Published online Oct 7, 2005. doi: 10.3748/wjg.v11.i37.5807
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: October 7, 2005
AIM: To describe the imaging features of hepatic mesenchymal hamartoma with emphasis on magnetic resonance imaging (MRI) compared to histopathologic results.
METHODS: Spin-echo sequence(SE),fast spin-echo sequence(FSE) were detected in 12 children (7 males,5 females) with mesenchymal hamartoma of the liver (MHL), aged 1.2 months to 12 years,( mean age, 6.3 years) by axial, saggital, coronary plain imaging with an Elscint 2.0T MR equipment. Their main symptoms were abdominal mass (5 cases), enlarged liver (8 cases), abdominal pain (1 case) and anemia (2 cases), and negative alpha-fetoprotein. Dynamic enhancement examination was added in 2 cases.
RESULTS: Six cases had single mass type of MHL, in which 3 cases had solid masses showing slight low-signal-intensity in T1WI, and irregular high-signal-intensity in T2WI, 1 case had a cystic-solid mixed mass showing several border-clear cysts in a solid mass, 2 cases had cystic masses with multi-septa. Five cases had diffuse and multifocal lesions type of MHL with its signal intensity being similar to that of the solid mass. One case had a combined diffuse and single cystic mass. In the early dynamic enhancement examination, the lesions were slightly circum-enhanced , and the center was enhanced in the later scan images. Inner hepatic vessels were compressed in 5 cases, vena cava and abdominal aortae were compressed in 3 cases. Pathological findings included fiber hyperplasia, hyaline degeneration, biliary duct hyperplasia, lobule-like array.
CONCLUSION: MR imaging is a better way to differentiate and diagnose MHL. MHL may be recognized by its characteristic occurrence in infancy and MR imaging features.
- Citation: Ye BB, Hu B, Wang LJ, Liu HS, Zou Y, Zhou YB, Kang Z. Mesenchymal hamartoma of liver: Magnetic resonance imaging and histopathologic correlation. World J Gastroenterol 2005; 11(37): 5807-5810
- URL: https://www.wjgnet.com/1007-9327/full/v11/i37/5807.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i37.5807
Mesenchymal hamartoma of the liver (MHL) is one of the rare hepatic benign tumors in children, which was first reported by Edmanson in 1956. It is usually present under 5 years of age, mostly under 2 years of age. One hundred and fifty cases of MHL are reported in Britain, 30 cases in Japan, 21 cases in China[1-3]. A few reports have discussed the X-ray, ultrasound and CT characteristics of MHL[5-9]. MHL is often misdiagnosed as other pathological lesions, because of its diversified image characteristics. MR has been widely used in the diagnosis of MHL . We have reviewed 12 MHL cases with MR scan in our hospital since 1996. The MR imaging features can help recognize and diagnose MHL.
Twelve children with MHL (7 males and 5 females) were reviewed from January 1996 to 2002.They aged 1.2 months to 12 years with a mean age of 6.3 years.Their clinical manifestations are listed in Table 1.
| Age | Cases | Gender | Clinical manifestations | |||||
| Male | Female | Abdominalmass | Mega-liver | Abdominalpain | Anemia | Alphafetoproteinnegative | ||
| 1 mo¬12 mo | 3 | 2 | 1 | 1 | 3 | 3 | ||
| 1 yr–2 yr | 6 | 4 | 2 | 3 | 3 | 1 | 6 | |
| 3 yr–4 yr | 2 | 1 | 1 | 1 | 1 | 1 | 2 | |
| 12 yr | 1 | 1 | 1 | 1 | 1 | |||
MR imaging was performed with an Elscint 2.0T MR equipment. Patient was on his back, and chloral hydrate lysis was performed before scanning. Spin-echo sequence (SE) and fast spin-echo sequence (FSE) were performed with body coil. For T1-weighted MR imaging, the repetition time (TR) was 500 ms and the echo time (TE) was 15 ms. For T2-weighted imaging, the TR was 2500–3000 ms and the TE was 96 ms. Routine axial and coronary scan was performed 1-2 times. Dynamic enhancement examination was added in 2 cases, and CT scan was performed in 2 cases.
The pathologic types of MHL are shown in Table 2. The envelopes of most masses were intact in general. Sections of the solid type mass were white and tough. The fluid in the cystic type was clear or turbid and pale yellow or coffee-like.
| Cases | Hepatic lobes | Pathologic types | ||||
| Right | Left | Solid | Cystic | Mixed cystic-solid | ||
| Single | 6 | 5 | 2 | 3 | 2 | 1 |
| Diffuse and multifocal | 5 | 5 | 5 | 5 | ||
| Diffuse and single combined | 1 | 1 | 1 | 1 | ||
Fiber hyperplasia, hyaline degeneration, biliary duct hyperplasia and lobule-like array were seen. Lymphatic dilation was observed in a few cases. In crystic type, simple pavement epithelium or bile duct epithelium was seen at the inner layer of the cystic wall. The cyst contained fluid or gelatinoids. The separates consisted of mesenchyme and fibrostroma. In solid type, fibroadenoid structure consisting of remarkable hyperplastic biliary duct and fibrosis around it was seen. There were compacted hepatic tissues between the masses.
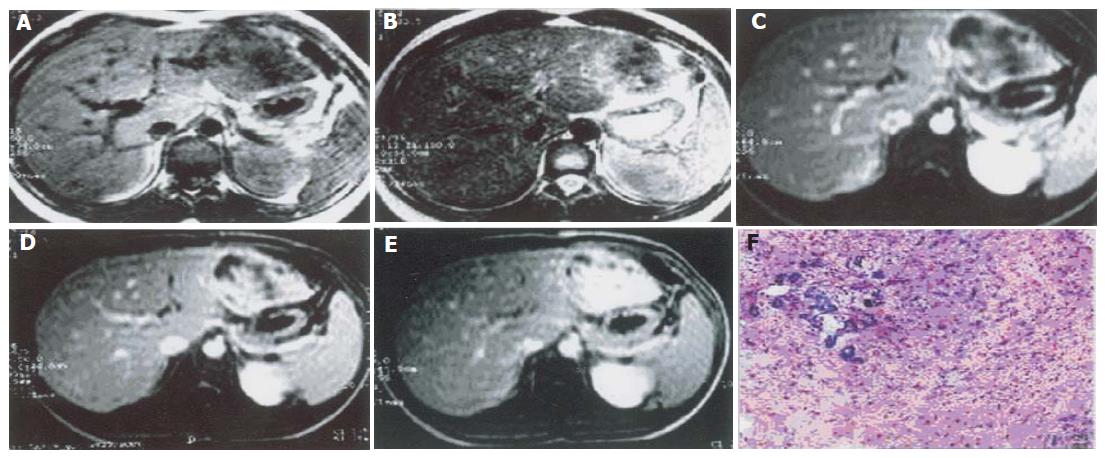
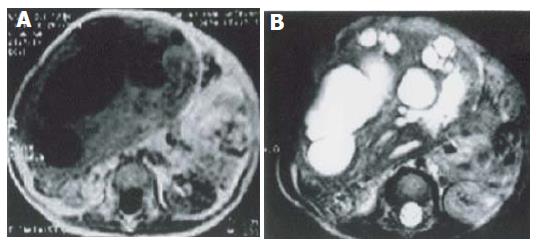
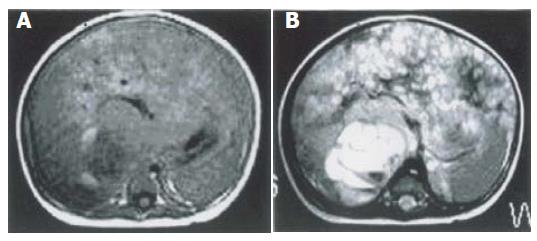
Single focal type of MHL was found in the right hepatic lobe of 5 cases, in the left hepatic lobe of 1 case with a big size and a clear border. The biggest one involved both lobes, with its diameter being 28 cm. Solid, cystic and mixed cystic-solid subtypes were classified based on the pathologic examination. In all lesions of solid subtype (n = 3), MR images showed a slightly low signal intensity on T1-weighted images, an irregular high signal intensity on T2-weighted images, and a higher signal intensity area inside. After intravenous administration of a gadolinium-based contrast agent, the lesions demonstrated mild annular enhancement in artery phase, moderate enhancement in portal phase, magnificent enhancement in delay phase (Figure 1). In the lesion of mixed cystic-solid subtype (n = 1), MR images showed a slightly low signal intensity on T1-weighted images, a slightly high signal intensity on T2-weighted images. There were several cystic foci with varying sizes and clear borders. The gallbladder-like signal intensity was scattered or congregated, but not syncretized (Figure 2).
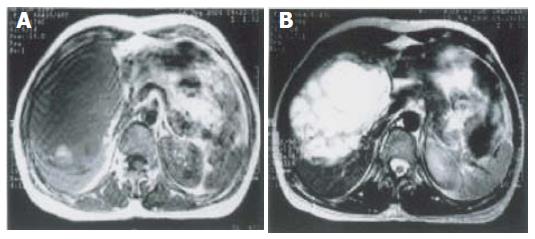
In the lesions of cystic subtype (n = 2), MR images showed multi-cyst foci with varying sizes and thickness, low or normal signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and varying signal intensity in different cysts, fluid-fluid plane in some cysts (Figure 3).
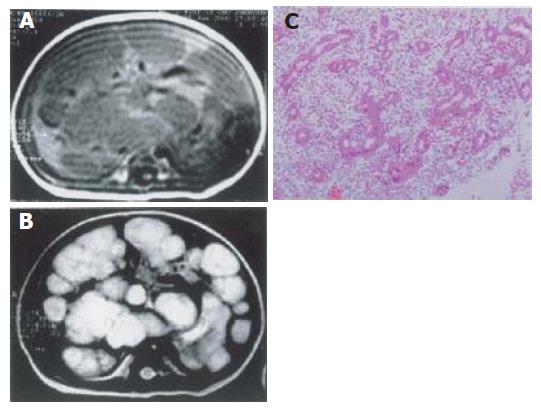
Diffuse and multifocal type of MHL was found in 4 case. MR images showed enlarged liver with smooth border, multiple foci with slightly low signal intensity on T1-weighted images, slightly high signal intensity on T2-weighted images. After intravenous administration of a gadolinium-based contrast agent, the lesions demonstrated annular enhancement in artery phase, no enhancement in early stage, enhancement in delay phase. In 1 case, MR images showed multifocal masses in the liver, slightly low and normal signal intensity on T1-weighted images, very high signal intensity on T2-weighted images. The cotton-like masses with clear borders, did not syncretize each other (Figure 4).
Combined diffuse and single type was found in 1 case. MR images showed big isolate multi-loculus cystic foci in the diffuse tubers, fluid-fluid plane in some cysts (Figure 5).
In the multifocal type, CT images showed enlarged liver, multifocal low density foci of varying sizes from 1 cm × 1 cm to 2 cm × 3 cm, lower density in the center. In the single focal type, CT images showed low density focus round in shape with a clear border.
Inner hepatic vessels were compressed in 5 cases, vena cava and abdominal aortae were compressed in 3 cases. No portal vein was compressed in all the 12 cases.
MHL is a rare hepatic benign tumor, occurring most in children and rarely in adults[4]. MHL in fetus has also been reported[1].
The exact pathologenesis of MHL is not clear. It has been reported that MHL is a kind of developmental deformity or the result of abnormal reaction procedure[8]. The abnormality appears in the late stage of embryo formation that grows along the portal vein system and surrounds the hepatic tissue island when the liver maintains lobular structure connecting to bile duct tree[7]. It was reported that MHL comes from the local area of bile duct atresia, inducing obstruction of distant bile ducts and necrosis of hepatic cells[5]. Some studies showed that MHL correlates with the developmental deformity of vessels and the rupture of 19q13.4[12,13]. It was reported that most of MHLs are diploid, but the set of chromosomes of MHL prompt that MHL is not the developmental deformity or the result of abnormal reaction procedure[7,12].
MHL has no special clinical manifestations. The most common symptom is an abdominal mass or an enlarged liver. The mass increases rapidly in specific cases, leading to inappetence, obvious dyspnoea or obvious obstruction of inferior vena cava. Other symptoms are fever, vomit, constipation, diarrhea and weight loss[6]. Malnutrition, anemia and cachexia are occasionally found . AFP is normal. Some reports indicates that AFP increases in the infancy under 4 months of age[1]. Surgical operation is the best choice of treatment.
Solid type of MHL may have a single focus or multiple foci. In our study MHL was located at the right lobe of liver in 5 of 6 cases of solid type, being consistant with other reports[6,8]. In the solid part of MHL, there were remarkably proliferated bile ducts and fibrosis around them to form fibroglandular structure. In 1 multifocal solid MHL, there was fluid retention in mesenchymal tissue (Figure 4). The higher the protein content in the fluid, the higher the signal intensity on T1-weighted images. Some studies showed that the solid part of MHL is formed during perinatal period. The mass enlarges rapidly, because of mesenchyme cystic degeneration with fluid retardation, obstruction and dilation of lymph duct or both[8]. With the growth of MHL, the cystic area in the mass enlarges significantly, taking up greater part of the lesion, because of mesenchyme atrophy, bile duct dilation, lymph retardation. Therefore, the lesion may be solid-cystic type or cystic type, which may be its pathological basis (Figure 3). There is no blood supply for MHL[6,8]. The masses shift the vessels in angiography. The septa are thick when there are more solid contents. There are areas surrounded by tumor vessels with no blood supply. Enhanced CT images can show cord-like enhancement[3]. We consider that the lesions with no blood supply may be cystic type, the lesions with less blood supply may be solid type. The part of annular enhancement may compress the hepatic tissue around the lesions. Intrahepatic vessels, inferior vena cava, abdominal aorta and other organs are compressed and shifted by huge MHL masses[1] with blood vessels or peripheral tissues invaded. Calcification is seldom found in MHL, but septum calcification is reported[9].
MHL seldom cancerates, but canceration can be found in specific cases[10]. In this research, no case was cancerated. Surgical operation is the best choice of treatment. MHL seldom relapses, and its prognosis is good. Surgery should be executed after diagnosis.
Due to the different imagings of MHL, it should be differentiated from hepatic abscess, metastasis, caroli’s disease, undifferentiated embryo sarcoma of the liver and hepatoblastoma.
In conclusion, MR imaging is a better way to differentiate and diagnose MHL. MHL may be recognized by its characteristic occurrence in infancy and MR imaging features.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Kamata S, Nose K, Sawai T, Hasegawa T, Kuroda S, Sasaki T, Okada A, Tawara M. Fetal mesenchymal hamartoma of the liver: report of a case. J Pediatr Surg. 2003;38:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yen JB, Kong MS, Lin JN. Hepatic mesenchymal hamartoma. J Paediatr Child Health. 2003;39:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Górnicka B, Ziarkiewicz-Wróblewska B, Wróblewski T, Koperski Ł, Pawlak J, Nyckowski P, Krawczyk M, Zimmermann A, Wasiutyński A. Myoid hamartoma of the liver--a novel variant of hamartoma developing in the hilar region and imitating a malignant liver tumor. Med Sci Monit. 2004;10:CS23-CS26. [PubMed] |
| 4. | Cook JR, Pfeifer JD, Dehner LP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum Pathol. 2002;33:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Wholey MH, Wojno KJ. Pediatric hepatic mesenchymal hamartoma demonstrated on plain film, ultrasound and MRI, and correlated with pathology. Pediatr Radiol. 1994;24:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ros PR, Goodman ZD, Ishak KG, Dachman AH, Olmsted WW, Hartman DS, Lichtenstein JE. Mesenchymal hamartoma of the liver: radiologic-pathologic correlation. Radiology. 1986;158:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | DeMaioribus CA, Lally KP, Sim K, Isaacs H, Mahour GH. Mesenchymal hamartoma of the liver. A 35-year review. Arch Surg. 1990;125:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Brunt EM. Benign tumors of the liver. Clin Liver Dis. 2001;5:1-15, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Konez O, Goyal M, Vyas PK, Boinapally SB. Mesenchymal hamartoma of the liver. Comput Med Imaging Graph. 2001;25:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ramanujam TM, Ramesh JC, Goh DW, Wong KT, Ariffin WA, Kumar G, Taib NA. Malignant transformation of mesenchymal hamartoma of the liver: case report and review of the literature. J Pediatr Surg. 1999;34:1684-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Otal TM, Hendricks JB, Pharis P, Donnelly WH. Mesenchymal hamartoma of the liver. DNA flow cytometric analysis of eight cases. Cancer. 1994;74:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Bove KE, Blough RI, Soukup S. Third report of t(19q)(13.4) in mesenchymal hamartoma of liver with comments on link to embryonal sarcoma. Pediatr Dev Pathol. 1998;1:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Mascarello JT, Krous HF. Second report of a translocation involving 19q13.4 in a mesenchymal hamartoma of the liver. Cancer Genet Cytogenet. 1992;58:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |