Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5423
Revised: March 6, 2005
Accepted: March 9, 2005
Published online: September 21, 2005
In this article, we have reviewed the main therapeutic measures for the treatment of Zollinger-Ellison syndrome (ZES). Review of the literature was based on computer searches (Pub-Med, Index Medicus) and personal experiences. We have evaluated all the measures now available for treating patients with sporadic gastrinomas or gastrinomas associated with Multiple Endocrine Neoplasia Type 1, (MEN 1) including medical therapy such as antisecretory drugs and somatostatin analogs (SST), chemotherapy and chemoembolization, and surgical procedures. In ZES patients, the best therapeutic procedure is surgery which, if radical, can be curative. Medical treatment can be the best palliative therapy and should be used, when possible, in association with surgery, in a multimodal therapeutic approach.
- Citation: Tomassetti P, Campana D, Piscitelli L, Mazzotta E, Brocchi E, Pezzilli R, Corinaldesi R. Treatment of Zollinger-Ellison Syndrome. World J Gastroenterol 2005; 11(35): 5423-5432
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5423.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5423
The Zollinger-Ellison Syndrome (ZES) represents a clinical, diagnostic and therapeutic challenge. In this paper, we have reviewed the recent advances on this fascinating syndrome. One MEDLINE (PubMed) search, was carried out using only MESH terms with the following strategy: Therapy AND (gastrinoma OR Multiple Endocrine Neoplasia Type 1 OR Zollinger-Ellison Syndrome). No limits were set and 4 369 papers were found. Only those papers in-extenso were considered. The authors reviewed all the papers and other papers or chapters in the published book were also manually searched in the references of the papers reviewed, if considered necessary for the present review.
The ZES, as described in 1955, is characterized by peptic ulcers of the upper gastrointestinal tract refractory to medical therapy, diarrhea and severe gastric acid hypersecretion associated with non-beta islet cell tumors of the pancreas[1]. It was later demonstrated that this pancreatic tumor, named gastrinoma, ectopically releases gastrin, which is responsible for the gastric hypersecretion. More than 80% of gastrinomas are localized in the anatomic area called the triangle of the gastrinomas. This triangle includes the convergence of the cystic duct and the common bile duct, the junction of the second and third portion of the duodenum, and the junction of the head and body of the pancreas[2-5]. Most gastrinomas arise from the duodenum (about 75%), whereas they are localized in the pancreas in 25% of cases[4]. Occasionally gastrinomas occur in the ovary, mesentery, liver, bile duct, gastric antrum/pyloric area, renal capsule, and in the jejunum[6,7]. Furthermore, extra-abdominal primary gastrinomas in the heart[8,9] and gastrin-releasing lung tumor causing ZES have recently been described[10].
The structure can be glandular or trabecular, and the cells are round with small nuclei and prominent nucleolus. The cells which produce gastrin are generally well-differentiated and contain the histological markers typical of neuroendocrine tumors, i.e., chromogranin A, neurospecific enolase and synaptophysin. These tumors are positive at immunohistochemistry not only for gastrin but, frequently, also for other peptides, including the pancreatic peptide (PP), somatostatin (SST), the adrenocorticotrophic hormone (ACTH) and the vasoactive intestinal peptide (VIP).
This tumor presents itself at various ages from 7 to 90 years, but the diagnosis is usually made between the ages of 30 and 50 years. There is no clear sex prevalence even if 60% of the patients affected are men[11-14].
Gastrinomas may behave in a benign or malignant manner, but usually, even when malignant, they can be slow growing. Numerous studies support the conclusion that gastrinomas pursue an aggressive growth pattern in 20-30% of cases and an indolent growth pattern in the rest; this seems to be independent of the presence or the absence of positive lymph nodes[3,15,16]. The most common site of metastases are the local lymph nodes, followed by the liver; these metastatic lesions, unlike those of adenocarcinomas, are vascular. Bone metastases are common in advanced disease, probably occurring in the majority of patients[17]. The percentage of patients presenting a malignant form of sporadic ZES is not clear; some data in the literature report that about 65% of gastrinomas are malignant, but perhaps this is overestimated[18]. Furthermore, it has been suggested that a poor prognosis is associated with the development of ectopic Cushing’s syndrome, reported in up to 5% of patients with ZES[19].
The gastrin secreting tumor responsible for ZES is the most common tumor in patients affected by Multiple Endocrine Neoplasia type 1 (MEN 1).
MEN 1 is an autosomal dominant inherited syndrome characterized mainly by the development of hyperplasia and/or multiple tumors in the parathyroid, endocrine pancreas, duodenum, anterior pituitary, foregut-derived neuroendocrine tissue, and adrenocortical glands. This syndrome, first described by Wermer in 1954, has a prevalence of 0.2-2/100 000 people/year, even if this is underestimated since, generally, the endocrinopathies which constitute this disease are not adequately classified[20,21]. MEN 1 is associated with inactivating mutations of a tumor suppressor gene (MEN1), mapped on chromosome 11q13 in 1988[22]. The MEN 1 gene was sequenced in 1997 and was found to encode for a transcriptional regulator, named “menin”[23]. In MEN 1 patients, one allele of the gene is inactivated by the germline mutation, and phenotype expression occurs with inactivation of the second allele by somatic cellular mutation. Somatic mutations of the MEN 1 gene have also been reported in sporadic forms of endocrine tumors with a variable incidence of 20-30% in the parathyroid, the endocrine pancreas (33% gastrinomas, 17% insulinomas), 25% in lung carcinoids, but less than 1% in pituitary and adrenocortical tumors[24-27].
In clinical practice, it is important to perform genetic analysis in order to diagnose MEN 1, but the diagnosis cannot be excluded with certainty, when a mutation is not found[28]. Therefore, the clinical screening of patients remains a prerequisite for genetic analysis. Screening for MEN 1 is a crucial step in the diagnosis and the management of neuroendocrine tumors, because prognosis and treatment may differ from the sporadic case.
Gastrinoma with ZES is found in 20-61% of patients with MEN 1[29,30]; conversely, MEN 1 is found in 30-38% of all patients with gastrinomas[30-32]. In MEN 1, gastrinomas are mainly localized in the duodenum, they are small and frequently multifocal and may therefore be difficult to detect either by traditional imaging methods or at surgery[33,34].
