotics may therefore exert benefits in IBD management by modulatory effects on intestinal flora. For example Lactobacillus GG, when administered to children with Crohn’s disease, increased mucosal IgA levels[41] improved intestinal permeability and reduced disease activity[110]. Further, the relapse rate in 32 adults with inactive Crohn’s disease was reduced to 6% when subjects in remission were treated with mesalazine and S. boulardii as compared to 38% with mesalazine alone[111]. However, in a placebo controlled study of 37 Crohn’s disease patients treated with Lactobacillus GG for 12 mo after curative resection, the probiotic did not prevent relapse: in fact more severe endoscopic findings were reported in the Lactobacillus group[112].
Recent clinical studies have evaluated the effect of non-pathogenic E. coli strain Nissle 1917 versus mesalazine for maintenance of remission in ulcerative colitis. Kruis et al[113] studied 120 and Rembacken et al[114] 116 patients with inactive ulcerative colitis over a period of 12 wk and 1 year respectively given either E. coli strain Nissle 1917 (MutaflorR) or mesalazine. These unblinded studies found a similar relapse) in both groups (73% in the mesalazine group and 67% in the E. coli group), suggesting that probiotic therapy may be an alternative maintenance therapy. Similar results were suggested in a third RCT study[115]. 327 patients were treated with mesalazine or E. coli Nissle 1917 for twelve months. Relapse rates were similar (45.1% in the probiotic group versus 36.4% in the mesalazine group). In a controlled trial by Tursi et al[116], low dose balsalazide with VSL#3 was shown to be more effective than balsalazide or mesalazine alone in patients with acute mild to moderate ulcerative colitis. The combination of a prebiotic and a probiotic (Bifidobacterium longum/Synergy 1) was associated with improvement in histological scores and measures of immune activation in a randomised controlled pilot study[117].
A recent study investigated the expression and function of CARD15/NOD2 in intestinal epithelial cell lines CARD15/NOD2 mRNA was expressed in both intestinal epithelial cell lines and primary intestinal epithelial cells. CARD15/NOD2 mRNA and protein were up-regulated by tumor necrosis factor alpha (TNF alpha) in SW480 cells. This study suggests that CARD15/NOD2 may serve as a key component of innate mucosal responses to luminal bacteria as an antibacterial factor[109]. Failure in this activity may contribute to the development of Crohn’s disease.
Pouchitis is an inflammation of an ileal reservoir surgically constructed in the management of IBD. It is associated with reduced counts of Bifidobacteria and Lactobacilli with increased numbers of Clostridia and anaerobes in faecal samples[118]. Increases in bile acids and decreases in short chain fatty acids, with a net increase in pH, may also be seen[118]. In a double blind randomized placebo controlled trial a probiotic mixture VSL#3 was studied over 9 mo in 40 patients with chronic relapsing pouchitis[119]. Relapse was defined by a pouch disease activity index (PDAI)[120] of 2 points or more and confirmed by endoscopy and histology, and was, strikingly, only identified in 15% of the VSL#3 group as against 100% in the placebo group. In a prophylactic study, 2 of 20 patients (10%) receiving 1 packet of VSL#3 1 year developed pouchitis, versus 40% of placebo treated patients[121].
Somewhat different conclusions were reached in two recently published studies[122,123]. In a group of 36 patients with recurrent or refractory pouchitis who had required antibiotics at least twice in the past year, patients were randomly assigned to VSL#3 or placebo after achieving remission, once daily for a year. More patients remained in remission in the probiotic group (85% vs 6%)[122].
In an observational study involving 31 patients treated with VSL#3 after achieving remission with Ciprofloxacin, only a minority of patients remained on probiotic therapy and in sustained remission, having stopped them due to recurrence of disease or adverse effects[123]. In summary then, the benefit of probiotics in Crohn’s disease remains unproven. The benefit of probiotics in ulcerative colitis remains unproven. E. coli Nissle 1917 appears promising and may be of value in patients intolerant of or resistant to 5-ASA preparations. Limited data from small controlled studies would suggest that VSL#3 is a reasonable therapy in the primary and secondary prevention of pouchitis.
