INTRODUCTION
Immunological liver injury is a key pathological change in liver disease, with a high mortality rate and histopathologically characterized by diffuse intrahepatic infiltration of inflammatory cells with massive multilobular necrosis. The details of the mechanisms involved in immunological liver injury remain to be elucidated. It was reported that injection of a small dose of lipopolysaccharide (LPS) into mice primed with Bacille Calmette-Guerin (BCG) causes lethal acute hepatic necrosis[1], mimicking fulminant hepatic failure. There is evidence that activated macrophages and T lymphocytes are essential for the induction of liver damage[2-5].
The current paradigm of T lymphocytes recruited to the inflamed liver is a multiple-step model, which describes the sequence of tethering/rolling, firm adhesion, and diapedesis/extravasation[6,7]. The chemokine family is a small secreted protein, that directs migration of various types of leukocytes and is involved in inflammatory and immunological processes including lymphocyte homing, angiogenesis and enhancement of cytotoxic T lymphocyte (CTL) responses[8,9]. Chemokines have been classified into four subfamilies, C, CC, CXC and CX3C, based on the motif formed by the conserved cysteine residues in the N-terminal region. The selective interaction of chemokines and their receptors in part contributes to the specificity by which leukocyte subsets might be preferentially determined[10,11].
CXCL16, with its structure similar to that of FKN[12], containing both membrane-anchored and soluble forms, is a ligand for CXC chemokine receptor 6 (CXCR6), which is expressed on natural killer T (NKT) cells, and type 1-polarized, activated CD4+ and CD8+ T cells. CXCL16 is markedly expressed in lung, small intestine and kidney, and weakly expressed in liver[13-18]. Because the pathological characteristics of immunological liver injury are associated with T-lymphocyte infiltration, and CXCL16 is chemotactic for T-lymphocyte migration to the liver tissue damage[4,5,19-21], we could reason that CXCL16 might play a crucial role in the pathogenesis of immunological liver injury. In the present study, we investigated the expression pattern and distribution of CXCL16, and studied the role of CXCL16 in liver T-cell infiltration, and the effect of anti-CXCL16 antibody on the pathogenesis of immunological liver injury.
MATERIALS AND METHODS
Mice
Specific pathogen-free BALB/c mice (6-7 wk old) obtained from Shanghai Animal Center of Chinese Academy of Sciences (Shanghai, China) were bred in pathogen-free conditions and allowed free access to food and water.
Reagents
LPS (Escherichia coli 055:B5) was purchased from Sigma (St. Louis, MO). BCG was from Institute of Biological Product (Shanghai, China). Rat immunoglobulin (goat IgG) was gifted by Dr. XL Wang. Rat anti-mouse CXCL16 mAb (clone 142417, rat IgG2a) was obtained from R&D systems. PE-labeled anti-CD4 mAb and FITC-conjugated anti-CD8 mAb were obtained from PharMingen (San Diego, CA, USA). TRIzol RNA extraction kit was purchased from Gibcol. All other chemicals used were of analytical grade.
Induction of liver injury by BCG and LPS
BCG was suspended in pyrogen-free physiological saline. To induce liver injury, mice were primed with intravenous injection of BCG (5×107 viable bacilli) via tail veins. After 12 d, they were given an intravenous injection of LPS (7.5 μg/mouse) suspended in pyrogen-free phosphate-buffered saline (PBS). To examine the effect of anti-CXCL16 antibodies on BCG-LPS-induced liver injury, monoclonal anti-CXCL16 antibody (0.2 mg/mouse) or nonimmune rat isotype IgG (0.2 mg/mouse) was administered intravenously 1-2 h before LPS challenge. Mice were killed to collect sera and liver specimens, and stored at -70 °C until use. Survival rate was determined 72 h after the LPS challenge.
Determination of serum alanine transferase (ALT)
Serum was collected for the measurement of ALT levels at various time points after LPS challenge.