ZES-MEN 1 is associated with gastric carcinoids, also called ECL-omas (type 2 carcinoids), due to chronic effect of hypergastrinemia on ECL cells. These tumors seem to be found in 13-30% of patients with ZES-MEN 1 and in 0-0.6% of patients without MEN 1[35,36]. ECL-cell tumors in MEN 1 are often multicentric and are generally characterized by a low mitotic index; most of these tumors are less than 1 cm in diameter and are limited to the mucosa and submucosa[37]. The percentage of patients in whom these tumors are invasive is unknown; metastases to local lymph nodes can be observed, but patient survival is usually excellent with tumor persistence for more than 25 years and very few related deaths[38].
The signs and symptoms of ZES are primarily due to gastric acid hypersecretion. The most common initial symptom is abdominal pain and 90-95% of patients develop peptic ulcers in the upper gastrointestinal tract. In 36% of cases, the ulcers are multiple or in an unusual site; however, there is a percentage of patients with ZES (from 18% to 25%) who do not have ulcers at diagnosis. The ulcer can appear as a common duodenal ulcer, but it is usually less responsive to therapy with respect to ulcers in patients without gastrinomas. The most common is a single ulcer of the duodenal bulb which can also appear in the second, third, or fourth portion of the duodenum or in the jejunum[39].
Gastroesophageal reflux, present in two-thirds of patients with ZES, can vary from light to severe forms and can be complicated by stenosis or Barrett’s mucosa[39]. Diarrhea is the second most common symptom, developing in 50-65% of patients, and can precede, accompany or follow the ulcerous disease; in 7-35%, it may be the only initial symptom[39]. The diarrhea is due to massive acid hypersecretion which activates pepsinogens which are responsible for mucosal damage; in addition, acid inactivation of the pancreatic enzyme and acid damage to enterocytes also contribute to it. Steatorrhea is a result of inactivation of pancreatic lipase by intraluminal acid in the upper small intestine; the low pH also renders some primary bile acids insoluble, reducing the formation of micelles, which are needed for absorption of fatty acid and monoglycerides[3].
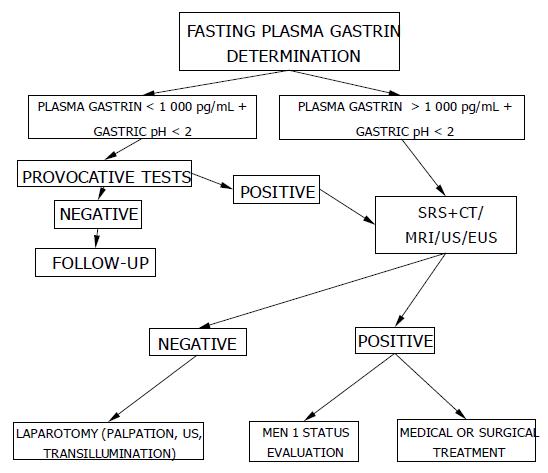
The diagnosis of ZES is based on the finding of elevated fasting serum gastrin associated with gastric acid hypersecretion. Other conditions exist in which gastrin levels can be elevated; these are hypochlorhydria in the course of chronic atrophic gastritis, the prolonged use of proton pump inhibitors or the presence of Helicobacter pylori (H pylori) infection. When the diagnosis is not completely clear, a reliable test is the measurement of basal acid secretion (BAO) or, alternatively, it can be useful to determine the pH of the gastric juice which can be obtained during endoscopy or through a nasogastric tube. If the gastric pH is >2 in the absence of antisecretory therapy, the patient is hypochlorhydric and, therefore, ZES can be excluded; if the pH is <2 and the plasma gastrin is higher than 1 000 pg/mL, the diagnosis of ZES is certain while, if the pH is <2 and the plasma gastrin concentration is between 100 and 1 000 pg/mL, a secretin test must be carried out[40]. The secretin test is performed by the administration of 2 IU/kg of secretin intravenously in 2 min, after having taken a blood sample in order to perform the basal gastrin concentration; after the injection, blood samples are taken after 2.5, 5, 10, 15 and 30 min. An increase of plasma gastrin >200 pg/mL is diagnostic of ZES. The test can give a false positive result in some patients with Type A chronic atrophic gastritis, but this is identified by measuring the gastric pH[41]. The secretin test can also give a false negative result in 10% of patients with ZES, and this is usually associated with a malignant course of the disease[42]. The calcium test might be of value in those patients in whom ZES is strongly suspected when the secretin test is negative. There is agreement in the literature that calcium and meal tests are less useful than secretin for detecting ZES.
To localize and stage gastrinomas, conventional non-invasive and occasionally invasive diagnostic modalities may be required. At present, the most important study should be somatostatin receptor scintigraphy (SRS) using 111In-pentetreotide with single photon emission tomography (SPECT) scanning[4]. Recent studies demonstrated that SRS allows total body localization study simultaneously at one time, thus allowing detection of liver and distant metastases and it is more sensitive in both localizing the primary gastrinoma and identifying patients with liver metastases than conventional methods such as CT, MRI, US[43]. Unfortunately, SRS detects 30-75% of gastrinomas measuring less than 1 cm, primarily duodenal gastrinomas; a negative scan in the presence of a visible tumor suggests either a poorly-differentiated neuroendocrine tumor or a pancreatic adenocarcinoma[44].
Ultrasonography can detect gastrinomas in 30% of cases and the detection rates are better for lesions greater than 3 cm in diameter and are poor for lesions smaller than 1 cm[45].
Using EUS, the sensitivities as high as 79-82% can be obtained[46,47]. A recent prospective study found a sensitivity of 93% and a specificity of 95% in the localization of intrapancreatic lesions[48]. EUS, in combination with SRS up to 69%, although it may still miss up to 50% of duodenal gastrinomas[49]. Therefore, when a gastrinoma is suspected, endoscopy or EUS should also be performed to identify duodenal as well as pancreatic gastrinomas, especially when multiple lesions are suspected as in MEN 1[33,34].
Regarding CT and MRI, the most recently published series showed that CT with bolus of contrast administration can achieve sensitivities ranging from 82% to 92%[50,51] and MRI, using T1-weighed fat suppression images, has a 91% sensitivity[52,53]. A comparative study demonstrated that MRI is equivalent to dynamic CT. Furthermore, MRI is considered the most sensitive technique for demonstrating liver and bone metastases in patients with neuroendocrine tumors and is recommended for monitoring the response to therapy. However, CT and MRI are both very sensitive and specific methods for demonstrating hepatic metastases and pancreatic tumors, and they show specific advantages in routine tumor staging and monitoring of therapy[54,55].