Some advocates propose that probiotics have an important emerging role in managing critical illnesses originating in the gastrointestinal tract. In a recent study from Hungary patients with severe acute pancreatitis were randomised upon arrival to hospital to receive one week of treatment with a twice daily administration of a freeze dried preparation containing 109 live L. plantarum 299 with a substrate of 10 g oat fibre, or a similar preparation containing Lactobacillus which had been inactivated[124]. The study was stopped when the infection rate showed a significant difference in the two groups. This occurred when 45 patients had completed the study, 22 had received treatment with live and 23 with heat killed L. plantarum 299. Infected pancreatic necrosis occurred in 1 out of 22 subjects (4.5%) in the treatment group as against 7 of 23 (30%) in the heat killed group. The length of hospital stay was shorter in the live LAB group but it did not reach statistical significance due to small sample size[124].
Studies on cirrhotic patients have shown a decrease in the incidence of encephalopathy. A Chinese study recently reported 55 patients with minimal hepatic encephalopathy who were randomised to receive a synbiotic preparation, fermentable fibre alone or placebo. Synbiotic treatment was associated with a reduction in serum ammonia, endotoxaemia, reversal of encephalopathy and improvement in Child-Turcotte-Pugh score in 50% patients[125]. Following liver transplantation, bacterial and fungal infections may occur in the first month despite extensive antibiotic treatment and selective digestive tract decontamination. A German study showed a reduction in post-transplant infective complications using probiotics[126]. Another recent study looked at the effects of a synbiotic preparation on gut barrier function and in critically ill patients admitted to the Intensive Care Unit (ICU). Ninety patients admitted to ICU were randomised (45 in each group) to receive either synbiotic preparations (Lactobacillus acidophilus La5, Bifidobacterium lactis Bb12, Streptococcus thermophilus and Lactobacillus bulgaricus with oligofructose (as a prebiotic) or placebo. Patients in the symbiotic group had a lower incidence of potentially pathogenic bacteria (43% vs 75%, P = 0.01) and multiple organisms (39% vs 75%, P = 0.01) but there were no significant differences in both groups in terms of intestinal permeability, septic complications or mortality[127]. It should be pointed out that the study lasted only one week.
Colon cancer is one of the leading causes of death in the Western world. Dietary intake of red meat is probably associated with a higher risk whereas the intake of fruit, vegetables, fish and calcium are arguably associated with a lower risk. It is interesting that colon cancer risk is also lower in countries such as Netherlands and Finland where a larger quantity of yoghurt is consumed[128,129]. Could diet exert its effects via the microflora If so, mechanisms involved might include altered metabolic activity of the intestinal microflora, binding and degradation of potential carcinogens, production of antitumorigenic or mutagenic compounds and enhancement in host immune response. In animals where colon cancer was induced by chemical carcinogens, administration of lactic acid bacteria resulted in a suppression of DNA damage, tumour formation and growth[130-133].
One bio-epidemiological study showed a higher risk of colon cancer in the simple presence of Bacteroides species, but lower risk with Lactobacillus acidophilus, Lactobacillus S06 and Eubacterium aerofaciens identifiable in faecal flora[134]. This raises the intriguing hypothesis that similarly shifting the resident bacterial populations may be accompanied by parallel reductions in neoplastic risk. However, biodiversity in the colon may merely represent an epiphenomenological consequence of the dietary and environmental risk factors listed above.
Probiotics may have a promising role in certain aspects of human digestion as illustrated by some interesting studies. Lactose digestion has been shown to improve in lactose malabsorbers who consume live yoghurt rather than milk[135,136]. The beneficial effects of yoghurt in lactose malabsorbers may result from improved digestion of lactose in the colon from stimulation of colonic bacterial activity by lactic acid bacteria[137].