Histology
Liver specimens were fixed in 10% neutrally buffered formalin and embedded in paraffin. Deparaffinized thin sections from each paraffin block were stained with hematoxylin and eosin for histologic examination and analyzed by light microscopy.
Analysis of specific gene expression in liver tissue by real-time quantitative RT-PCR
Total RNA was isolated from liver specimens using TRIzol, according to the manufacturer’s instructions. The specific mRNA level was quantified by real-time quantitative PCR using a LightCycler instrument (Roche Molecular Biochemicals, Germany) with the DNA binding dye SYBR Green I as described with minor modifications[22]. The sense primer for CXCL16 was 5’-AAC CAG GGC AGT GTC GC-3’, and the antisense primer was 5’-AGG CAA ATG TTT TTG GTG G-3’ (position: 79-342). The sense primer for β-actin was 5’-GCT ACA GCT TCA CCA CCA CAG-3’, and the antisense primer was 5’-GGT CTT TAC GGA TGT CAA CGT C-3’ (position: 590-877). Expression of mRNA was normalized by β-actin. In brief, external standards were plasmids containing CXCL16 gene fragment (and β-actin). Plasmid DNA was purified and quantified photometrically and serially diluted. One microgram total RNA was reverse transcribed to cDNA. One microliter cDNA or external standard was added to capillary to a final reaction volume of 20 μL. The primer final concentration was adjusted to 0.5 μmol/L. The amplification conditions were initial denaturation at 95 °C for 30 s, 45 cycles at 95 °C for 0 s, at 60 °C for 3 s, at 72 °C for 12 s. Melting curve analysis was performed at the end of amplification. The acquisition temperature for the fluorescence signal was adjusted to 84 °C. PCR products were electrophoresed on 2% agarose gels and photos were taken under ultraviolet light to identify their specificity and sensitivity.
Immunohistochemistry
Experiments were conducted on frozen sections as described in detail elsewhere[23]. Sections were incubated with primary antibody, and then with HRP-conjugated anti-rat IgG antibodies. After being visualized using DAB containing 0.01% hydrogen peroxide and counterstained with hematoxylin, sections were microscopically examined. Control sections were treated with omission of the primary antibody.
Preparation of intrahepatic infiltrating leukocytes and FACS analysis
Liver-infiltrating leukocytes were prepared as previously described[24]. In brief, livers were taken from mice, pressed through the nylon mesh, and suspended in 2% fetal calf serum (FCS). The cell suspension was treated with Percoll density (1.09) gradient centrifugation. Mononuclear cells (MNCs) were isolated from parenchymal hepatocytes by centrifugation at 2 500 r/min for 30 min. The pellet was washed thrice in DMEM and resuspended in 10% FCS-DMEM. Flow cytometric immunofluorescence analysis of liver-infiltrating cells was performed with FACS calibur (BD Corp.). In brief, 4×105 cells were incubated with a PE-labeled anti-CD4 Ab and FITC-conjugated anti-CD8 Ab or FITC-conjugated anti-CD3 Ab at 4 °C for 30 min after washing twice with PBS solution.
Statistical analysis
All statistical calculations were performed using GraphPad PRISMTM (Graphpad Software Inc.). The survival rates were analyzed using the log-rank test. Student’s t test was used to test for significance. P<0.05 was considered statistically significant.
RESULTS
Establishment of immunological liver injury model in mice
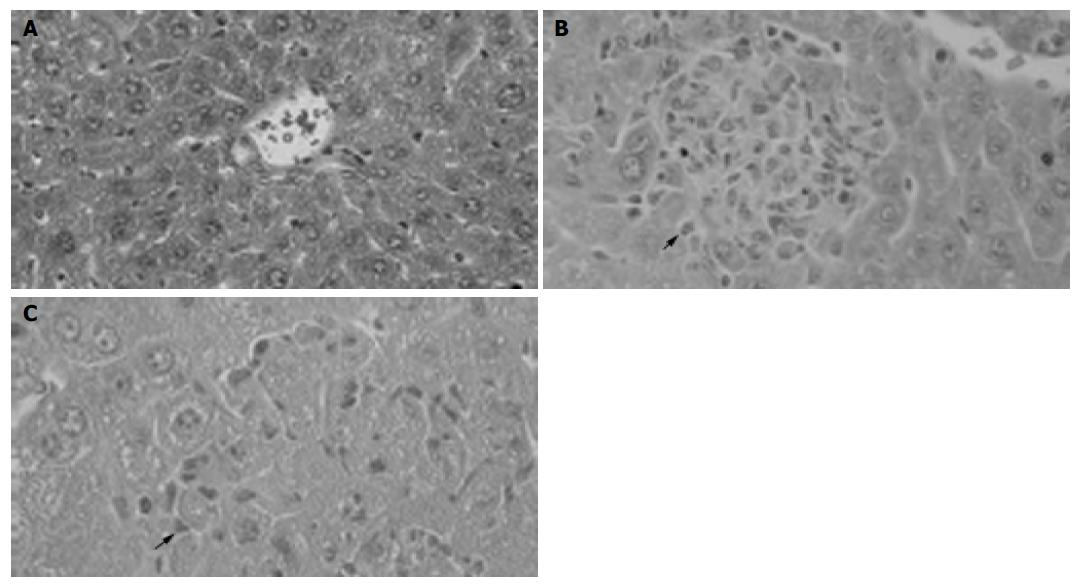
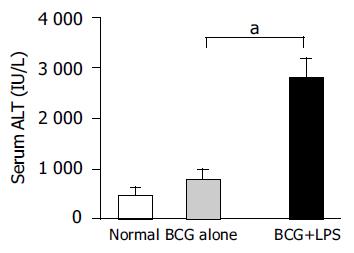
BCG treatment alone induced MNC infiltration into hepatic lobes and formed granuloma (Figure 1B). Subsequent LPS challenge in BCG-primed mice induced diffuse infiltration of MNCs and multilobular hepatocellular necrosis in liver tissue (Figure 1C). The ALT level dramatically increased in BCG-primed mice after LPS challenge (Figure 2) compared to BCG treatment alone (P<0.05). All these data confirmed that the murine immunological liver injury model was successfully conducted.
Figure 1 Pathological examination showing granuloma formation with BCG administration and hepatocellular necrosis followed by LPS injection.
H&E staining of cross-sections of normal liver (A), 12 d after BCG injection (B) and BCG-primed mice sequentially challenged with LPS (C). Bars = 20 mm.
Figure 2 Levels of serum ALT in mice.
The serum ALT levels of mice (n = 5 at each group) were measured as described in Materials and methods. Data represent mean±SD. aP<0.05 vs BCG-primed mice.
Expression pattern and distribution of CXCL16 in murine injured liver model
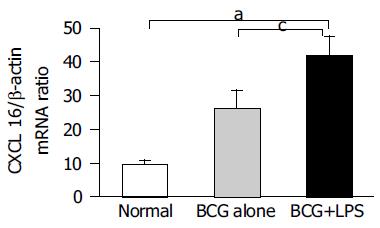
Total RNA were isolated from liver tissue of normal, BCG-treated and BCG-primed mice after LPS challenge. CXCL16 mRNA was quantified by real-time quantitative PCR. CXCL16 was expressed in BCG-primed mice and strongly upregulated after LPS challenge, compared to that in normal liver tissue (Figure 3). These results suggested that upregulation of CXCL16 expression might be associated with liver injury.
Figure 3 Detection of CXCL16 mRNA in the injured liver by real-time quantitative RT-PCR analysis.
Total RNA was isolated from the liver tissues at the different group as indicated and then reverse transcribed with reverse transcriptase and amplified by real-time quantitative PCR specific for CXCL16. Amplification of b-actin by same approach was used as control. The results represent three independent experiments. aP<0.05 vs normal mice, cP<0.05 vs BCG-primed mice.
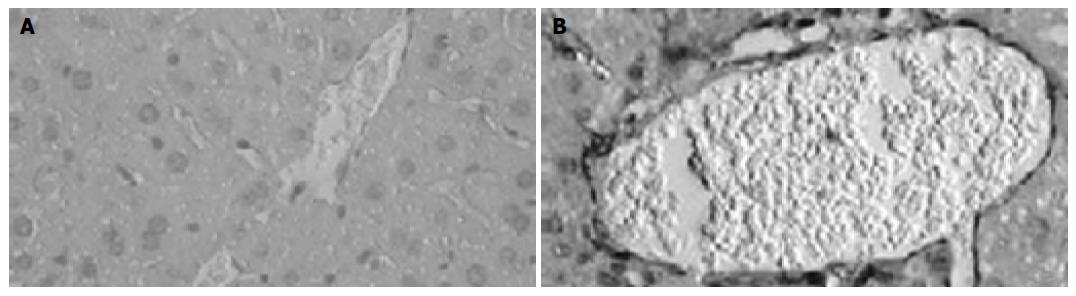
Immunohistochemical analysis further showed that expression of CXCL16 in the liver significantly increased after LPS challenge in BCG-primed mice, compared to that in normal mice (Figure 4). CXCL16 was predominantly distributed in periportal area, hepatic sinusoids and vascular endothelia (Figure 4).
Figure 4 Immunohistochemical examination of CXCL16 expression in liver tissue.
Sections of livers were obtained from normal mice without any treatment (A) and BCG-primed mice sequentially injected with LPS (B). CXCL16-positive (brown) cells are shown in the liver tissue with hematoxylin counterstaining. Bars = 20 mm.
Infiltration of T lymphocytes in injured liver tissue
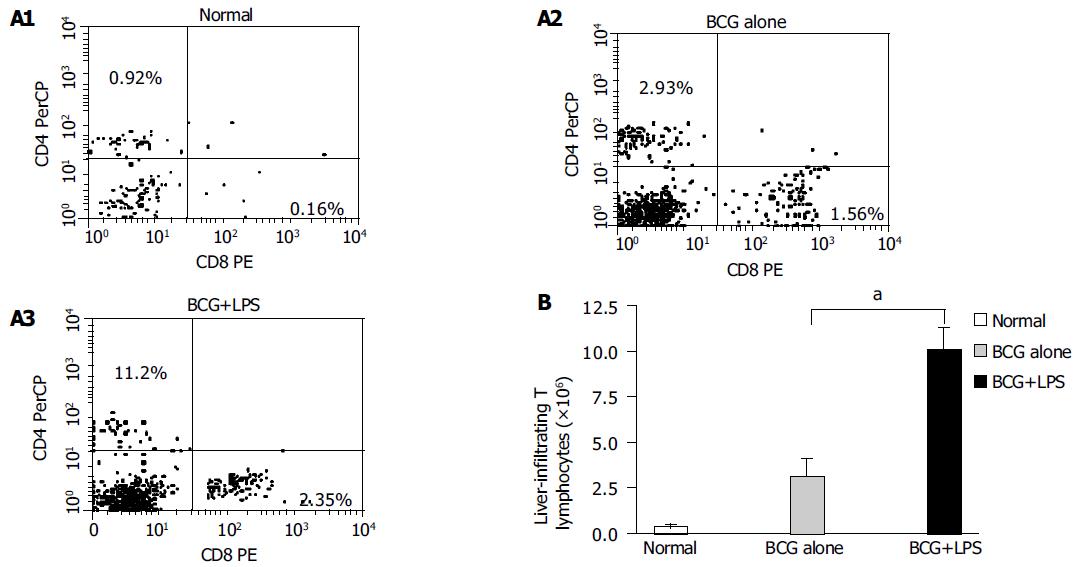
To investigate the characteristics of circulating T lymphocyte recruitment and infiltration in immunological liver injury, cell surface expressions of CD3, CD4 and CD8 in the infiltrated MNCs were analyzed using a flow cytometer. The results revealed that the CD4+ T lymphocyte population increased from 0.92% to 2.93%, and further increased to 11.2% in BCG-primed mice after LPS challenge. CD8+ T lymphocyte population also increased from 0.16% to 1.56%, and reached 2.35% after LPS challenge (Figure 5A). The total absolute number of T lymphocytes increased from 0.399×106 to 3.179×106/liver and reached 1.01×107/liver in BCG-primed mice injected with LPS (Figure 5B). These findings demonstrated that T lymphocytes might be involved in the pathogenesis of immunological liver injury.
Figure 5 Analysis of liver-infiltrating CD4+ and CD8+T lymphocytes population in liver injury induced by BCG and LPS.
Mice were primed with BCG and sequentially challenged by LPS, 12 d after priming. MNCs derived from normal (without any treatment, open bars); BCG-primed (horizontal bars) and BCG-primed plus LPS challenged mice (hatched bars) were harvested for FACS analysis. A1-A3: The CD4+ and CD8+T lymphocytes population were analyzed with FACS; B: the absolute number of T lymphocytes was determined by multiplying the total MNC number by the fraction of CD4+, CD8+T-lymphocytes population. aP<0.05 vs BCG-primed mice.
Anti-CXCL16 antibody increased survival of mice with immunological liver injury
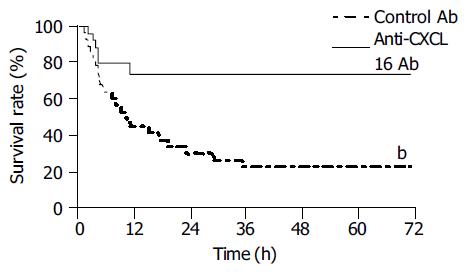
Neutralizing anti-CXCL16 antibody could protect mice against lethal liver failure induced by BCG and LPS. Approximately 70% of the mice (n = 10) survived for 72 h, whereas most (80%) of the mice treated with control Ab died within 72 h (Figure 6).
Figure 6 Protective effect of anti-CXCL16 antibody on survival rate for murine lethal hepatic failure.
Immunological liver injury was induced by BCG and LPS treatment and anti-CXCL16 Ab (solid line, n = 10) or control Ab (dashed line, n = 10) was injected as indicated in Materials and methods. The anti-CXCL16 antibody significantly protected the mice from lethality (bP<0.01 vs control Ab).
Anti-CXCL16 antibody reduced infiltration of T lymphocytes in liver injury
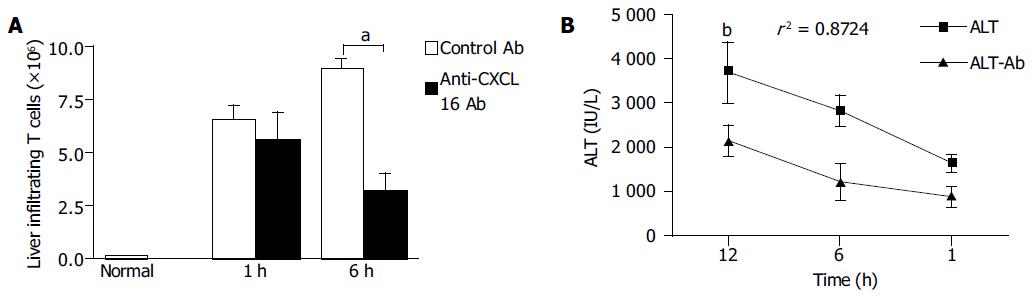
Infiltrated MNCs in the liver were observed and granuloma formed after BCG treatment. The diffuse infiltration of MNCs resulted in multilobular necrosis after LPS challenge. To investigate the effects of anti-CXCL16 antibody on intrahepatic infiltrating T lymphocytes, liver MNCs were isolated and T lymphocytes were analyzed. The results revealed that anti-CXCL16 antibody in vivo significantly decreased the number of T-infiltrating lymphocytes from 1.01×107 to 3.52×106/liver in BCG-primed mice after LPS challenge (Figure 7A, P<0.05).
Figure 7 Effects of anti-CXCL16 antibody on intrahepatic infiltration of T lymphocytes and serum ALT level in the mice.
Mice were primed with BCG and sequentially challenged by LPS 12 d after priming. Anti-CXCL16 antibody and isotype antibody were administrated into the mice, respectively. A: T lymphocytes derived from the mice treated with either anti-CXCL16 antibody (closed bars) or isotype antibody (hatched bars) was harvested for FACS analysis. The absolute number of T lymphocytes was determined by multiplying the total MNC number by the fraction of CD3+T lymphocytes population. aP<0.05 vs control Ab treatment. B: Anti-CXCL16 antibody significantly suppressed the elevation of serum ALT. bP<0.01 vs control Ab group. Results were obtained from three independent experiments.
Change in serum ALT levels was characteristic of the immunological liver injury model in mice. The serum ALT level significantly decreased, when anti-CXCL16 antibody was applied in vivo compared to that after treatment with control Ab. The correlation coefficient of specific Ab and control Ab was 0.8724 (P<0.01, Figure 7B). These data indicated that T cells might play a key role in the BCG and LPS-induced immunological liver injury.
Anti-CXCL16 antibody protected mice against multilobular necrosis in liver injury
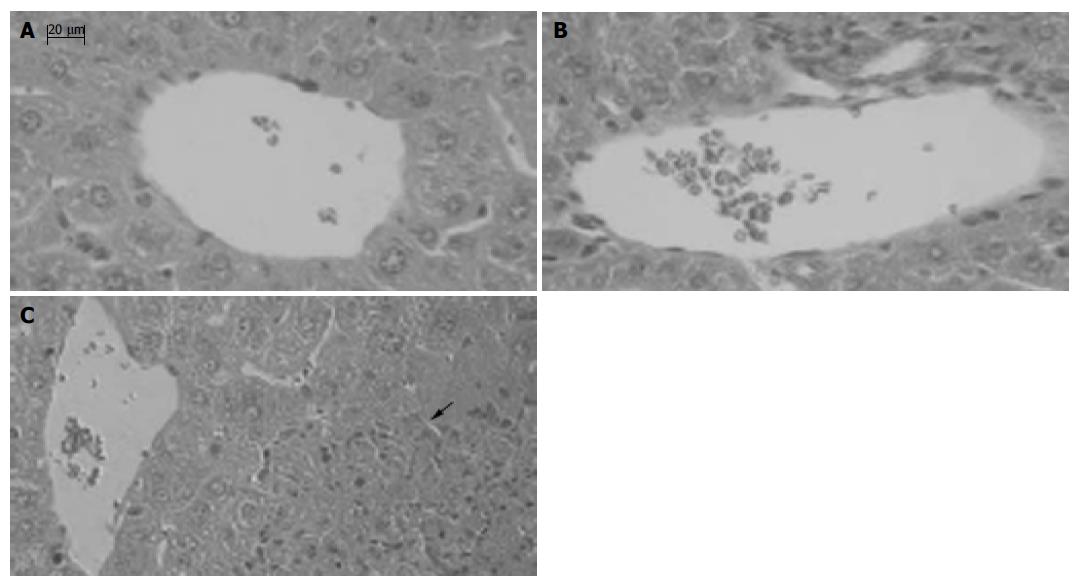
MNCs infiltrated into liver lobes and formed granuloma in liver 12 d after BCG treatment, compared to the normal mice (Figure 8A). Massive necrosis with diffuse infiltration of MNCs was observed in the livers of BCG-primed mice after LPS challenge (Figure 8C). In contrast, liver necrosis was significantly reduced by anti-CXCL16 Ab administration, and the specific Ab blocked the infiltration of MNCs infiltration in liver lobes and portal areas (Figure 8B).
Figure 8 Effect of anti-CXCL16 antibody on the formation of hepatic lesion in liver injury induced by BCG and LPS.
Sections of livers were obtained as follows: A: The liver tissue without any treatment; B: The liver tissue of mice primed with BCG was treated with the anti-CXCL16 Ab followed by LPS challenge; C: The liver tissue of mice primed with BCG was treated with nonimmune rat IgG followed by LPS challenge. An arrow shows a necrotic lesion. Tissues were stained with H&E and examined histologically. Bars = 20 mm.
DISCUSSION
In the present study, accumulation of T lymphocytes and massive hepatic necrosis were found and CXCL16 was strongly upregulated in the injured liver tissue in murine model. Upregulation of CXCL16 might be associated with necrosis and liver injury. Administration of anti-CXCL16 antibody markedly inhibited the intrahepatic infiltration of T lymphocytes, and prevented lethal hepatic failure. These findings demonstrate that CXCL16 may play an important role in T lymphocyte infiltration and hepatic necrosis in immunological liver injury.
Serum ALT level is an important characteristic of hepatitis accompanied with pathologic changes of liver. Our results demonstrate that intravenous administration of anti-CXCL16 Ab can significantly decrease serum ALT level and hepatic necrosis, suggesting that CXCL16 plays a role in liver injury through some mechanisms. CXCL16 is predo-minantly expressed on APCs, existing in both secretory and transmembrane forms, and selectively chemoattracts CXCR6+T lymphocytes, which are potential cytotoxic effector cells, such as CD4+Th1, activated CD8+T cells and NKT cells[13,14]. It was reported that these effector cells are closely associated with massive liver injury[25-28]. These effector T lymphocytes, perhaps accompanying other leukocytes, might be recruited and involved in the pathogenesis of acute liver injury.
Activated CD4+T, CD8+T and NKT cells express TNF-α and FasL molecules associated with liver injury[3,15,21,29-31]. Therefore, infiltrated T lymphocytes may mediate cytotoxicity to Fas-expressing hepatocytes and cause liver damage. Injection of LPS activates lymphocytes and triggers the release various soluble factors such as TNF-α, IFN-γ and IL-1, resulting in hepatocyte apoptosis and severe liver injury[2,31,32]. All these and sequential cytokine production are the key factors in the pathogenesis of acute hepatic failure. Anti-CXCL16 antibody can decrease the number of intrahepatic infiltrating T lymphocytes, especially CD4+ and CD8+T-lymphocyte population. These might explain in part the reason, why specific antibody can rescue mice from death. Some leukocytes, such as neutrophils, are involved in liver injury. However, available evidence suggests that a small dose of LPS, that does not cause overt organ injury, can effectively inhibit extravascular recruitment of neutrophils[33,34].
It is well known that many chemokines contribute to chemoattraction to trafficking leukocytes during liver inflammation[8,35,36]. However, anti-CXCL16 antibody could significantly reduce the number of intrahepatic T lymphocytes and liver necrosis, indicating that CXCL16 might play other roles besides chemotactic function. There is evidence that supports these functions[17,37]. Our experiments have confirmed that macrophages infected with Mycobacterium tuberculosis significantly increase the expression of CXCL16 and release chemokines and cytokines (data not shown). CXCL16 function in macrophages needs to be further studied.
Anti-CXCL16 Ab could not completely protect mice against death, suggesting that other chemokines or cytokines can also induce liver injury. Recently, it was reported that chemokines such as migration inhibitory factor, macrophage inflammatory protein-2 and thymus and activation-regulated chemokine are involved in the pathogenesis of liver injury[1,38,39]. Although these facts can explain in part the possible reason why anti-CXCL16 Ab can protect mice from death, the precise mechanism needs to be elucidated further.