INFLAMMATION: THE RELATIONSHIP BETWEEN TISSUE INJURY, REPAIR AND CANCER
Proliferation of cells alone is not sufficient to cause cancer, rather proliferation in the setting of altered growth signals from inflammatory cell infiltrates and DNA damaging agents released from infiltrating leukocytes promote malignant degeneration. Wound healing, which requires subversion of usual growth programs, mobilization and migration of cells and a heightened resistance to apoptosis, embodies all the properties of malignant cells with one exception, that is, healing is self limited. Successful wound healing results in restoration of tissue integrity and resolution of inflammation, reinstating homeostatic growth control. The tissue environments of longstanding unrelenting chronic infection or idiopathic chronic inflammation are persistent states of inadequate wound healing. Within this setting, inflammatory cells produce highly reactive oxygen and nitrogen species, which interact with cellular DNA inducing point mutations, deletions or rearrangements. Usually DNA damage such as this triggers apoptosis. However, in areas of chronic inflammation and repair, growth programs are corrupted and the environment is poised to allow replication and survival of cells, which under normal situations would either be quiescent or lost to apoptosis. Allowing cells to continually divide in an environment conducive to DNA damage may result in the accumulation of genetic defects and the emergence of malignant cells.
Which cell is the target of malignant transformation has been the area of much debate. Original thought was that a terminally differentiated cell would acquire enough genetic damage to replicate endlessly. This would require multiple genetic alterations in key cell signaling cascades to allow autonomous growth. A more likely scenario, however, is that these cells would undergo apoptosis or be sloughed off as a normal part of organ turnover prior to “backing up” the differentiation ladder sufficiently to acquire independent growth. More recently, focus has been on tissue-derived stem cells as the source of cancer. Tissue stem cells possess several important features making them attractive candidates for malignant degeneration. These include long life span, relative apoptosis resistance and ability to replicate for extended periods of time. In the setting of chronic inflammation, progenitor or stem cells within the peripheral tissue are forced to undergo multiple rounds of cell division predisposing to the accumulation of mutations. While restricted progenitors or even differentiated cells can still become transformed, in most cases, it has been believed that early stem cells are the targets of transformation.
If one looks at areas of high proliferative capacity (high BrdU or PCNA staining in tissue) as the stem cell zone, then this theory of peripheral stem cells as the cells of origin of cancer must be reconciled with the fact that this supposed stem cell compartment is often the most damaged and depleted by agents thought to be carcinogens[5]. With chronic inflammation leading to atrophic changes attributed to peripheral stem cell exhaustion, the very cell thought to be transformed is lost - leading us to investigate if an alternate stem cell compartment is responsible for peripheral cancers arising in the setting of inflammation. In order to understand this concept, and why the bone marrow-derived stem cell (BMDC) is a “logical choice” for a cancer stem cell, we need to understand a few points about cancer stem cells, and BMDCs in general.
Stem cells in cancer - cancer as an abnormal organ
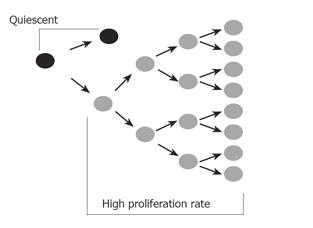
A tumor mass can be compared to an abnormal organ; in that it is composed of a heterogeneous mixture of cell types and of cells possessing different proliferative capacities and different levels of differentiation. Tumor cells are admixed with fibroblasts, endothelial cells and inflammatory cells comprising the tumor stroma. This tumor stroma is being increasingly recognized as a critical contributor to malignant growth and survival of the tumor mass; however, stromal cells themselves are usually not malignant. Likewise, not all cells within a tumor are malignant - meaning not all cells can form tumors if transplanted at another site, or into a secondary host. In fact, the majority of cells within a tumor are incapable of independent growth and are readily susceptible to apoptosis. Only a small fraction of cells within a tumor are capable of independent growth, and fulfill the criteria described for cancer stem cells[6-8]. These cells have metastatic potential, form tumors in secondary hosts and are believed to be responsible for continual renewal of cells within the tumor mass. These cells are likely to proliferate slowly and asymmetrically, self renewing the stem cell population and giving rise to daughter cells, which proliferate to sustain tumor growth (Figure 1). Conventional anti-cancer chemotherapy and radiotherapy target rapidly dividing daughter cells, affecting the bulk of the tumor mass, but leave the cancer stem cell intact, explaining the often rapid recurrence of tumor bulk, once therapy is stopped (Figure 2). At present, the most pressing issue for cancer research is to identify the cancer stem cell and exploit its unique characteristics with targeted therapies.