Irritable bowel syndrome (IBS) is a collection of functional gastrointestinal symptoms such as abdominal pain, defaecatory frequency and or constipation. This area is inevitably contentious, since it remains unclear how much intrinsic intestinal pathology exists in IBS. However, changes in gut sensitivity and defaecatory function are clearly present. Alterations in the composition of intestinal flora have been reported but not proven including a decrease in faecal Lactobacilli, E. coli and Bifidobacteria[41,110,111] and an increase in other faecal anaerobes[138-140]. Symptomatic improvement was noted in a very small crossover trial of 18 patients with IBS given L.acidophilus[141] and in an uncontrolled study using E. faecium PR88[142]. On the other hand, no improvement was seen in bloating, pain or urgency to defaecate after the consumption of Lactobacillus GG for 8 wk[143,144].In a study reported by Saggioro et al[145], 50 patients with IBS according to RomeIIcriteria were randomly assigned to a probiotic preparation (a combination of Lactobacillus plantarum LPO 1 and Bifidobacterium breve Bro or placebo for 4 wk). Pain and severity scores decreased significantly after 14 d of treatment. In a more recent study, 77 patients were randomly assigned to a malted drink containing Lactobacillus salivarus UCC4331 or Bifidobacterium infantis 35 624 or a malted drink alone[146]. Significant improvement in symptoms was noted in the B. infantis group. A corresponding normalisation in the ratio of IL-10/IL 12 was also noted suggesting that the probiotic may help reduce a proinflammatory state associated with IBS.
However the heterogeneity of the various studies makes it difficult to draw conclusions on the effect of probiotics in IBS, and the field is bedevilled by the fact that all therapeutic interventions in IBS produce a 30%-50% placebo response.
H pylori infection is associated with gastritis, gastro duodenal ulcers and gastric malignancies. The majority of H Pylori hosts become hypochlorhydric with time. Clinical studies and experimental models have shown that the secreted products of Lactobacillus acidophilus can suppress H Pylori growth in vitro and in vivo L. johnsonii[147] and LG21[148] are effective in suppressing the growth of H pylori and reducing gastric inflammation. Placebo controlled studies have demonstrated a reduction in side effects of standard triple therapy if probiotics were administered concurrently[149-151]. Daily intake of inactivated L. acidophilus was shown to improve the efficacy of eradication treatment[152]. Only one study[153] showed that supplementation with fermented milk, containing live special probiotic L. casei DN-114001, confers an enhanced therapeutic benefit on H pylori eradication in children with gastritis on triple therapy. The theory that probiotic therapy enhances the disappearance of H pylori does not gain any strength from the available literature. Further clinical studies would be needed to evaluate the effects of long term ingestion of probiotics in preventing Helicobacter-associated diseases, but are unlikely to supplant H pylori eradication which is rapidly and highly effective.
In conclusion probiotics are live microbial food supplements or components of bacteria which may have beneficial effects on gastrointestinal health. Innate bacterial floras clearly play an important role in reinforcement of the physical gut barrier, affecting paracellular permeability, mucosal trophic action and microbiological interactions with mucosal lining of the gut. The key and unanswered question is whether the deliberate manipulation of the bacterial complement in the gut can confer clinical benefit. Probiotics do now appear to have a potential role in the prevention and treatment of various gastrointestinal illnesses, but it is likely that benefits achieved are specific to the bacterial species used and to the underlying disease context. Much further work is required from bench to bedside before we can realise the potential of these new interventions.