Functional studies, measuring hormonal gradients by transhepatic portal venous sampling, seem to be more sensitive; however, this method is invasive, and does not lead to the exact location and requires considerable expertise. A modification of the hormonal gradient during angiography has been developed; in brief, secretin is injected intra-arterially selectively into different vessels and hepatic venous samplings are collected and assayed for hormonal gradients. Selective angiography can identify the site of the tumor in up to 75% of cases, but has largely been supplanted by the combination of Octreoscan, EUS and CT[56,57].
In about 20% of patients, a gastrinoma cannot be identified by conventional imaging techniques and SRS[58]. In these cases, during laparotomy, operative techniques such as duodenal transillumination and intraoperative ultrasound can be used[4].
A suggested algorithm for the diagnosis of ZES is reported in Figure 1.
In patients with ZES, the two main principal therapeutic objectives are to control the gastric acid hypersecretion which causes the most debilitating symptoms (ulcers, diarrhea and dehydration) and to control the growth of the tumor which, even if slow-growing, is able to produce early and diffuse hepatic metastases.
Until the end of the 1970s, the only effective therapy for controlling acid hypersecretion was total gastrectomy. Currently, after the introduction of potent antisecretory drugs, such as H2 antagonists and proton pump inhibitors, the most important aspect for these patients is the growth of the neoplasm. In fact, half of the patients die from causes related to the tumoral mass and not from the effects of the massive gastric acid hypersecretion[59]. Thus, at present, a large number of treatment options are available for the therapy of patients with ZES: control of the gastric acid hypersecretion; control of the gastrin hypersecretion; surgical resection or cytoreduction of the tumor; control of the tumor size by using chemotherapy or interferon alfa; control of the hepatic metastases with chemoembolization and/or embolization.
If ZES is suspected, it is important, while waiting for the conclusive results of the diagnostic tests, to prevent the complications which could arise. For this reason, it is advised to rapidly start an antisecretory therapy which is usually well-tolerated and without particular contraindications.
Since proton pump inhibitors have been available commercially, H2 receptor antagonists are no longer the drugs of choice for patients with ZES, even if they have actually been proven effective in alleviating the symptoms and healing ulcers in gastrinomas. Ranitidine is the drug of choice among the H2 antagonists, due to its low side effects and its limited interaction with other drugs. However, despite the first excellent results, long-term studies have shown that the use of these drugs is limited by many factors such as poor control of the gastric acid hypersecretion and sometimes the need for high and frequent doses of the drug[60]. The pharmacological basis of these observations is unknown, even if various possibilities have been suggested, such as a diminished bioavailability due to reduced absorption, an altered metabolism of the drug with increased excretion, the development of tachyphlaxis and the hypersecretory state itself[61]. The poor effectiveness of H2 antagonists is usually evident in ZES associated with severe gastroesophageal reflux, MEN 1 with hyperparathyroidism and partial gastric resection[62].
Omeprazole, the first proton pump inhibitor available, belongs to a new class of drugs which has proven to be safe and effective in controlling gastric hypersecretion in patients with ZES and has completely substituted the use of H2 antagonists. The PPIs commonly used are omeprazole, lansoprazole, pantoprazole, rabeprazole and esomeprazole[63]. These drugs show differences in pharmokinetics and pharmodynamics, but it is not always clear, if these fine variations have clinical importance[64].
The goal of ZES therapy is to obtain improvement of the symptoms and healing of the ulcers by controlling the gastric hypersecretion. Various studies have shown that a reliable criterion for the control of the hypersecretion is the reduction of the BAO below 10 mEq/h in the hour preceding the next administration of the drug in cases of uncomplicated ZES and below 5 mEq/h in cases of ZES associated with MEN 1, gastro-esophageal reflux disease or in patients undergoing a partial gastrectomy[2,65].
Using the acute upward dose titration method, widely accepted for establishing the initial dosage of omeprazole, some studies have demonstrated that the average dosage of omeprazole capable of controlling gastric hypersecretion in the majority of patients is between 60 and 100 mg of the drug per day[66]. The only reliable parameter able to demonstrate the absence of damage to the mucosa is the level of acid inhibition when the “steady state” has been reached; therefore, the BAO must be measured every 3-4 wk and the patient should be evaluated by endoscopy and acid secretion analysis at intervals from 3 to 6 mo and, successively, from 6 to 12 mo. The objective of the therapy is not achlorhydria, but a BAO between 1 and 10 mmol/h; if complete inhibition of the acid hypersecretion is seen, the dosage of omeprazole should be reduced by 50% and the patient should be re-evaluated. If the BAO is greater than 10 mmol/h, the dosage of the PPI should be increased gradually and, for doses of omeprazole over 60 mg (or equivalent doses of another PPI), it would be advisable to administer the drug in two divided doses. The lack of control of gastric acid secretion could be a high risk for patients affected by ZES[66,67] and some data in the literature have demonstrated that an initial dose of 20 mg is not able to control the acid secretion and the symptoms linked to the disease[68].
Many data have been reported on the effectiveness and safety of the PPIs, when these drugs are administered on a long-term basis in patients with ZES. After having established the initial dosage and having monitored the gastric secretion, the long-term maintenance dosages can be reduced significantly in the majority of patients, when control of the gastric secretion is stabilized[69,70]. This can be easily explained by the fact that the effectiveness of omeprazole increases with time[70-72]. In fact, Metz et al [70], have shown that the maintenance dosages of omeprazole can be partially or completely reduced in all patients with ZES, above all in those who are not affected by MEN 1, serious gastro-esophageal reflux or those who have undergone a partial gastrectomy. Some studies have shown that, in the long-term, it is not necessary to increase the dosage of omeprazole; if, however, during the first year, repeated increments in therapy are necessary, it means that the initial dose was inappropriate and there was no progressive development of pharmaceutical resistance[73]. While the H2 antagonists have demonstrated the phenomenon of tachyphylaxis with prolonged use, only in 10% of patients is it necessary to increase the dose of the PPIs[74]. PPIs have been proven to be safe and effective in the maintenance therapy of patients with ZES for more than 10 years, without any effect of related toxicity[65]. It does not seem that treatment with PPIs in ZES has induced any significant increase in the concentration of plasma gastrin or alterations in the percentage of argyrophil cells[75].