Figure 1 A proposed model for the cancer stem cell.
The cancer stem cell replicates itself asymmetrically, thus maintaining one daughter stem cell identical to itself. This remains in a relatively quiescent state. The asymmetric division also produces another daughter cell with a high proliferative rate which rapidly divides to sustain the tumor mass.
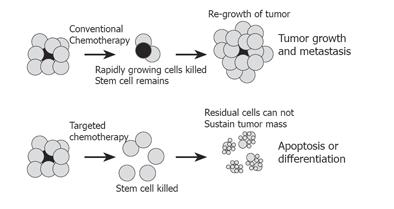
Figure 2 Conventional versus stem cell-targeted chemotherapy.
Conventional chemotherapy and radiotherapy targets rapidly dividing cells, and may shrink tumor mass substantially. However, the stem cell (gray), which is relatively quiescent, is not affected. Regrowth of tumor from surviving stem cell leads to regrowth of tumor and treatment failure. Chemotherapy targeted at the stem cell would remove the source of new cell growth, and allow residual cells within the tumor to be targeted with chemotherapy, differentiating agents or therapy aimed at inducing apoptosis, thus successfully eliminating the tumor.
Bone marrow stem cells
BMDCs are a heterogenous group of cells isolated from the bone marrow which are capable of repopulating the hematopoietic system of a lethally irradiated immunologically compatible secondary host. These cells have been divided into at least two main categories; the hematopoietic and mesenchymal stem cells (MSC). Hematopoietic stem cells are traditionally regarded as the cells which give rise to the formed elements of the blood and have been used extensively in human bone marrow transplantation. Thus, hematopoietic stem cells have been extensively studied and defined with regard to surface markers, growth characteristics and repopulation potential[9]. Less well defined are the MSC. This term MSC, as defined in the literature, is the heterogenous population of cells isolated as the adherent population, when total marrow is placed in culture[10]. These cells give rise to adipocyte, chondrocyte, cells of osteocyte lineages and the marrow mesenchyma, which is vital for optimal hematopoiesis[11-13].
Work from multiple laboratories demonstrates surprising roles for marrow-derived stem cells in addition to hematopoiesis, stressing that the potential for differentiation may be much greater than originally believed. Markers defining cell subpopulations within the marrow are not standardized in these studies, making direct comparison of data between laboratories challenging; however, one thing remains consistent - cells within the bone marrow have a markedly greater differentiation potential than originally believed. For the purposes of this discussion, the term BMDCs will be broadly used to refer to cells derived from the marrow, and will encompass hematopoietic stem cells, MSC multipotent progenitor cells and whole marrow.
In vitro and in vivo studies-plasticity of BMDCs
Multiple and elegant studies from independent groups have shown quite clearly that bone marrow stem cells can differentiate along multiple diverse lineage pathways[14-17]. These findings challenge the conventional view that bone marrow stem cells give rise only to the marrow mesenchyma or formed elements in the peripheral blood. In vitro, BMDCs have been shown to differentiate at the single cell level and acquire characteristics of mesoderm, neuroectoderm and endoderm[15,16]. These cells appear to use culture environmental factors to guide lineage decisions. Strikingly, in vivo studies in the mouse model have confirmed this plasticity. Elegant studies utilizing transplanted single cells demonstrate differentiation along multiple lineages, supporting a central role for the local tissue environment in dictating differentiation of stem cells, confirming that a single cell is multipotent, and supporting the assertion that experimental findings demonstrating multiple cell lineage differentiation is not due to circulating tissue specific progenitor cells, but rather to a single multipotent cell. In these studies, multiple types of epithelial cells have been shown to be derived from BMDCs including epithelium of the lung, gastrointestinal tract and skin after transplantation of a single bone marrow-derived stem cell[14]. This is not a transient event, as cells can be recovered nearly a year after transplantation. In the gastrointestinal tract, engrafted cells are seen as isolated epithelial cells in the gastric pits of the stomach, the small intestinal villi, the colonic crypt, and rarely in the esophagus. Under these experimental conditions, cells were recovered as single differentiated epithelial cells, and did not appear to engraft into the stem cell niche as clonal expansion was not seen. Infusion of labeled BMDCs into a non-irradiated host, also led to the engraftment (albeit to a lesser degree) and differentiation as epithelial cells of the liver, lung and gut in a similar pattern to that seen with marrow ablation and transplantation[15], demonstrating that engraftment and differentiation are true physiological events and not merely artifacts of irradiation and experimental manipulation. While epithelial cell damage is not necessary for engraftment, studies support the notion that damage to the epithelium increases engraftment.