S- Editor Pan BR L- Editor Misra SP E- Editor Bai SH
| 1. | Metchnikoff E. The prolongation of life. Optimistic studies New York: Putman's Sons 1908; 161-183. [Cited in This Article: ] |
| 2. | Lilly DM, Stilwell RH. Growth promoting factors produced by probiotics. Science. 1965;147:747-748. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Parker RB. Probiotics, the other half of the antibiotic story. Animal nutr Health. 1974;29:4-8. [Cited in This Article: ] |
| 4. | Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365-378. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80 Suppl 1:S147-S171. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Marteau P, Cuillerier E, Meance S, Gerhardt MF, Myara A, Bouvier M, Bouley C, Tondu F, Bommelaer G, Grimaud JC. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16:587-593. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Charteris WP, Kelly PM, Morelli L, Collins JK. Selective detection, enumeration and identification of potentially probiotic Lactobacillus and Bifidobacterium species in mixed bacterial populations. Int J Food Microbiol. 1997;35:1-27. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] [Cited in This Article: ] |
| 9. | Long SS, Swenson RM. Development of anaerobic fecal flora in healthy newborn infants. J Pediatr. 1977;91:298-301. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317-321. [PubMed] [Cited in This Article: ] |
| 11. | Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61-67. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19-25. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Simhon A, Douglas JR, Drasar BS, Soothill JF. Effect of feeding on infants' faecal flora. Arch Dis Child. 1982;57:54-58. [PubMed] [Cited in This Article: ] |
| 14. | Lundequist B, Nord CE, Winberg J. The composition of the faecal microflora in breastfed and bottle fed infants from birth to eight weeks. Acta Paediatr Scand. 1985;74:45-51. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Adlerberth I, Carlsson B, de Man P, Jalil F, Khan SR, Larsson P, Mellander L, Svanborg C, Wold AE, Hanson LA. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr Scand. 1991;80:602-610. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Ducluzeau R. [Development, equilibrium and role of microbial flora in the newborn]. Ann Pediatr (Paris). 1993;40:13-22. [PubMed] [Cited in This Article: ] |
| 18. | Ouwehand A, Vesterlund S. Health aspects of probiotics. IDrugs. 2003;6:573-580. [PubMed] [Cited in This Article: ] |
| 19. | Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250-G254. [PubMed] [Cited in This Article: ] |
| 20. | Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072-6076. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439-451. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516-523. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | García-Lafuente A, Antolín M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001;48:503-507. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613-G626. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197-216. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966-972. [PubMed] [Cited in This Article: ] |
| 29. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882-1885. [PubMed] [Cited in This Article: ] |
| 31. | Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609-1616. [PubMed] [Cited in This Article: ] |
| 32. | Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589-593. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168-38178. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol. 2002;160:165-173. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224-238. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444S-450S. [PubMed] [Cited in This Article: ] |
| 37. | Fukushima Y, Kawata Y, Mizumachi K, Kurisaki J, Mitsuoka T. Effect of bifidobacteria feeding on fecal flora and production of immunoglobulins in lactating mouse. Int J Food Microbiol. 1999;46:193-197. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Rodrigues AC, Cara DC, Fretez SH, Cunha FQ, Vieira EC, Nicoli JR, Vieira LQ. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol. 2000;89:404-414. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Shu Q, Gill HS. A dietary probiotic (Bifidobacterium lactis HN019) reduces the severity of Escherichia coli O157: H7 infection in mice. Med Microbiol Immunol. 2001;189:147-152. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157: H7 infection in mice. FEMS Immunol Med Microbiol. 2002;34:59-64. [PubMed] [Cited in This Article: ] |
| 41. | Malin M, Suomalainen H, Saxelin M, Isolauri E. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab. 1996;40:137-145. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71-80. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Mao Y, Nobaek S, Kasravi B, Adawi D, Stenram U, Molin G, Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996;111:334-344. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Isolauri E. Probiotics in human disease. Am J Clin Nutr. 2001;73:1142S-1146S. [PubMed] [Cited in This Article: ] |
| 46. | Alam M, Midtvedt T, Uribe A. Differential cell kinetics in the ileum and colon of germfree rats. Scand J Gastroenterol. 1994;29:445-451. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Cummings JH, Englyst HN. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45:1243-1255. [PubMed] [Cited in This Article: ] |
| 49. | Frankel WL, Zhang W, Singh A, Klurfeld DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:375-380. [PubMed] [Cited in This Article: ] |
| 50. | Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottière HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut. 2000;46:507-514. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Gibson PR, Moeller I, Kagelari O, Folino M, Young GP. Contrasting effects of butyrate on the expression of phenotypic markers of differentiation in neoplastic and non-neoplastic colonic epithelial cells in vitro. J Gastroenterol Hepatol. 1992;7:165-172. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Conly JM, Stein K, Worobetz L, Rutledge-Harding S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994;89:915-923. [PubMed] [Cited in This Article: ] |
| 53. | Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6 Suppl 1:S43-S45. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Roberfroid MB, Bornet F, Bouley C, Cummings JH. Colonic microflora: nutrition and health. Summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutr Rev. 1995;53:127-130. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Miyazawa E, Iwabuchi A, Yoshida T. Phytate breakdown and apparent absorption of phosphorus, calcium and magnesium in germ free and conventionalized rats. Nutr Res. 1996;16:603-613. [DOI] [Cited in This Article: ] |
| 56. | Younes H, Coudray C, Bellanger J, Demigné C, Rayssiguier Y, Rémésy C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 2001;86:479-485. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;167:185-189. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Jin LZ, Marquardt RR, Zhao X. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl Environ Microbiol. 2000;66:4200-4204. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90-97. [PubMed] [Cited in This Article: ] |
| 60. | Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333-338. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, Hart CA. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162-165. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Isolauri E, Kaila M, Mykkänen H, Ling WH, Salminen S. Oral bacteriotherapy for viral gastroenteritis. Dig Dis Sci. 1994;39:2595-2600. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Isolauri E, Kaila M, Arvola T, Majamaa H, Rantala I, Virtanen E, Arvilommi H. Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling rats. Pediatr Res. 1993;33:548-553. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25:516-519. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54-60. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046-1049. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Oberhelman RA, Gilman RH, Sheen P, Taylor DN, Black RE, Cabrera L, Lescano AG, Meza R, Madico G. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr. 1999;134:15-20. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Szajewska H, Kotowska M, Mrukowicz JZ, Armańska M, Mikołajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361-365. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Chapoy P. [Treatment of acute infantile diarrhea: controlled trial of Saccharomyces boulardii]. Ann Pediatr (Paris). 1985;32:561-563. [PubMed] [Cited in This Article: ] |
| 70. | Hochter W, Chase D, Hagenhoff G. Saccharomyces boulardii bei acuter Erwachsenen diarrhoea Wirkamkeit and Vertraglichkeit der Behandlung. Munchen Medizinische Wochenschr. 1990;188-192. [Cited in This Article: ] |
| 71. | Buydens P, Debeuckelaere S. Efficacy of SF 68 in the treatment of acute diarrhea. A placebo-controlled trial. Scand J Gastroenterol. 1996;31:887-891. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Mitra AK, Rabbani GH. A double-blind, controlled trial of bioflorin (Streptococcus faecium SF68) in adults with acute diarrhea due to Vibrio cholerae and enterotoxigenic Escherichia coli. Gastroenterology. 1990;99:1149-1152. [PubMed] [Cited in This Article: ] |
| 73. | Wunderlich PF, Braun L, Fumagalli I, D'Apuzzo V, Heim F, Karly M, Lodi R, Politta G, Vonbank F, Zeltner L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 1989;17:333-338. [PubMed] [Cited in This Article: ] |
| 74. | McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16:292-307. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | McFarland LV. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control. 1995;23:295-305. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Adam J, Barret A, Barret-Bellet C. Essais cliniques controles en doubles inso de lultra-leur lyophyilisec. Etude multi-centrique par 25; medicins de 388 cas. Gaz Med Francaise. 1977;84:2072-2078. [Cited in This Article: ] |
| 77. | Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Borgia M, Sepe N, Brancaro V, Simone P, Borgia R. A controlled clinical study on Streptococcus faecium preparation for the prevention of side reactions during long term antibiotic treatments. Curr Ther Res. 1982;1:265-271. [Cited in This Article: ] |