Patients affected by ZES who are not able to take PPIs orally and who need a therapy which primarily suppresses gastric acid secretion; patients with active peptic ulcer, recent hemorrhage and endoscopic stigmata that puts them at high risk of rebleeding, are candidates to receive PPIs intravenously. Another possible means of administration of solutions prepared with oral PPIs is intragastric, which can be utilized as an alternative to the intravenous route in critically ill patients or in whom esophageal stenosis is present.
During prolonged treatment with proton pump inhibitors, reduced serum levels of vitamin B12, but not of folate, have occasionally been documented[76]. This phenomenon seems to be related to the achlorhydria induced by the drug which, moreover, is not common during therapy with PPIs.
Some studies have considered the interactions between ZES, H pylori and PPIs, pointing out how the Helicobacter infection does not constitute a risk factor for the onset of peptic ulcers in these patients. In fact, the prevalence of H pylori infection in these subjects is lower than in the overall population and is much lower than in patients with common peptic ulcer disease. Indeed long term PPIs therapy in HP-positive patients with ZES may lead to the reduction of parietal cell mass[77].
Somatostatin analogs It is well-known that somatostatin and its analogs are able to reduce gastric acid and serum gastrin levels in patients with ZES, with both short-term and long-term administration[78]. In addition to immediate-release subcutaneous octreotide, other long-acting somatostatin analogs such as lanreotide, which can be administered every 10-14 d, and octreotide LAR, which can be administered every 28 d, are currently available on the market[79,80]. In 1993, Rusniewsky noted an average decrease in serum gastrin of 87% in patients with ZES treated with octreotide subcutaneously for a period of 9–12 mo. It was also seen that octreotide was capable of controlling gastric hypersecretion without an “escape phenomenon” and was able to reduce, and sometimes eliminate, the hyperplasia of the ECL cells when this was present before treatment[81]. In the same period, Bordi reported that prolonged treatment with octreotide is able to significantly decrease the fundic argyrophil cells in patients with a gastric pathology[82].
Thereafter, several studies have shown that treatment with somatostatin analogs has an inhibiting effect on tumoral growth in patients with malignant gastrointestinal neuroen-docrine neoplasias, such as pancreatic tumors or carcinoids, and is able to stabilize the tumoral growth in 37-80% of patients; in only a few patients (0-17%), a reduction of the tumor dimensions has been observed[83,84].
Moreover, up to now, only one study on the effectiveness of octreotide in controlling tumoral growth in patients with metastatic gastrinomas has been carried out. In this study, performed on 15 patients, it was shown that, in 53% of these patients with malignant gastrinomas, the tumoral growth progressed, 47% of patients presented stabilization and 6% had a reduction of the tumor size[85]. In patients who responded to treatment, stabilization of the tumor was long lasting and the incidence of side-effects was lower in comparison to the group treated with chemotherapy. In this study, it was concluded that, in gastrinomas, somatostatin analogs are not the drugs of first choice in controlling gastric acid hypersecretion, but are very useful in controlling tumoral growth.
The most common side-effects of somatostatin analogs are gallstones, abdominal pain, diarrhea and pain in the injection site.
Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Try3-octreotide for neuroendocrine tumors has recently been proposed as a palliative treatment; this modality of treatment can, however, cause myelotoxicity and nephrotoxicity[86]. In the past 3 years, several studies have been published on the use of this therapy in neuroendocrine tumors associated with syndromes, using not only ytrium but also lutetium which seems to be less toxic for the kidneys[87]. As regards the therapy for gastrinomas, a regression of metastases in 25% of the patients has been described[88].
As previously reported, long lasting somatostatin analogs have been shown to be capable of reducing the mass of gastric endocrine cells[89]; furthermore, it has been demonstrated that these drugs can inhibit growth in hypergastrinemic experimental models which develop gastric carcinoids[90,91]. This evidence supporting the use of octreotide can be utilized in patients with gastric carcinoids. These data have been confirmed in a recent study of our group which showed the regression of gastric carcinoids associated with ZES-MEN 1 after 1 year of treatment with long acting somatostatin analogs[92].
Surgery The role of surgery in the treatment of ZES has evolved considerably from 1955 to now[1]. Initially, the surgical therapy proposed was total gastrectomy with the aim of removing the high levels of gastrin from the target organ[93]; in the past 20 years, improved pharmacological control of gastric acid hypersecretion has eliminated the role of surgery in containing the hypersecretion itself. The availability of PPIs allows us to adequately control the symptoms in all patients with ZES, making the natural history of gastrinomas, the only determining factor in long-term survival[94].
Since adequate medical therapy is able to eliminate the symptoms in all patients and, although it is hard to treat the neoplastic disease with chemotherapeutic drugs, some authors claim that the risks and complications of exeresis of the gastrinoma exceed the actual benefits of this procedure[58]. More recently, however, some others have reported that surgical excision of a gastrinoma has proven highly effective in order to prevent hepatic metastases[93,95]. At present, surgical exploration plays a key role in identifying and removing the primary tumor, and in preventing the onset of liver metastases.
The main objectives of surgery are to stage the tumor, improve survival by removing the malignant tumor and control the acid hypersecretion syndrome. Since the majority of patients with ZES present either with occult disease or with localized disease, the surgeon has to search for and remove the non-localized lesion laparotomically using imaging techniques. Furthermore, intra-operative techniques such as palpation, ultrasonography and transillumination of the duodenal wall can contribute to the identification of pancreatic and duodenal gastrinomas, including lymph nodes and hepatic metastases. It is important to consider that a large percentage of primary occult tumors are found inside the duodenum. Gastrinomas commonly metastasize in the lymph nodes and sometimes arise in this site; hence, the resection of all the lymph nodes in the peripancreatic region is important, even if they do not appear swollen. Exploration of the liver and the rest of the abdomen is also recommended in order to identify possible metastatic disease[96].
It has also been reported that more than 50% of patients are free from disease after surgery and the majority of them remain so for the following 5-10 years[93,95].
Gastric acid hypersecretion caused by hypertrophy of parietal cells can persist, after surgery, despite biochemical evidence of cure; for this reason it is very important for all patients to continue maintenance, antisecretory therapy until the BAO determination is less than 10 mEq/h[97]. In those patients in whom there is a persistence of neoplastic tissue or post-operative relapse, debulking the tumor often leads to a proven benefit[95].
Liver transplant is characterized by a remarkable morbidity and, for the most part, post-operative immunosuppressive therapy stimulates the relapse of growth of the tumor[98]. A careful evaluation of the data found in the literature shows that a non-carcinoid histotype is present among the exclusion criteria for liver transplants in patients with hepatic metastases and neuroendocrine tumors. In this regard, French authors, in a retrospective study, have pointed out an unfavorable prognosis in patients with non-carcinoid endocrine tumors undergoing a liver transplant[99].