The mechanism by which the marrow-derived cells acquire the appropriate phenotype of epithelial cells is not known, with evidence supporting both direct differentiation or fusion with a peripheral cell[18-21]. The method of engraftment and differentiation may be specific to the individual tissues and/or may depend on the mechanism of injury inducing engraftment. Irrespective of the mechanism involved, BMDCs have been shown to engraft and take on the function of cells within the peripheral tissues[16,18-23].
HUMAN STUDIES - EVIDENCE FOR PLASTICITY OF BMDCS IN PATIENTS TRANSPLANTED WITH GENDER MISMATCHED BONE MARROW
Human studies have confirmed that plasticity of BMDCs is not restricted to mice, and may be a physiologically relevant phenomenon in man as well. Studies, examining peripheral tissue of female patients transplanted with bone marrow derived from male donors, have shown that BMDCs from the donor can differentiate into skin, gut epithelium and mature hepatocytes[24,25]. Identification of the Y chromosome in cells of these tissues confirms that BMDCs can substantially repopulate the GI tract epithelium[25], and this repopulation does not appear to be a rare event. Patients in these studies have some level of graft-versus-host disease, and the level of inflammation in the tissue correlates with the level of donor cell engraftment. These findings are consistent with the data derived from murine studies and suggest the inflammatory environment is crucial for optimal engraftment and differentiation of BMDCs. The fact that BMDCs have the capacity to differentiate along organ-specific lineages appropriate for the organ of engraftment, and are found in increasing numbers during chronic inflammation (a condition associated with cancer), places these cells “in the right place, at the right time” to be candidates for the cancer stem cell.
Similarities between BMDCs and cancer cells
In addition to what appears to be of immense plasticity of cells within the bone marrow, BMDCs have other traits which make them attractive candidates for cancer stem cells. BMDCs have the capacity for self renewal, are long lived, are chemoresistant, and may be inherently mutagenic[26-29]. Intriguing is the fact that similar growth regulators and control mechanisms are involved in both cancer and stem cell maintenance. For example, proteins from the polycomb group, the epigenetic chromatin modifiers, are involved in both cancer development and maintenance of embryonic and adult stem cells[30]. Also, pathways used by bone marrow stem cells for trafficking appear to be exploited by tumor cells for metastasis[31]. For instance, chemokines and cytokines produced during chronic inflammation (such as SDF-1) influence the behavior and migration of cancer cells. These are the same chemokines and cytokines responsible for physiological stem cells homing back to the marrow cavity[32-36]. Identification of bone marrow stromal cell-derived growth inhibitor as a potent inhibitor of breast cancer cell migration, and the capability of this protein to induce cell cycle arrest and apoptosis in breast cancer stem cells further supports the use of similar growth mechanisms between stem and cancer cells[37]. Inflammation of the GI tract is associated with IL-6 and IL-8 production which initiate neutrophil infiltration[39]. Interestingly, IL-6 is also chemotactic for MSC[33]. Other cytokines and chemokines prominent in the setting of mucosal inflammation such as VEGF and MIP-1α are also chemoattractants for MSC[33,34]. Receptors such as CXCR 2 and 4 are found on both cancer cells and stem cells, and influence the homing of stem cells, or invasion/metastases of cancer cells, suggesting a link between the two populations of cells. One might suppose that a mechanism similar to that used to regulate BMDC circulation and homing back to areas of bone may also facilitate migration and engraftment of BMDCs into peripheral tissues as a result of chronic inflammation, if the peripheral tissue secretes the appropriate homing signals.
Additionally, immune escape has long been a perplexing property of cancer cells; MSCs have unique immunological properties in that they are not immunogenic, they do not stimulate alloreactivity, and they escape lysis by cytotoxic T cells and natural killer cells[39]. This inherent ability to evade immune recognition may explain why many cancer cells evade the host immune response.
BMDCs as the origin of epithelial cancer: helicobacter induced gastric cancer as a model system
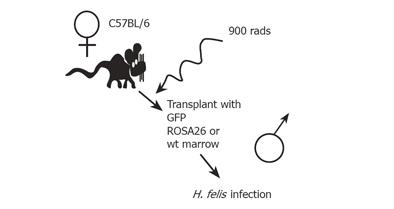
We reasoned that BMDCs, as the ultimate uncommitted adult stem cell, might represent the ideal candidate for transformation, if placed in a favorable environment. We used the well-described H. felis/C57BL/6 mouse model of gastric cancer to test this theory[40]. This model is optimal for studying the role of stem cells in inflammatory-mediated cancers because C57BL/6 mice do not develop gastric cancer under controlled conditions. With Helicobacter infection, however, the gastric mucosa progresses through a series of changes including metaplasia and dysplasia, culminating in gastrointestinal intraepithelial neoplasia (GIN)[41] by 12-15 mo of infection, thus reiterating human disease, where gastric cancer in the absence of Helicobacter infection is unusual, while longstanding infection carries a significant (up to 1-3%) risk of gastric cancer[42-47]. In order to test the role for BMDCs in gastric cancer (Figure 3), C57BL/6J mice were myeloablated and transplanted with gender-mismatched bone marrow from mice that expressed a non-mammalian beta-galactosidase enzyme [C57BL/6JGtrosa26 (ROSA 26)], mice that expressed green fluorescent protein [C57BL/6J-beta-actin-EGFP (GFP)], or control C57BL/6J liter mates. Engraftment of ROSA26 BMDCs into the gastric mucosa was confirmed by several independent methods including detecting enzyme activity, specific B-galactosidase immunohistochemistry (IHC, two cytoplasmic markers) (Figures 4 and 5), and detection of LacZNeo fusion gene sequence (nuclear marker) by PCR within beta-galactosidase positive gastric glands isolated by laser capture microscopy. In those mice transplanted with GFP marrow, GFP was detected by fluorescence activated cell sorting of cytokeratin positive-single cell preparations, and GFP immunohistochemistry of tissue sections. X and Y chromosome fluorescent in situ hybridization (X and Y-FISH) was used as an additional means to detect BMDCs in gender mismatched transplants[40].
Figure 3 An experimental mouse model for bone marrow transplantation and H.
felis-induced gastric carcinoma.
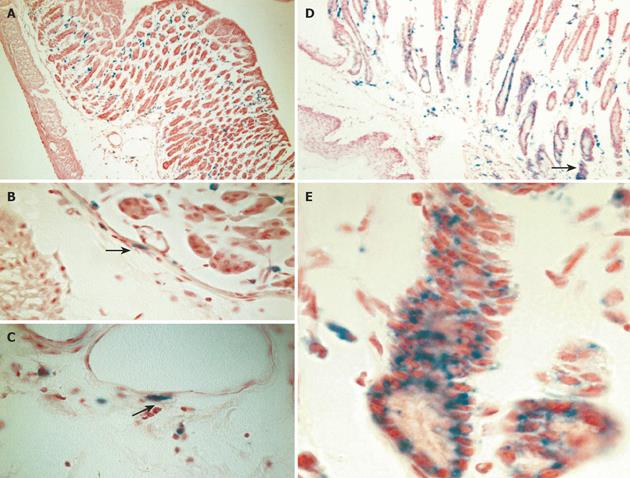
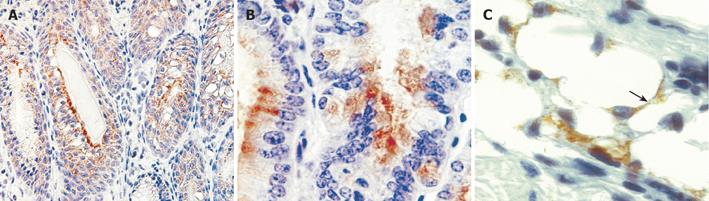
Figure 4 Engraftment of donor-derived ROSA-26 marrow by x-gal staining.
A: Mice transplanted with ROSA 26 marrow and infected with H. felis for 4 wk had donor-derived leukocytes (blue) infiltrating the gastric mucosa, and no engraftment into gland structures. B and C: A higher power view reveals myocytes and myofibroblasts in the submucosal tissue adjacent to vascular structures (arrows). D: After 30 wk of infection, marked architectural distortion is seen with antralization and appearance of metaplastic glands. Entire gland structures are derived from donor marrow (blue staining). Gland shown in panel D (arrow) is shown at higher power in E.
Figure 5 Immunohistochemistry for bacterial beta-galactosidase confirms uniform signal in gastrointestinal neoplasia.
Mice developed severe dysplasia and intraepithelial neoplasia derived from donor marrow, 12-15 mo after infection with H. felis (A) and (B). Immunohistochemistry for bacterial beta-galactosidase demonstrates cytoplasmic staining in dysplastic glands. A population of adipocytes in the submucosa are also stained for beta-galactosidase (arrow).
As expected, acute Helicobacter infection was associated with an influx of bone marrow-derived inflammatory cells (Figure 4A - blue staining) into the tissue. At early time points, we did not detect any engraftment or differentiation of BMDCs to an epithelial cell phenotype. At 20 wk of infection, rare glands entirely replaced by BMDCs were isolated, suggesting that engraftment into the stem cell niche had occurred. These findings were more pronounced at 30 wk, where antralized glands and metaplastic cells at the squamocolumnar junction were entirely replaced by marrow-derived cells (Figures 4D and 4E). The severity of intraepithelial dysplasia increased over time, and by one year of infection, most mice developed invasive neoplastic glands. All of the intraepithelial neoplasia in mice infected for 12-16 mo rose from donor marrow cells, strongly suggesting an inherent vulnerability of this population of cells to malignant progression. Progressive parietal and chief cell loss is a hallmark of chronic Helicobacter infection. Of the few parietal or chief cells which we isolated from the infected mice, none were derived from the bone marrow, strongly suggesting that marrow cells do not differentiate toward the parietal or chief cell phenotype under the experimental conditions that were used[40].
Normal healing of the gastric mucosa after iatrogenic ulceration likewise did not require BMDCs[40], nor did loss of specific cell lineages, such as targeted ablation of parietal cells, lead to marrow engraftment[40]. Rather, it seems that long standing inflammation and inflammatory mediated damage to the epithelium is required - an environment strongly linked to the development of cancer in many settings. In our Helicobacter-gastric cancer model, infection and inflammation reached a plateau at 8 wk; however, engraftment was not apparent until 20 wk, suggesting that events other than increased inflammation are responsible for engraftment. Between 8 and 20 wk, there is loss of the oxyntic glands, and a restructuring of the gastric architecture to include metaplastic cell lineages, reflecting the effects of an abnormal tissue milieu on rapidly proliferating cells[48]. Once engraftment began, however, the number of bone marrow-derived glands increased dramatically, suggesting that a threshold for recruitment had been reached[40].
In addition to epithelial cells within the tumor, BMDCs also comprise a subset of cells within the tumor stroma and within seemingly uninvolved epithelium and subepithelial spaces adjacent to the tumors. We have recovered adipocytes (Figure 5C), fibroblast, endothelial cells and myofibroblasts (Figures 4B and 4C) derived from bone marrow precursors in areas adjacent to dysplasia and neoplasia.
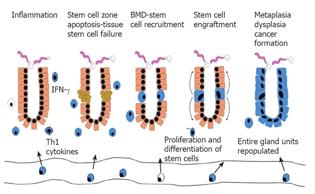
Based on these experiments, we have proposed a new paradigm for epithelial cancer (Figure 6). Chronic tissue inflammation leads to tissue injury and with time, to tissue stem cell failure. Peripheral stem cell failure leads to recruitment and permanent engraftment of BMDCs into the tissue stem cell niche, where the BMDCs essentially take over the function of the tissue stem cell. In the setting of inflammation, specifically with Th1 type cytokines and an abnormal tissue environment (for example, one lacking chief and parietal cells), the BMDCs initiate differentiation, but fail to regulate growth programs appropriately and progresses through stages of metaplasia and dysplasia. We speculate that the inappropriate retention of primitive growth programs in a stem cell forced to replicate may permit survival despite otherwise lethal mutations, thus allowing transformation. This new model brings together previously unexplained observations regarding the behavior of cancer, and presupposes that properties inherent to cancer such as their resistance to apoptosis, their unlimited growth potential and their ability for local spread and distant metastasis are fundamental to the origin of the cell, rather than traits acquired. The concept of cancer initiation and promotion can also be viewed within the context of this model. Initiation may represent BMDCs trafficking into the stem cell niche as a result of tissue stem cell damage. In the absence of continued inflammation and injury, these engrafted cells may behave in a way indistinguishable from endogenous tissue stem cells. Promotion may represent an additional stimulus received at a later time that allows sustained proliferation of BMDCs and transformation.
Figure 6 A new paradigm proposed for epithelial cancer.
In vitro experiments and animal models supporting the BMDC-epithelial cancer model
In addition to the Helicobacter-gastric cancer model, other studies have begun to address the role of BMDCs in cancer using various in vitro and in vivo models. For example, BMDCs have been shown to localize to a known stem cell niche within the epidermis known as the CD34 positive bulge region of the hair follicle, and clonally expand to repopulate portions of the epidermis, functioning as an epidermal stem cell[49]. Similar to our findings, engraftment of BMDCs to the stem cell niche is dramatically increased with injury severe enough to deplete peripheral stem cells in the region. However, these are short-term studies. Longer term studies utilizing carcinogen exposure will determine the eventual fate of these BMDCs, and determine if BMDCs in the stem cell niche behave differently from peripheral stem cells occupying the same niche. It is intriguing, however, to speculate the ultimate fate of these stem cells given the prevalence of BMDC-skin carcinoma in solid organ recipients (see below).
In addition to residing in the epithelial stem cell niche, bone marrow-derived myofibroblasts have been recovered within the colonic subepithelial compartments in both mice and human beings[50,51]. Interestingly, Direkze et al. observed that in the IL-10 knockout mouse model of colitis, up to 45% of subepithelial myofibroblasts were marrow derived[51], suggesting that in the setting of chronic inflammation, damaged tissue is replaced by BMDCs. When the same group looked at tumor-associated myofibroblasts and fibroblasts, they also found a significant portion of these cells derived from bone marrow cells[50]. It is not clear from these data, if tumors recruit bone marrow cells into the stromal compartment or if resident myofibroblasts and fibroblasts derived from marrow contribute to tumor formation because of abnormal signaling behavior.
Adenocarcinoma of the distal esophagus (Barretts’ adenocarcinoma) results from reflux-induced mucosal damage followed by healing with a metaplastic intestinal cell lineage. This intestinal metaplasia is prone to malignant degeneration and is another ideal model to test the role of BMDCs in inflammatory-mediated cancers. Using a rat model of Barrett’s metaplasia, a significant contribution of BMDCs to the stroma and the metaplastic epithelium has been demonstrated, supporting a role for BMDCs in these pre-neoplastic lesions[52]. Though these findings have only been reported in an abstract form so far, this information is especially exciting because it provides evidence of direct BMDC involvement in carcinogenesis from both an additional species (rat) and tissue type (esophagus), providing further support for our BMDC-epithelial cancer model.
Human data supporting the BMDC-epithelial cancer model
In human beings, the incidence of solid tumors is significantly increased following bone marrow transplantation[53] and may be related to persistent chronic inflammation of graft vs. host disease. The data on BMDCs in human cancers, however, have been conflicting. First, it is difficult to examine the contribution of donor marrow to tumor formation in human beings because of a paucity of cell markers to consistently identify autologous BMDCs or donor cells after BM transplantation. The most reliable marker we have to date is identification of the sex chromosomes in sex mismatched transplants. However, there are inherent difficulties with using Y-chromosome identification. X/Y fluorescent in situ hybridization (FISH) analysis of archived tumors is estimated to miss more than 50% of Y-positive cells due to sectioning bias, where only a portion of the nucleus and thus only a portion of the chromosomes are included in the tissue section. Additionally, females with a history of carrying a male fetus may show peripheral blood chimerism confounding interpretation of data, and eliminating this population from the study. Also, tumors identified within a short time after transplant may reflect the effects of immunosuppression on previously undetected early malignancy and not newly formed tumors, and may explain why some studies conclude tumors in these patients are host derived[54], while other studies demonstrate a definite contribution of donor’s-BMDCs[55]. Studies utilizing larger numbers of patients followed for longer periods of time will better address this new and controversial area, and determine if the BMDCs are confined to the stroma, involved in angiogenesis or constitute the epithelial component of the tumor mass in human beings.
In addition to patients receiving bone marrow transplants, recipients of solid organ transplants also have a higher incidence of secondary malignancy. Interestingly, in solid organ transplant recipients, hematopoietic cells of donor origin are often found in the circulation, indicating that hematopoietic stem cells are transferred with the transplanted organ[56,57]. These transferred stem cells have been shown to give rise to Kaposi sarcoma (KS), a vascular tumor[58], and skin carcinoma[59]. The detected KS lesions occurred distal to the graft site, and formed presumably via mobilization of donor progenitor cells with subsequent transformation at a distant site. Donor-derived stem cells contribute to skin carcinomas, and have been recovered as components of squamous cell carcinoma, basal cell carcinoma, actinic keratosis, keratoacanthomas and benign cutaneous lesions[59], attesting to the great potential for abnormal differentiation of these cells. BMDCs as terminally differentiated cells in other organs including hepatic endothelial cells, hepatocytes and biliary epithelial cells[60], suggesting that these cells may play a role in transformation within these organs as well, if subjected to the appropriate environmental conditions.
CONCLUSION
One of the greatest and most elusive challenges in cancer biology has been to identify the cellular origin of cancer. We have identified the bone marrow stem cell as the cell of origin of Helicobacter-induced gastric cancer in a mouse model, radically altering our current view of gastric cancer formation in particular, and of inflammation-mediated cancers in general. The concept of BMDC plasticity is being increasingly recognized and validated by independent groups. Our recent observation that BMDCs are the origin of Helicobacter-induced gastric cancer[40] combined with supporting observations of BMDCs in other tumors such as benign and malignant tumors of the skin[59], Kaposis sarcoma[58] Barretts’ adenocarcinoma of the esophagus[52] as well as demonstration of BMDCs as constituents of tumor stroma and tumor vascular structures[51-55] suggests exciting approaches for cancer therapy. If the propensity for BMDCs to transform is based on inappropriate regulation of immature growth programs, with growth programs left “turned on” rather than the previously held concept of mutation driven-reactivation of programs, can we target these pathways Undoubtedly, genetic mutations have occurred which are irreversible; but if we can target and switch off inappropriately activated growth cascades, perhaps we can push these damaged cells into apoptosis or enhance the sensitivity to conventional chemo- and radiotherapy. These approaches may lead to novel and more efficacious cancer therapy.
Presently, our laboratory is involved in identifying the cell population within the bone marrow capable of cancer formation as well as defining the homing and differentiation signals which allow these cells to access to gastric mucosa, and to differentiate as metaplastic and dysplastic cells. Studies designed to determine if fusion is a means of bone marrow cell integration into gastric mucosa and gastric cancer are underway. The applicability of these findings to other epithelial cancers will be tested as well as our ability to control the growth of these cells by manipulations of the local tissue environment. These efforts are aimed at identifying cell-specific targets for chemotherapy. Findings from these studies will radically alter our approach to the treatment of gastric cancer as well as other solid tumors, and offer hope for improved survival and potential cure.