| 79. | Gotz V, Romankiewicz JA, Moss J, Murray HW. Prophylaxis against ampicillin-associated diarrhea with a lactobacillus preparation. Am J Hosp Pharm. 1979;36:754-757. [PubMed] [Cited in This Article: ] |
| 80. | Siitonen S, Vapaatalo H, Salminen S, Gordin A, Saxelin M, Wikberg R, Kirkkola AL. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann Med. 1990;22:57-59. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439-448. [PubMed] [Cited in This Article: ] |
| 82. | Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883-889. [PubMed] [Cited in This Article: ] |
| 84. | Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Tankanow RM, Ross MB, Ertel IJ, Dickinson DG, McCormick LS, Garfinkel JF. A double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhea. DICP. 1990;24:382-384. [PubMed] [Cited in This Article: ] |
| 86. | Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, Gasbarrini A. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16:1461-1467. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324-333. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Aronsson B, Barany P, Nord CE, Nyström B, Stenvinkel P. Clostridium difficile-associated diarrhoea in uremic patients. Eur J Clin Microbiol. 1987;6:352-356. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Biller JA, Katz AJ, Flores AF, Buie TM, Gorbach SL. Treatment of recurrent Clostridium difficile colitis with Lactobacillus GG. J Pediatr Gastroenterol Nutr. 1995;21:224-226. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Bennet RG, Laughton B, Lindsay J et al. Lactobacillus GG treatment of Clostridium difficile infection in nursing home patients. Abstract of the 3rd International Conference on Nosocomial Infection, Atlanta, Georgia 1990. . [Cited in This Article: ] |
| 93. | Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000;95:S11-S13. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Surawicz CM, McFarland LV, Elmer G, Chinn J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol. 1989;84:1285-1287. [PubMed] [Cited in This Article: ] |
| 95. | Popoola J, Swann A, Warwick G. Clostridium difficile in patients with renal failure - management of an outbreak using biotherapy. Nephrol Dial Transplant. 2000;15:571-574. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913-1918. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012-1017. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: a systematic review. CMAJ. 2005;173:167-170. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Muñoz P, Bouza E, Cuenca-Estrella M, Eiros JM, Pérez MJ, Sánchez-Somolinos M, Rincón C, Hortal J, Peláez T. Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis. 2005;40:1625-1634. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945-953. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Rath HC, Schultz M, Dieleman LA, Li F, Kolbl H, Falk W, Scholmerich J, Sartor RB. Selective vs. broad spectrum antibiotics in the prevention and treatment of experimental colitis in two rodent models. Gastroenterology. 1998;114:A1067. [DOI] [Cited in This Article: ] |
| 103. | Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, Aerts R, Kerremans R, Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771-774. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Janowitz HD, Croen EC, Sachar DB. The role of the fecal stream in Crohn's disease: an historical and analytic review. Inflamm Bowel Dis. 1998;4:29-39. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Wellmann W, Fink PC, Benner F, Schmidt FW. Endotoxaemia in active Crohn's disease. Treatment with whole gut irrigation and 5-aminosalicylic acid. Gut. 1986;27:814-820. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995;108:1617-1621. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39:1193-1196. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Solomon MJ, McLeod RS, O'Connor BI et al. Combination ciprofloxacin and metronidazole in severe perianal Crohn's disease. Can J Gastroenterol. 1993;7:571-573. [Cited in This Article: ] |
| 109. | Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993-1000. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Gupta P, Andrew H, Kirschner BS, Guandalini S. Is lactobacillus GG helpful in children with Crohn's disease Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31:453-457. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci. 2000;45:1462-1464. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405-409. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853-858. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:PI126-PI131. [PubMed] [Cited in This Article: ] |