Surgery in ZES associated with MEN 1 The indications for surgery in patients with ZES-MEN 1 are controversial, since previous experience has shown that surgery rarely cures these patients[100,101]. Some authors have carried out an exploratory laparotomy on patients with ZES-MEN 1 in which the tumor, studied using imaging techniques, had a diameter greater than 2.5 cm. A direct relationship between the size of the tumor and the onset of hepatic metastases has been hypothesized and, as in other malignant solid tumors, the effectiveness of the cure is expected to be greater, if the tumors are resected, when they are still small[36,102].
In a recent study, a group of Swedish researchers established that the best surgical procedures include enucleation of the tumor in the head of the pancreas, excision of duodenal gastrinomas together with the removal of the metastasized lymph nodes and, as prophylaxis of tumoral relapse, the combination of these procedures with an 80% subtotal resection of the distal pancreas. It is thought that a more extensive resection of the tumor reduces the risk of the malignant evolution of pancreatic and duodenal tumors[101].
In patients with ZES-MEN 1, hyperparathyroidism is usually present at the moment of diagnosis[103]; therefore, in patients with hypercalcemia, a parathyroidectomy should be performed before any other surgical procedure in order to better control the pathological effects of hypercalcemia. It has been demonstrated that a parathyroidectomy is capable of reducing the fasting serum levels of gastrin and the BAO, and this, therefore, improves the response to the antisecretory therapy[32].
Systemic chemotherapy Therapeutic strategies for the management of patients with metastatic gastroenteropancreatic endocrine tumors have to take into consideration the fact that controlling the hormone-mediated symptoms often improves the quality of life, to the point that the patients feel well despite the extensive metastatic disease[104]. At best, chemotherapy has furnished only unclear results utilizing the classical anti-tumoral agents, such as 5-fluorouracil, in controlling metastatic endocrine tumors. Systemic chemotherapy reached a new therapeutic dimension immediately after the introduction of streptozotocin (STZ) into clinical use[105]; there have been several studies on the effects of this drug on metastatic islet-cell tumors which have confirmed the effectiveness of streptozotocin. Despite this, there are a number of acute and chronic side effects, such as serious renal or hematologic toxicity[106]. Various studies have standardized the dosage and administration schedule of streptozotocin and have suggested its combination with other cytotoxic drugs such as 5 fluorouracil (5-FU) and doxorubicin (adriamycin); the combination of STZ and doxorubicin has been superior to other regimens, mainly STZ and 5-FU, and seems to be associated with a 69% objective response lasting for 18 mo and overall median survival of 2.2 years[107].
In a detailed study conducted on 10 patients with metastatic gastrinomas and treated with streptozotocin, 5-fluorouracil and doxorubicin, 4 patients showed a partial response to the treatment, but the survival rate was the same both in the 4 responders and in the 6 non-responders[108]. Another type of chemotherapy proposed is monotherapy with chlorozotocin, a nitrosourea having a composition similar to streptozotocin characterized by less gastrointestinal toxicity but increased myelosuppression or monotherapy with dacarbazine[109].
In conclusion, chemotherapy is not the treatment of first choice in patients with gastrin secreting tumors, but seems to be indicated in rapidly evolving tumors in which the mass of the primary tumor increases more than 25% in a period of follow-up of 12 mo or in which the tumoral symptoms cannot be treated by other means[110].
Interferon In recent years, the validity of interferon has been sustained in neuroendocrine tumors, especially in the carcinoid syndrome. The data in the literature demonstrate that therapy with interferon-alpha leads to the stabilization of the tumor in 20-40% of patients with different gastrointestinal neuroendocrine tumors, including those with metastatic gastrinomas, but it did not result in an increase in the survival rate of these patients[111]. It has been proposed, therefore, that gastrinomas can be treated with chemotherapy and/or interferon when they grow and metastasize[111].
Role of chemoembolization or embolization Selective embolization of the hepatic arteries causes transient and complete ischemia with an objective, symptomatic and hormonal response of 65% and 81%, respectively; this treatment has been demonstrated to be useful in patients with liver metastases from neuroendocrine pancreatic tumors. Unfortunately, only a small number of gastrinomas have been treated with chemoembolization and there are no sufficient data on long-term survival after this procedure. Chemoembolization, which uses a combination of gelfoam and chemotherapeutic agents (streptozotocin or doxorubicin), may determine a notable improvement in the quality of life of the patient and is usually accompanied by a reduction in circulating peptide serum levels and the size of the tumor. However, it has not been clearly demonstrated that the inhibition of tumoral growth improves the survival rate[112-115].
Chemoembolization is an alternative to chemotherapy for progressive liver metastases in patients with gastrinomas.
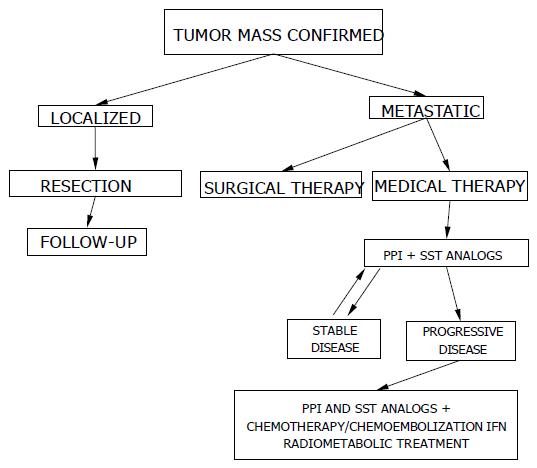
A suggested algorithm for medical and surgical treatment of ZES is reported in Figure 2.
Proton pump inhibitors have been used to more drastically inhibit gastrin action on the parietal cells, thus allowing the patient to rapidly return to a normal clinical condition without the need for surgery. If it is possible to eliminate the morbidity and mortality caused by acid hypersecretion, the majority of patients affected by ZES should be able to be maintained in good clinical condition, without symptoms. In ZES, antisecretory drugs have been proven to be safe and effective, without particular side effects over a long period of time, even 15 years or more.
Recent developments in surgical techniques, and pre-and intra-operatory imaging investigation, have permitted the identification and successive resection of more than 95% of gastrinomas. Therefore all localized gastrinomas should be excised, if possible. If hepatic metastases are also present, their enucleation may improve the symptoms and the survival rate as well as facilitate medical treatment. Another possibility is to carry out surgery, only when the tumor is greater than 2 cm in diameter, because these dimensions are associated with a greater risk of metastases in the liver. However, there is some controversy about the surgical approach to gastrinomas associated with MEN 1.
Somatostatin analogs can be useful in reducing gastric acid hypersecretion, serum gastrin and gastric ECL-cells, thus contributing to curing the disease more effectively. Furthermore, thanks to their antiproliferative effects, these drugs are used in treating patients with hepatic metastases and in those affected by ZES-MEN 1 with gastric carcinoids.
Chemotherapy may be particularly indicated in the malignant evolving form, but not in slow-growing tumors or as first choice therapy in gastrinomas.
Embolization and/or chemoembolization may be a palliative procedure for patients with hepatic metastases.
Finally, we would like to emphasize that the choice of treatment must involve a multidisciplinary approach.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. 1955. CA Cancer J Clin. 1989;39:231-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 2. | Jensen RT, Gardner JD, Raufman JP, Pandol SJ, Doppman JL, Collen MJ. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med. 1983;98:59-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Jensen RT, Doppman JL, Gardner JD. Gastrinoma. LW, Brooks FA, DiMagno EP, Gardner JD, Lebenthal E, Scheele GA, eds. The exlocrine pancreas: biology, pathobiology and disease. New York: Raven Press 1986; 727-744. |
| 4. | Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1). Surg Oncol. 2003;12:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, Polak JM, Häcki WH, Stamm B, Heitz PU. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 243] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Jensen RT. Zollinger-Ellison syndrome. Surgical endocrinology: clinical syndromes. Philadelphia 2001; 291-344. |
| 7. | Soga J, Yakuwa Y. The gastrinoma/Zollinger-Ellison syndrome: statistical evaluation of a Japanese series of 359 cases. J Hepatobiliary Pancreat Surg. 1998;5:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Gibril F, Curtis LT, Termanini B, Fritsch MK, Lubensky IA, Doppman JL, Jensen RT. Primary cardiac gastrinoma causing Zollinger-Ellison syndrome. Gastroenterology. 1997;112:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Noda S, Norton JA, Jensen RT, Gay WA. Surgical resection of intracardiac gastrinoma. Ann Thorac Surg. 1999;67:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Abou-Saif A, Lei J, McDonald TJ, Chakrabarti S, Waxman IF, Shojamanesh H, Schrump DS, Kleiner DE, Gibril F, Jensen RT. A new cause of Zollinger-Ellison syndrome: non-small cell lung cancer. Gastroenterology. 2001;120:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zollinger RM, Ayres HP. The Zollinger-Ellison syndrome. In: Nyhus LM, Wastell C, Donahue PhE, eds. Surgery of the Esophagus, Stomach and Small Intestine 1987; 491-513. |
| 12. | Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology. 1973;65:140-165. [PubMed] |
| 13. | Ellison EC, Wilson SD. The Zollinger-Ellison syndrome: re-appraisal and evaluation of 260 registered cases. Ann Surg. 1964;160:512-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 283] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Stage JG, Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl. 1979;53:79-91. [PubMed] |
| 15. | Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615-630. [PubMed] |
| 16. | Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Durieux S, Lioté F, Timsit MA, Duet M, Valleur P, Lemaire V, Dryll A. Bone metastases in epidural invasion of gastrinoma. Rev Rhum Ed Fr. 1994;61:453-455. [PubMed] |
| 18. | Maton PN. Gastrinoma and other hypergastrinemic syndrome. Gut Peptides. New York: Raven Press 1994; 675-700. |
| 19. | Ruszniewski P, Girard F, Benamouzig R, Mignon M, Bonfils S. Long acting somatostatin treatment of paraneoplastic Cushing's syndrome in a case of Zollinger-Ellison syndrome. Gut. 1988;29:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 480] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Shepherd JJ. The natural history of multiple endocrine neoplasia type 1. Highly uncommon or highly unrecognized? Arch Surg. 1991;126:935-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 598] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 23. | Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1389] [Cited by in RCA: 1254] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 24. | Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, Manickam P, Olufemi SE, Skarulis MC, Doppman JL. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet. 1997;16:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 256] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Zhuang Z, Vortmeyer AO, Pack S, Huang S, Pham TA, Wang C, Park WS, Agarwal SK, Debelenko LV, Kester M. Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas. Cancer Res. 1997;57:4682-4686. [PubMed] |
| 26. | Debelenko LV, Brambilla E, Agarwal SK, Swalwell JI, Kester MB, Lubensky IA, Zhuang Z, Guru SC, Manickam P, Olufemi SE. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Hum Mol Genet. 1997;6:2285-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 157] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Rindi G, Villanacci V, Ubiali A. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion. 2000;62 Suppl 1:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Calender A. Molecular genetics of neuroendocrine tumors. Digestion. 2000;62 Suppl 1:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Norton JA, Jensen RT. Multiple endocrine neoplasia. Cancer Principies and practice of oncology. Philadelphia: Lippincott Raven Publisher 1997; 1723-1729. |
| 30. | Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83:43-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Benya RV, Metz DC, Venzon DJ, Fishbeyn VA, Strader DB, Orbuch M, Jensen RT. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type I. Am J Med. 1994;97:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Mignon M, Cadiot G, Rigaud D, Ruszniewski PH, Jais P, Lehy T, Lewin MJM. Management of islet cell tumors in patients with multiple endocrine neoplasia type 1. Endocrine tumors of the pancreas: Recent advances in research and management. Karger[23] 1995; 342-359. |
| 33. | Tomassetti P, Migliori M, Lalli S, Campana D, Tomassetti V, Corinaldesi R. Epidemiology, clinical features and diagnosis of gastroenteropancreatic endocrine tumours. Ann Oncol. 2001;12 Suppl 2:S95-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Jensen RT. Gastrointestinal endocrine tumours. Gastrinoma. Baillieres Clin Gastroenterol. 1996;10:603-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Lehy T, Cadiot G, Mignon M, Ruszniewski P, Bonfils S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut. 1992;33:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 279] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Solcia E, Rindi G, Larosa S, Capella C. Morphological, molecular, and prognostic aspects of gastric endocrine tumors. Microsc Res Tech. 2000;48:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Roy PK, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore). 2000;79:379-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Roy PK, Venzon DJ, Feigenbaum KM, Koviack PD, Bashir S, Ojeaburu JV, Gibril F, Jensen RT. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis-a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine. 2001;80:189-222. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Frucht H, Howard JM, Slaff JI, Wank SA, McCarthy DM, Maton PN, Vinayek R, Gardner JD, Jensen RT. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome. A prospective study. Ann Intern Med. 1989;111:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Tartaglia A, Bianchini S, Vezzadini P. Biochemical diagnosis of gastroenteropancreatic endocrine tumors. Minerva Med. 2003;94:1-7. [PubMed] |
| 43. | Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, Weber HC, Stewart CA, Jensen RT. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas. A prospective study. Ann Intern Med. 1996;125:26-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 282] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 44. | van Eijck CH, Lamberts SW, Lemaire LC, Jeekel H, Bosman FT, Reubi JC, Bruining HA, Krenning EP. The use of somatostatin receptor scintigraphy in the differential diagnosis of pancreatic duct cancers and islet cell tumors. Ann Surg. 1996;224:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | London JF, Shawker TH, Doppman JL, Frucht HH, Vinayek R, Stark HA, Miller LS, Miller DL, Norton JA, Jensen RT. Zollinger-Ellison syndrome: prospective assessment of abdominal US in the localization of gastrinomas. Radiology. 1991;178:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 410] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 47. | Glover JR, Shorvon PJ, Lees WR. Endoscopic ultrasound for localisation of islet cell tumours. Gut. 1992;33:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 49. | Ruszniewski P, Amouyal P, Amouyal G, Grangé JD, Mignon M, Bouché O, Bernades P. Localization of gastrinomas by endoscopic ultrasonography in patients with Zollinger-Ellison syndrome. Surgery. 1995;117:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Van Hoe L, Gryspeerdt S, Marchal G, Baert AL, Mertens L. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. AJR Am J Roentgenol. 1995;165:1437-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 255] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 52. | Semelka RC, Cumming MJ, Shoenut JP, Magro CM, Yaffe CS, Kroeker MA, Greenberg HM. Islet cell tumors: comparison of dynamic contrast-enhanced CT and MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1993;186:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 101] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Debray MP, Geoffroy O, Laissy JP, Lebtahi R, Silbermann-Hoffman O, Henry-Feugeas MC, Cadiot G, Mignon M, Schouman-Claeys E. Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Wiedenmann B, Jensen RT, Mignon M, Modlin CI, Skogseid B, Doherty G, Oberg K. Preoperative diagnosis and surgical management of neuroendocrine gastroenteropancreatic tumors: general recommendations by a consensus workshop. World J Surg. 1998;22:309-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Ricke J, Klose KJ, Mignon M, Oberg K, Wiedenmann B. Standardisation of imaging in neuroendocrine tumours: results of a European delphi process. Eur J Radiol. 2001;37:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Maton PN, Miller DL, Doppman JL, Collen MJ, Norton JA, Vinayek R, Slaff JI, Wank SA, Gardner JD, Jensen RT. Role of selective angiography in the management of patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:913-918. [PubMed] |
| 57. | Roche A, Raisonnier A, Gillon-Savouret MC. Pancreatic venous sampling and arteriography in localizing insulinomas and gastrinomas: procedure and results in 55 cases. Radiology. 1982;145:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 59. | Lew EA, Pisegna JR, Starr JA, Soffer EF, Forsmark C, Modlin IM, Walsh JH, Beg M, Bochenek W, Metz DC. Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 2000;118:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Howard JM, Chremos AN, Collen MJ, McArthur KE, Cherner JA, Maton PN, Ciarleglio CA, Cornelius MJ, Gardner JD, Jensen RT. Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology. 1985;88:1026-1033. [PubMed] |
| 61. | Jensen RT, Collen MJ, McArthur KE, Howard JM, Maton PN, Cherner JA, Gardner JD. Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric hypersecretory states. Am J Med. 1984;77:90-105. [PubMed] |
| 62. | Jensen RT, Maton PN, Gardner JD. Current management of Zollinger-Ellison syndrome. Drugs. 1986;32:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40:52-62; quiz 121-3. [PubMed] |
| 64. | Thomson AB. Are the orally administered proton pump inhibitors equivalent? A comparison of lansoprazole, omeprazole, pantoprazole, and rabeprazole. Curr Gastroenterol Rep. 2000;2:482-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of omeprazole in Zollinger-Ellison syndrome: a prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Jensen RT, Gardner JD. Zollinger-Ellison syndrome: clinical presentation, pathology, diagnosis and treatment. In: Zakim D, Dannenberg AJ, eds. Peptic ulcer disease and other acid-related disorder. New York Accademic Research Associates 1991; 117-246. |
| 67. | Metz MD, Jensen RT. Advances in gastric antisecretory therapy in Zollinger-Ellison syndrome. Mignon M, Jensen RT, eds. Endocrine tumors of the pancreas: Recent advances in research and management. Karger[23] 1995; 240-257. |
| 68. | Termanini B, Gibril F, Stewart CA, Weber HC, Jensen RT. A prospective study of the effectiveness of low dose omeprazole as initial therapy in Zollinger-Ellison syndrome. Aliment Pharmacol Ther. 1996;10:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Stadil F, Stage JG. The Zollinger--Ellison syndrome. Clin Endocrinol Metab. 1979;8:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Feigenbaum KM, Koviack PD, Jensen RT. Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology. 1992;103:1498-1508. [PubMed] |
| 71. | Andersson T, Bergstrand R, Cederberg C, Eriksson S, Lagerström PO, Skånberg I. Omeprazole treatment does not affect the metabolism of caffeine. Gastroenterology. 1991;101:943-947. [PubMed] |
| 72. | Jansen JB, Lundborg P, Baak LC, Greve J, Ohman M, Stöver C, Röhss K, Lamers CB. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut. 1988;29:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Lloyd-Davies KA, Rutgersson K, Sölvell L. Omeprazole in the treatment of Zollinger-Ellison syndrome: a 4-year international study. Aliment Pharmacol Ther. 1988;2:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Wolfe MM, Jensen RT. Zollinger-Ellison syndrome. Current concepts in diagnosis and management. N Engl J Med. 1987;317:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 141] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Maton PN, Lack EE, Collen MJ, Cornelius MJ, David E, Gardner JD, Jensen RT. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990;99:943-950. [PubMed] |
| 76. | Howden CW. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. J Clin Gastroenterol. 2000;30:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Weber HC, Venzon DJ, Jensen RT, Metz DC. Studies on the interrelation between Zollinger-Ellison syndrome, Helicobacter pylori, and proton pump inhibitor therapy. Gastroenterology. 1997;112:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Ruszniewski P, Laucournet H, Elouaer-Blanc L, Mignon M, Bonfils S. Long-acting somatostatin (SMS 201-995) in the management of Zollinger-Ellison syndrome: evidence for sustained efficacy. Pancreas. 1988;3:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Tomassetti P, Migliori M, Gullo L. Slow-release lanreotide treatment in endocrine gastrointestinal tumors. Am J Gastroenterol. 1998;93:1468-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Tomassetti P, Migliori M, Corinaldesi R, Gullo L. Treatment of gastroenteropancreatic neuroendocrine tumours with octreotide LAR. Aliment Pharmacol Ther. 2000;14:557-560. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Arnold R, Simon B, Wied M. Treatment of neuroendocrine GEP tumours with somatostatin analogues: a review. Digestion. 2000;62 Suppl 1:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Bordi C, Azzoni C, Pilato FP, Robutti F, D'Ambra G, Caruana P, Rindi G, Corleto VD, Annibale B, Delle FG. Morphometry of gastric endocrine cells in hypergastrinemic patients treated with the somatostatin analogue octreotide. Regul Pept. 1993;47:307-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C, Jurgensen R, Stein K, Schafer H, Bruns C. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430-438. [RCA] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Jensen RT. Carcinoid and pancreatic endocrine tumors: recent advances in molecular pathogenesis, localization, and treatment. Curr Opin Oncol. 2000;12:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2002;94:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, Bartolomei M, Orsi F, De Cicco C, Mäcke HR. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol. 2001;12:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 88. | Leimer M, Kurtaran A, Smith-Jones P, Raderer M, Havlik E, Angelberger P, Vorbeck F, Niederle B, Herold C, Virgolini I. Response to treatment with yttrium 90-DOTA-lanreotide of a patient with metastatic gastrinoma. J Nucl Med. 1998;39:2090-2094. [PubMed] |
| 89. | Peghini PL, Annibale B, Azzoni C, Milione M, Corleto VD, Gibril F, Venzon DJ, Delle FG, Bordi C, Jensen RT. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Cadiot G, Lehy T, Bonfils S. Action of somatostatin analogue (SMS 201-995) on the growth-promoting effect resulting from sustained achlorhydria in rat gastric mucosa, with special reference to endocrine cell behaviour. Eur J Clin Invest. 1988;18:360-368. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Modlin IM, Kumar R, Nangia A, Soroka CJ, Pasikhov D, Goldenring JR. Gastrin-dependent inhibitory effects of octreotide on the genesis of gastric ECLomas. Surgery. 1992;112:1048-1056; discussion 1048-1056;. [PubMed] |
| 92. | Tomassetti P, Migliori M, Caletti GC, Fusaroli P, Corinaldesi R, Gullo L. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N Engl J Med. 2000;343:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320-38; discussion 320-328;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Hirschowitz BI. Clinical course of nonsurgically treated Zollinger-Ellison syndrome. Mignon M, Jensen RT, eds. Endocrine tumors of the pancreas: Recent advances in research and management. Karger[23] 1995; 360-371. |
| 95. | Thompson NW, Bondeson AG, Bondeson L, Vinik A. The surgical treatment of gastrinoma in MEN I syndrome patients. Surgery. 1989;106:1081-105; discussion 1081-1085;. [PubMed] |
| 96. | Delcore R, Cheung LY, Friesen SR. Outcome of lymph node involvement in patients with the Zollinger-Ellison syndrome. Ann Surg. 1988;208:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Fraker DL, Norton JA, Saeed ZA, Maton PN, Gardner JD, Jensen RT. A prospective study of perioperative and postoperative control of acid hypersecretion in patients with Zollinger-Ellison syndrome undergoing gastrinoma resection. Surgery. 1988;104:1054-1063. [PubMed] |
| 98. | Azoulay D, Bismuth H. Role of liver surgery and transplantation in patients with hepatic metastases from pancreatic endocrine tumors. Mignon M, Jensen RT, eds. Endocrine tumors of the pancreas: Recent advances in research and management. Karger[23] 1995; 461-476. |
| 99. | Le Treut YP, Delpero JR, Dousset B, Cherqui D, Segol P, Mantion G, Hannoun L, Benhamou G, Launois B, Boillot O. Results of liver transplantation in the treatment of metastatic neuroendocrine tumors. A 31-case French multicentric report. Ann Surg. 1997;225:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Akerström G, Hessman O, Skogseid B. Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN 1. Langenbecks Arch Surg. 2002;386:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993;17:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 907] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 104. | Arnold R, Frank M. Systemic chemotherapy for endocrine tunors of the pancreas. Mignon M, Jensen RT, eds. Endocrine tumors of the pancreas: Recent advances in research and management. Karger[23] 1995; 431-438. |
| 105. | Murray-Lyon IM, Eddleston AL, Williams R, Brown M, Hogbin BM, Bennett A, Edwards JC, Taylor KW. Treatment of multiple-hormone-producing malignant islet-cell tumour with streptozotocin. Lancet. 1968;2:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 154] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Broder LE, Carter SK. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med. 1973;79:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 205] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 584] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 108. | von Schrenck T, Howard JM, Doppman JL, Norton JA, Maton PN, Smith FP, Vinayek R, Frucht H, Wank SA, Gardner JD. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology. 1988;94:1326-1334. [PubMed] |
| 109. | Kvols LK, Buck M. Chemotherapy of endocrine malignancies: a review. Semin Oncol. 1987;14:343-353. [PubMed] |
| 110. | Rougier P, Mitry E. Chemotherapy in the treatment of neuroendocrine malignant tumors. Digestion. 2000;62 Suppl 1:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Oberg K. Advances in chemotherapy and biotherapy of endocrine tumors. Curr Opin Oncol. 1998;10:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Clouse ME, Perry L, Stuart K, Stokes KR. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Digestion. 1994;55 Suppl 3:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Perry LJ, Stuart K, Stokes KR, Clouse ME. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Surgery. 1994;116:1111-1116; discussion 1111-116;. [PubMed] |
| 114. | Drougas JG, Anthony LB, Blair TK, Lopez RR, Wright JK, Chapman WC, Webb L, Mazer M, Meranze S, Pinson CW. Hepatic artery chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg. 1998;175:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors--a retrospective single-center analysis. Digestion. 2003;68:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |