Copyright
©The Author(s) 2005.
World J Gastroenterol. Oct 14, 2005; 11(38): 5966-5972
Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5966
Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5966
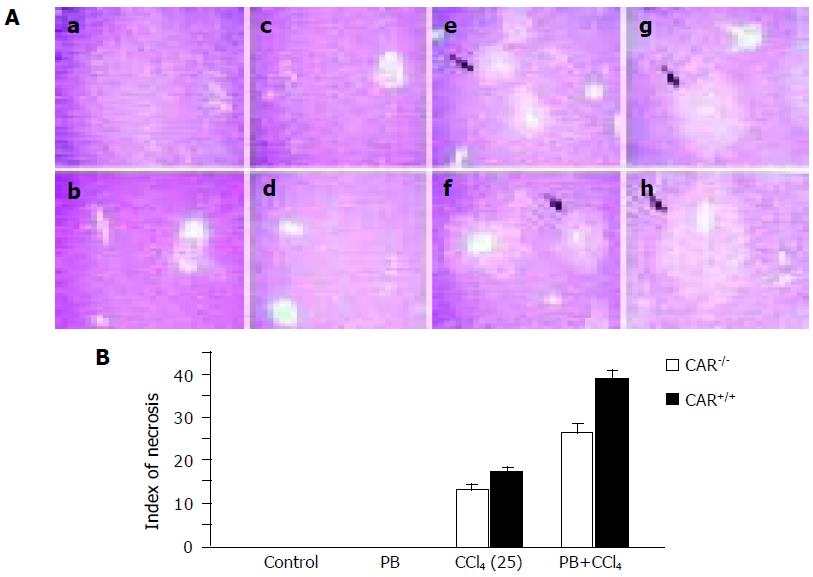
Figure 2 Histological changes associated with CCl4 toxicity.
(A) Histological findings in the liver; (a) CAR–/– mice, control; (b) CAR+/+ mice, control; (c) CAR–/– mice, PB; (d) CAR+/+ mice, PB; (e) CAR–/– mice, CCl4; (f) CAR+/+ mice, CCl4; (g) CAR–/–mice, PB plus CCl4; and (h) CAR+/+ mice, PB plus CCl4. Liver sections from each treatment were examined by hematoxylin and eosin staining. Liver samples from all treated animals were analyzed, but only representative histology is presented. The extent of the necrotic area in the CAR+/+ liver was larger than that in CAR–/– mice. PB pretreatment caused extensive centrilobular necrosis in CAR+/+ mice. The arrows indicate centrilobular necrosis. Magnification, ×100. (B) The indices of centrilobular necrosis in CAR+/+ mice and CAR–/– mice. The indices of centrilobular necrosis were higher in CAR+/+ mice than that in CAR–/– mice with PB pretreatment. The index in CAR+/+ mice treated with CCl4 plus PB was significantly increased. The index of centrilobular necrosis was scored as follows: area of centrilobular necrosis divided by whole area. Data are mean±SD.
- Citation: Yamazaki Y, Kakizaki S, Horiguchi N, Takagi H, Mori M, Negishi M. Role of nuclear receptor CAR in carbon tetrachloride-induced hepatotoxicity. World J Gastroenterol 2005; 11(38): 5966-5972
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/5966.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.5966