| 117. | Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242-249. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Ruseler-van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance. Gut. 1994;35:658-664. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409-415. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE, Sherman K, Lashner BA. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721-728. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103-1107. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Rayes N, Seehofer D, Hansen S, Boucsein K, Müller AR, Serke S, Bengmark S, Neuhaus P. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123-127. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Jain PK, McNaught CE, Anderson AD, MacFie J, Mitchell CJ. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr. 2004;23:467-475. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Shahani KM, Ayebo AD. Role of dietary lactobacilli in gastrointestinal microecology. Am J Clin Nutr. 1980;33:2448-2457. [PubMed] [Cited in This Article: ] |
| 129. | Malhotra SL. Dietary factors in a study of cancer colon from Cancer Registry, with special reference to the role of saliva, milk and fermented milk products and vegetable fibre. Med Hypotheses. 1977;3:122-126. [PubMed] [DOI] [Cited in This Article: ] |
| 130. | Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, Moretti M, Vilarini I, Scassellati-Sforzolini R, Rowland I. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26:365-380. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Reddy BS, Rivenson A. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Res. 1993;53:3914-3918. [PubMed] [Cited in This Article: ] |
| 132. | Sekine K, Toida T, Saito M, Kuboyama M, Kawashima T, Hashimoto Y. A new morphologically characterized cell wall preparation (whole peptidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Res. 1985;45:1300-1307. [PubMed] [Cited in This Article: ] |
| 133. | Reddy BS. Prevention of colon cancer by pre- and probiotics: evidence from laboratory studies. Br J Nutr. 1998;80:S219-S223. [PubMed] [Cited in This Article: ] |
| 134. | Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202-3207. [PubMed] [Cited in This Article: ] |
| 135. | de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. Probiotics--compensation for lactase insufficiency. Am J Clin Nutr. 2001;73:421S-429S. [PubMed] [Cited in This Article: ] |
| 136. | Gaudichon C, Mahé S, Roos N, Benamouzig R, Luengo C, Huneau JF, Sick H, Bouley C, Rautureau J, Tome D. Exogenous and endogenous nitrogen flow rates and level of protein hydrolysis in the human jejunum after [15N]milk and [15N]yoghurt ingestion. Br J Nutr. 1995;74:251-260. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | Marteau P, Pochart P, Flourié B, Pellier P, Santos L, Desjeux JF, Rambaud JC. Effect of chronic ingestion of a fermented dairy product containing Lactobacillus acidophilus and Bifidobacterium bifidum on metabolic activities of the colonic flora in humans. Am J Clin Nutr. 1990;52:685-688. [PubMed] [Cited in This Article: ] |
| 138. | Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185-194. [PubMed] [Cited in This Article: ] |
| 139. | Wyatt GM, Bayliss CE, Lakey AF, Bradley HK, Hunter JO, Jones VA. The faecal flora of two patients with food-related irritable bowel syndrome during challenge with symptom-provoking foods. J Med Microbiol. 1988;26:295-299. [PubMed] [DOI] [Cited in This Article: ] |
| 140. | Bradley HK, Wyatt GM, Bayliss CE, Hunter JO. Instability in the faecal flora of a patient suffering from food-related irritable bowel syndrome. J Med Microbiol. 1987;23:29-32. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Halpern GM, Prindiville T, Blankenburg M, Hsia T, Gershwin ME. Treatment of irritable bowel syndrome with Lacteol Fort: a randomized, double-blind, cross-over trial. Am J Gastroenterol. 1996;91:1579-1585. [PubMed] [Cited in This Article: ] |
| 142. | Hunter JO, Lee AJ, King TS, Barratt MEJ, Linggood MA, Blades JA. Enterococcus faecium strain PR88: An effective probiotic. Gut. 1996;38:A 62. [Cited in This Article: ] |
| 143. | Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615-2620. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294-301. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004;38:S104-S106. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [PubMed] [DOI] [Cited in This Article: ] |
| 147. | Felley CP, Corthésy-Theulaz I, Rivero JL, Sipponen P, Kaufmann M, Bauerfeind P, Wiesel PH, Brassart D, Pfeifer A, Blum AL. Favourable effect of an acidified milk (LC-1) on Helicobacter pylori gastritis in man. Eur J Gastroenterol Hepatol. 2001;13:25-29. [PubMed] [DOI] [Cited in This Article: ] |
| 148. | Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709-710. [PubMed] [DOI] [Cited in This Article: ] |
| 149. | Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744-2749. [PubMed] [DOI] [Cited in This Article: ] |
| 150. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [PubMed] [DOI] [Cited in This Article: ] |
| 151. | Shimbo I, Yamaguchi T, Odaka T, Nakajima K, Koide A, Koyama H, Saisho H. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol. 2005;11:7520-7524. [PubMed] [Cited in This Article: ] |
| 152. | Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625-1629. [PubMed] [DOI] [Cited in This Article: ] |
| 153. | Sýkora J, Valecková K, Amlerová J, Siala K, Dedek P, Watkins S, Varvarovská J, Stozický F, Pazdiora P, Schwarz J. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39:692-698. [PubMed] [DOI] [Cited in This Article: ] |