Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7165
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: December 7, 2005
AIM: (1) To gain information on immune responses to an accelerated schedule of 0, 1, and 2 mo in paramedical staff and BDS students who are at an increased risk of getting hepatitis B infection and come under high risk groups. (2) To assess the efficacy and safety of Enivac-HB in different age groups, using genetically modified yeast strain Pichia pastoris, a new recombinant hepatitis B vaccine developed and manufactured in India.
METHODS: A prospective, comparative, and single blinded trial of rapid (0, 1, and 2 mo) hepatitis B immunization schedulewas reported. A total of three hundred and seven (212 females and 95 males) healthy volunteers divided into three age groups (18-29, 30-39, and 40-49) were enrolled after screening for markers of hepatitis B. All the volunteers received 20 mg of the vaccine intramuscularly at 0, 1, and 2 mo.
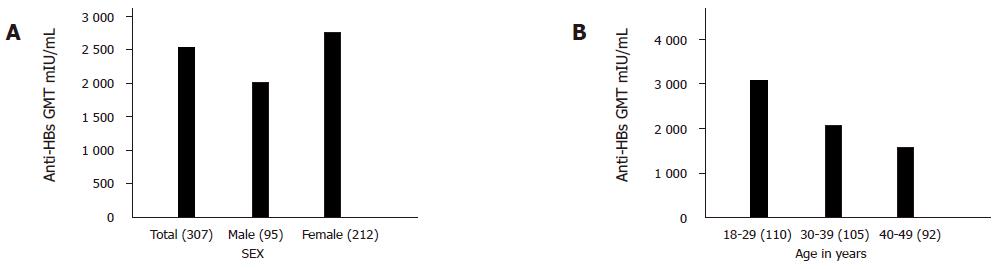
RESULTS: Geometric mean titers were calculated pre and post vaccination. Before immunization the GMT was 0.0124 mIU/mL. One month after the administration of the third dose of recombinant vaccine 296/307 (96.5%) subjects achieved seroprotective levels of anti-HBs. The geometric mean anti-HBs titers achieved after one month of the third dose was 2 560.0 mIU/mL. The geometric mean anti-HBs titer of males was 2 029.0 mIU/mL, while that of the females was 2 759.0 mIU/mL. In the age group of 18-29 years, anti-HBs titer was 3 025.0 mIU/mL, while that in the age group of 30-39 years was 2 096.0 mIU/mL. In third age group of 40-49 years, anti-HBs titer was 1 592.0 mIU/mL. Hyper-responses (anti-HBs≥100 mIU/mL) were shown in 88.0% (271/307) of subjects. Eleven (3.5%) subjects responded poorly to the vaccine in the age group of 40-49 years. There was only mild pain at the site of injection otherwise there were no other adverse drug reactions (ADRs).
CONCLUSION: This vaccine (Enivac-HB) is safe and efficacious, providing significant protection after the third dose and rapid hepatitis B immunization schedule of 0, 1, and 2 mo can be recommended whenever rapid protection is the goal.
- Citation: Hussain Z, Ali SS, Husain SA, Raish M, Sharma DR, Kar P. Evaluation of immunogenicity and reactogenicity of recombinant DNA hepatitis B vaccine produced in India. World J Gastroenterol 2005; 11(45): 7165-7168
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7165
Hepatitis B virus (HBV) infection is responsible for a high proportion of the worlds cases of cirrhosis, and is the cause of up to 80% of all cases of hepatocellular carcinoma (HCC)[1,2]. The situation is grim in developing countries like India, where blood bank infrastructure is non-existent outside the major metropolitan cities and safe blood handling practice standards are low[3]. The weighed prevalence of hepatitis B in India has been estimated to be 4.7%[4,5], which makes this an intermediate prevalence country.
Hepatitis B vaccination has been one of the success stories of the 20th century and has been extensively used in a wide range of groups throughout the world. Hepatitis B vaccination program have successfully reduced the prevalence of hepatitis B, in Taiwan[6] where universal HBV vaccination has led to a significant reduction of hepatitis B prevalence and incidence of hepatocellular carcinoma in children. The immunogenicity, efficiency, and safety profile of hepatitis B vaccine has been well established. More than 90% seroconversion has been achieved in adult populations consistently[7-10]. The safety profile of the recombinant hepatitis B vaccine has been very good[11].
Numerous genetically engineered hepatitis B vaccines are available in India today. However, vaccines do differ in terms of latency (time taken for the production of effective antibodies), reactogenicity and price. Newer technologies involving more advanced yeast strains like Pichia pastoris[12] used for manufacturing the vaccines are today revolutionizing genetically engineered hepatitis B vaccines.
The aim of the study was (1) to gain information on immune responses to an accelerated schedule of 0, 1, and 2 mo in paramedical staff and BDS students who are at an increased risk of getting hepatitis B infection and come under high risk groups. (2) To assess the efficacy and safety of Enivac-HB in different age groups, using genetically modified yeast strain P pastoris, a new recombinant hepatitis B vaccine developed and manufactured in India.
The study was conducted in Maulana Azad Medical College, New Delhi for over the period of 6 mo (October 2002-April 2003). Five milliliters of blood sample was taken by venipuncture with the informed consent of all the 317 volunteers. Serum sample was separated and stored at -20 °C to perform various serological tests.
Permission to conduct this study on adult human volunteers was obtained from Drugs Controller of India. The Institute Ethics Committee approved the study protocol. The study population consisted of 317 volunteers. The following inclusion criteria had to be met. The subjects had to be tested negative for hepatitis B surface antigen (HBsAg), anti-hepatitis B surface antibody (anti-HBs), and anti-hepatitis B core antibody, IgM anti-HBe, IgG anti-HBe and subjects had to be at least 18 years of age and had to sign an informed consent. Subjects were excluded from the study if they had been previously vaccinated with HBV vaccine; or if they were presently taking immunosuppressive drugs; or had a history of hypersensitivity to yeast; or had to receive immunoglobulins, blood or blood products within the previous 6 mo. Subjects who were pregnant, who had significant hematological, hepatic, renal, cardiac or respiratory diseases or who had participated in any other trial 30 d before or during the present study were excluded. Physical examination such as pulse, blood pressure, temperature, and edema were checked. All the 307 healthy seronegative volunteers of the age ranging between 18 and 49 years were recruited for participating in the vaccination schedule of 0, 1, and 2 mo.
Of the 317 volunteers, 307 volunteers, who were negative for all the serological markers of hepatitis B infection, completed the hepatitis B vaccination program. Seven were positive for HBV markers and three volunteers had high anti-HBs titers. All the 307 volunteers received the three doses of HBV vaccination. The subjects were administered 1 mL (20 mg of recombinant DNA) of hepatitis B (Enivac-HB) vaccine intramuscularly in the deltoid muscle according to the following schedule: 1st dose-0 d, 2nd dose -30 d after the 1stdose, 3rddose-60 d after the 1stdose. Vaccinees were monitored for adverse events closely for 3 d after each dose, i.e. fever, pain at the site of injection, erythema, and swelling, nausea, rash, fatigue, bodyache, and scored as absent, mild, moderate or severe. Samples for anti-HBs antibody titers were determined at 0 and 90 d. Anti-HBs antibodies were done using a commercially available quantitative ELISA kit (AUSAB-EIA, Abbot Labs, USA). Protection with hepatitis B vaccination was considered to be achieved when the concentration of anti-HBs antibody titers was ≥10 mIU/mL. A non-response was defined as anti-HBs antibody titers ≤10 mIU/mL[13], responders were those with titer levels ≥10 mIU/mL and ≤100 mIU/mL, high responders were those with anti-HBs titers ≥100 mIU/mL, and those with titers ≥1 000 mIU/mL was hyper-responder[13].
A new recombinant DNA hepatitis B vaccine (Enivac-HB, Panacea Biotec, India) containing hepatitis B surface antigen (s-gene) produced in genetically engineered yeast P pastoris cells[12] was evaluated. A derivative of the plasmid V322 carrying hepatitis B viral DNA was used to amplify the HBsAg. The s-gene isolated from hepatitis B was placed in P pastoris cell. The HBsAg nucleotide sequence[14] was purified to >95% by adsorption by the antigen in the cellular extract to colloidal silica, followed by its desorption, ion exchange chromatography and ultracentrifugation. The vaccine was prepared by adsorbing the purified HBsAg onto aluminum hydroxide gel (0.5 mg aluminum per dose) and thiomersal was added as a preservative to a final concentration of 0.05 mg/dL. P pastoris is the most advance second generation yeast strain with proven advantages over other yeast strains like Saccharomyces cerevisiae[12] which is used in the manufacturing process of many commercially available genetically engineered hepatitis B vaccines in India. The yeast strain P pastoris can grow at a highly acidic pH that becomes a barrier for growth of many contaminating microbes during the process of fermentation[12]. In short, the manufacturing process itself is a purification procedure. The yield of hepatitis B surface antigen obtained with this strain of P pastoris is higher and it means per liter more surface antigen is obtained with P pastoris than with many other strains[12]. Enivac-HB is manufactured using a technology that is free from toxic substances like cesium chloride[12]. Some commercially available vaccines in India are known to use technology that involves the usage of cesium chloride[12]. This advantage automatically translates into lesser contamination and hence, lesser chances of reactogenicity[12].
Seroconversion and seroprotection were compared by descriptive statistics. Geometric mean titers (GMTs) pre and post-vaccinations were compared by Student’s t test. P<0.05 was compared as statistically significant (95%CI).
A total of 317 subjects were screened, 307 subjects negative for all the serological markers of hepatitis B were enrolled in the study. The mean age of the study group was 33.5±9.4 years, with 95 male subjects and 212 female subjects. Dose schedules were 0, 1, and 2 mo. Adverse events were recorded by specifically interviewing each subject during the entire duration of the study. The adverse drug reactions (ADRs) were assessed closely for 3 d after each dose and that no vaccinee had fever or any other designated systemic complaint with only mild pain at the site in 79%, 84.9%, and 75.4% of the vaccinees following doses 1, 2, and 3, respectively. Hematological and biochemical parameters were normal in all the subjects at the end of the study compared to the baseline value. None of the patients became HBsAg and/or anti HBe positive during the follow-up period. “The geometric mean anti-HBs titer” at the initiation of the vaccination program was 0.124 mIU/mL. One month after the administration of the third dose of recombinant vaccine, 296/307 (96.5%) subjects achieved seroprotective levels of anti-HBs antibodies. “The geometric mean anti-HBs titer” achieved after one month of the third dose was 2 560.0 mIU/mL geometric mean titers. “The geometric mean anti-HBs titer” of males was 2 029.0 mIU/mL, while that of females was 2 759.0 mIU/mL as shown in Figure 1A. In the age group of 18-29 years, anti-HBs titer was 3 025.0 mIU/mL, while that of 30-39 years was 2 096.0 mIU/mL. In 40-49 years of age, anti-HBs titer was 1 592.0 as shown in Figure 1B. The above value is statistically significant with P<0.05, and had 95%CI. Hyper-responses (anti-HBs ≥100 mIU/mL) was shown by 88.0% (271/307; P<0.05) of subjects. Twenty subjects all in the age group of 40-49 were (11/307; 3.5%) non-responders, with the geometric mean anti-HBs titers 8.60 mIU/mL. The mean age of the 11 non-responders was 46.4±6.9 years, 1.3% (4) males and 2.2% (7) females were non-responders but the difference was not statistically significant.
Accelerating the vaccination schedule against hepatitis B is appealing because it may increase patient compliance and provide earlier protection for the people who are already in a high risk group or environment. A comparative Indian study of HBV vaccine in three age groups, 18-29, 30-39, and 40-49 years, was done. The experimental data obtained during the course of the trial indicated that seroprotection one month[15] after the third dose was (96.5%) with mean geometric anti-HBs titers 2 560.0 mIU/mL. Twenty subjects all in the age group of 40-49 years (11/307; 3.5%) showed low response to the vaccine and demonstrated an antibody titer of 8.60 mIU/mL. While all the subjects in rest of the age group achieved 100% seroconversion. Risk factors that have been associated with non-response to hepatitis B vaccine include increasing age, male gender, obesity, history of smoking, administration of vaccine in buttock rather than deltoid[3]. The relationship of hepatitis B vaccination response with age is controversial. Our study suggests that seroconversion in age group >40 years is 79% which is considered high compared to most other studies[16,17] where seroconversion rate of 60% has been reported. However, as all the above studies, we too found a decreasing seroconversion rate with increasing age. These findings favor the hypothesis that increasing age decreases seroprotective antibody formation after vaccination.
In the present clinical study, it was observed that female volunteers showed a better response in comparison to male volunteers (P<0.001). Jilg et al[18] reported a slightly lower response in males and Dentico et al[19] also reported that the sex factor is one of the parameters influencing the response following vaccination. Whether or not there exists a sex related variation of the immunogenic response is still controversial.
The vaccination schedule (0, 1, and 2 mo) employed in the present study has been well studied in the other trials[15-19]. Marsano et al[20] have established that the 0, 1, and 2 mo schedule of vaccination gives a rate that is quicker than and identical to the rate of seroprotection of the standard schedule of vaccination of 0, 1, and 6 mo and is much quicker. The recombinant yeast-derived vaccine evaluated did not produce any severe adverse reactions. There was only mild pain at the site of injection otherwise it was completely safe with no adverse drug reactions.
From the present data, we can confirm that the Enivac-HB is highly immunogenic. This new indigenously manufactured vaccine using P pastoris as yeast strain is safe and provides effective titers against hepatitis B. In a country with an estimated 40 million or more carriers of the hepatitis B virus and an estimated 18 million newborns each year, the availability of an indigenously manufactured vaccine would probably make it easier to include the vaccine in a community-based program.
The study conclusively proves that the recombinant DNA hepatitis B vaccine (Enivac-HB) produced using genetically engineered yeast cell P pastoris appears to be highly immunogenic and safe and confers a seroprotection of 96.5% of the subjects with 88.0% showing hyper-response. The study suggests that the vaccine appears to be well tolerated and the rapid vaccination schedule of 0, 1, and 2 mo can be recommended whenever rapid protection is the goal.
The vaccines and finance for the clinical study were provided by Panacea Biotec Limited, New Delhi, India. The authors are grateful to Mrs Alice Jacob, a member of PCR Hepatitis Laboratory, New Delhi, India for helping us during the study.
Co-first author: Zahid Hussain
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Report of a WHO meeting: prevention of liver cancer, technical report no. 691, World Health Organization, Geneva. 1983;8-9. [Cited in This Article: ] |
| 2. | Ghendon Y. WHO strategy for the global elimination of new cases of hepatitis B. Vaccine. 1990;8 Suppl:S129-S33; discussion S129-S33;. [PubMed] [Cited in This Article: ] |
| 3. | Das K, Gupta RK, Kumar V, Kar P. Immunogenicity and reactogenicity of a recombinant hepatitis B vaccine in subjects over age of forty years and response of a booster dose among nonresponders. World J Gastroenterol. 2003;9:1132-1134. [PubMed] [Cited in This Article: ] |
| 4. | Thyagarajan SP, Jayararn S, Mohanvalli B. Prevalence of HBV in the general population of India. Hepatitis B in India: problems and prevention. New Delhi: CBS Publishers 1996; 5-16. [Cited in This Article: ] |
| 5. | Tandon BN. Dimension and issues of HBV control in India Hepatitis B in India: problems and prevention (ed) Sarin SK and Singal AK 1996. 1996;. [Cited in This Article: ] |
| 6. | Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32 Suppl:S66-S81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Arslanoğlu I, Cetin B, Işgüven P, Karavuş M. Anti-HBs response to standard hepatitis B vaccination in children and adolescents with diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15:389-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Cook IF, Murtagh J. Comparative immunogenicity of hepatitis B vaccine administered into the ventrogluteal area and anterolateral thigh in infants. J Paediatr Child Health. 2002;38:393-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Abraham B, Baine Y, De-Clercq N, Tordeur E, Gerard PP, Manouvriez PL, Parenti DL. Magnitude and quality of antibody response to a combination hepatitis A and hepatitis B vaccine. Antiviral Res. 2002;53:63-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cassidy WM. Adolescent hepatitis B vaccination. A review. Minerva Pediatr. 2001;53:559-566. [PubMed] [Cited in This Article: ] |
| 11. | Elkayam O, Yaron M, Caspi D. Safety and efficacy of vaccination against hepatitis B in patients with rheumatoid arthritis. Ann Rheum Dis. 2002;61:623-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology (N Y). 1993;11:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 441] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Belloni C, Pistorio A, Tinelli C, Komakec J, Chirico G, Rovelli D, Gulminetti R, Comolli G, Orsolini P, Rondini G. Early immunisation with hepatitis B vaccine: a five-year study. Vaccine. 2000;18:1307-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Valenzuela P, Gray P, Quiroga M, Zaldivar J, Goodman HM, Rutter WJ. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979;280:815-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Elavia AJ, Marfatia SP, Banker DD. Immunization of hospital personnel with low-dose intradermal hepatitis B vaccine. Vaccine. 1994;12:87-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Looney RJ, Hasan MS, Coffin D, Campbell D, Falsey AR, Kolassa J, Agosti JM, Abraham GN, Evans TG. Hepatitis B immunization of healthy elderly adults: relationship between naive CD4 T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J Clin Immunol. 2001;21:30-36 DOI : 10.1023/A: 1006736931381. [Cited in This Article: ] |
| 17. | Bennett RG, Powers DC, Remsburg RE, Scheve A, Clements ML. Hepatitis B virus vaccination for older adults. J Am Geriatr Soc. 1996;44:699-703. [PubMed] [Cited in This Article: ] |
| 18. | Jilg W, Lorbeer B, Schmidt M, Wilske B, Zoulek G, Deinhardt F. Clinical evaluation of a recombinant hepatitis B vaccine. Lancet. 1984;2:1174-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Dentico P, Buongiorno R, Volpe A, Zavoianni A, Pastore G, Schiraldi O. Long-term immunogenicity safety and efficacy of a recombinant hepatitis B vaccine in healthy adults. Eur J Epidemiol. 1992;8:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Marsano LS, Greenberg RN, Kirkpatrick RB, Zetterman RK, Christiansen A, Smith DJ, DeMedina MD, Schiff ER. Comparison of a rapid hepatitis B immunization schedule to the standard schedule for adults. Am J Gastroenterol. 1996;91:111-115. [PubMed] [Cited in This Article: ] |