Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3135
Revised: July 18, 2004
Accepted: September 4, 2004
Published online: May 28, 2005
AIM: To study the nervous-pathways of Fengch’ih acupuncture by means of anterograde transport of aqueous solution of horseradish peroxidase (HRP).
METHODS: Fifty Wistar rats were randomly divided into 1, 2, 3, 4, and 5 d groups, and every group had 10 animals. HRP (30% aqueous solution) was injected into a Fengch’ih. Serial, transverse or capital, 40 μm sections of the cervical spinal ganglia, cervical and thoracic spinal cord segment and brain were cut on a cryotome. Sections were incubated for HRP histochemistry according to the tetramethylbenzidine (TMB). Part of the sections were counterstained with neutral red.
RESULTS: After 1 d of survival times, many labeled cell bodies were found in 1-4 cervical spinal ganglia, anterior horn of 1-4 cervical spinal cord, ventromedial division of facial nucleus, accessory facial nucleus ipsilaterally. With increasing survival times, the intensity of labeled cells were slightly decreased.
CONCLUSION: Fengch’ih may bring into full play its effect by correlation of posterior ear branch of facial nerve and anterior branch of 2-3 cervical nerve with 1-4 cervical the anterior horn of the spinal cord, ventromedial division of facial nucleus, accessory facial nucleus.
- Citation: Xi GM, Wang HQ, He GH, Huang CF, Yuan QF, Wei GY, Li H, Liu WW, Fan HY. Nerve-pathways of acupoint Fengch’ih in rat by anterograde transport of HRP. World J Gastroenterol 2005; 11(20): 3135-3138
- URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3135.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3135
The acupoint of Fengch’ih, also known as Refu, is derived from the medical literature Miraculous Pivot: Febrile Disease, and held to be on the Gallbladder Channel of foot-Shaoyang, including hand- and foot-Shaoyang, Yangwei and Yanghui, one of the most commonly used acupoints in clinical treatment. As is documented, Fengch’ih can be used to treat many diseases, especially those resulting from pathogenic wind. Its clinical application is also extensive in modern time for treating such diseases as dyspepsia, stomach-ache, gastric ulcer, common cold, headache, cervical spondylosis, trigeminal neuralgia, hypertension, apoplexia, epilepsia, optic atrophy, myopia, tinnitus, painful heels[1]. However, little is known about its nerve-pathway. In the present study, we aim to illustrate the nerve-pathways and therapeutic mechanisms of Fengch’ih acupuncture, by means of anterograde transport of aqueous solution of horseradish peroxidase (HRP).
A total of 50 adult Wistar rats in good condition, each body weighing 250-300 g, were divided randomly into control and acupuncture group regardless of the sex, and each group subdivided into 1, 2, 3, 4, and 5 d with 10 rats in each subgroup. The animals were anesthetized with 10% chloral hydrate (0.3 mL/100 g) intraperitoneally to surgery.
The animals were fixed in prone position on the table. Fengch’ih was ascertained at the intersection of sternocleid-omastoid muscle and trapezius muscle. After conventional sterilization, 30% HRP50 μL was injected into a Fengch’ih acupoint. As for the controls, equal quantity of HRP was injected into Fengch’ih.
After survival times of 1-5 d, the animals were deeply anesthetized with 10% chloral hydrate, and thoracotomy was done quickly, and intubation was performed via left ventricle to aorta, and perfused with 50-150 mL 0.9% NaCl (till the liver whitened), followed by 4 °C 1.0% paraformal-dehyde-1.25% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.4, of 500 mL, first rapidly and then slowly, lasting no less than 30 min. Soon, the brainstem with attached cerebellum, cervical and upper thoracic spinal cord, and the first to eighth bilateral cervical dorsal root ganglia were removed quickly and stored overnight in 0.1 mol/L phosphate buffer containing 30% sucrose.
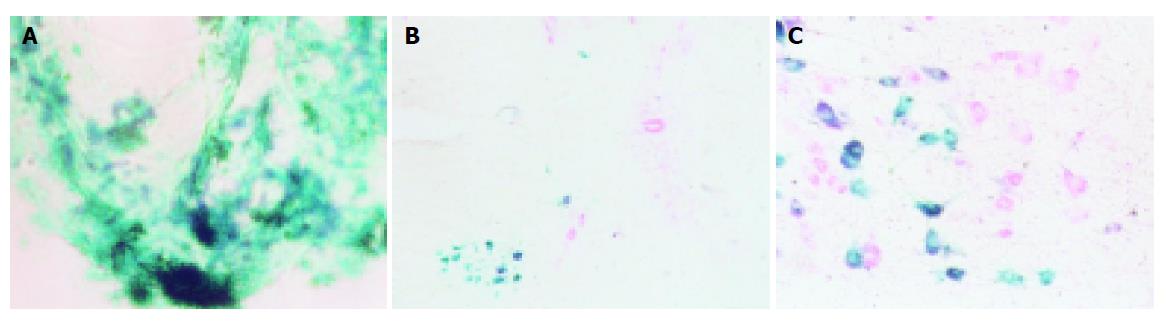
In order to locate the positive nuclear groups, serial, capital or transverse (brainstem and spinal cord) or longitudinal (dorsal root ganglion) 40-μm sections were cut on a cryotome. Sections were incubated for HRP histochemistry according to the tetramethylbenzidine-sodium tungstate (TMB-ST) protocol[2], and mounted on neutral gum-coated slides. Part of the sections was counterstained with neutral red. The sections were examined with bright field microscopy. All photomicrographs (Figures 1A-1C) were taken with a photomicroscope (Olympus microscope) on panchromatic film (made in Japan). Magnified multiples of microscope and photograph were 20×, 10×, and 12.15×, respectively. The finally magnified multiple of the neurons was 2430.
To count numerical density on volume (Nv), numerical density on area (Na) and mean volume (v) of the HRP-positive neurons in the photograph of cervical dorsal root ganglia, anterior horn of spinal cord and facial nucleus of nerve, a square-net-test-system with 2500 counting grid was applied. The border length of the counting grid was 2 mm, and its area was 4 mm2. The value of area divided by magnified multiple was named practice value. The section number of the HRP-positive neurons, the crossing number falling on the section and reference testing crossing number were counted. And then, the Novena and v were computed according to stereological formula[3]. Every stereological parameter was expressed by mean±SD.
HRP positive neurons were green when they were not counterstained, and changed into dark green after being counterstained. In cytoplasm and processes many HRP positive particles were found, and those in the processes were in the pattern of a pearl string. One day after injection, the HRP positive neurons began to appear in 1-4 homolateral cervical dorsal root ganglia, the anterior horn of the 1-4 cervical spinal cord (layer VIII and IX), the ventromedial division of the facial nucleus, the accessory facial nucleus (Figure 1). The HRP positive particles in the HRP neurons of the above-mentioned regions declined with the increase of survival time, and those HRP positive particles in the cytoplasm and the process faded away on the 5th d. The Nv, Na, v of the HRP-positive neurons in cervical dorsal root ganglia, anterior horn of the 1-4 cervical spinal cords, and the ventromedial division of the facial nucleus referred to in Table 1.
| Nv (×103 mm-3) | Na (mm-2) | v (×10-5 mm3) | |
| Dorsal root ganglia | 2.65±2.39 | 31.85±20.46 | 5.12±3.27 |
| Anterior horn | 2.06±1.30 | 88.00±54.53 | 5.96±2.38 |
| Facial nucleus | 2.43±0.09 | 91.49±11.08 | 2.91±1.19 |
Acupunctures on the Fengch’ih acupoint can be categorized as: (1) the superficial needling, the most common one, with the needlepoint slanted toward the apex of nose, for treating many diseases; (2) deep needling, the special one, with the needle pointed straight toward homolateral nose for treating sequelae of apoplexy, toward the bottom of homolateral angularis for treating diseases in trunk, neck, limbs, throat, and trachea, toward throat for treating pseudobulbar paralysis, or slightly toward temporal for hemicrania, and (3) penetration needling, toward opposite side of Fengch’ih (Fengfu), mainly for cervical spondylosis. Then, what is the functioning of Fengch’ih? The following is an illustration of its nerve pathway based on the anatomy of Fengch’ih and the findings of the present experiment.
The acupoint of Fengch’ih is located at the intersection of trapeziums muscle and the upper end of sternocleidomastoid muscle, under the superior nuchal line pitting of inside the hairline. The sensory fibers of its skin and subcutaneous fascia come from the posterior branch of the 3rd cervical nerve and the branches of lesser occipital nerve. The sympathetic fibers originates from 8 and 1-2 thoracic lateral horn cells. At the acupoint is located lesser occipital nerve trunk, and deeper are lateral suboccipital triangle, occipital artery and vein, vertebral artery, and the posterior branch of the 1st cervical nerve. Lesser occipital nerve originates either from the anterior branch of the 2nd and the 3rd cervical nerve, or from the nerve loop between the two, then, along the posterior border of the end of sternocleidomastoid muscle, extends to the lateral scalp, and distributes behind auricle and in the lateral skin of occipital nerve. Its ramie communicates with the branches of greater occipital nerve and auriculotemporal nerve[4,5].
In addition, facial nerve branches off the stylomastoid foramen. Posterior auricular nerve controls occipital muscle, periauricular muscles, posterior belly of digastrics muscles and stylohyoid muscle[5].
In the present study, after the injection of HRP into Fengch’ih acupoint, HRP positive neurons were found in the regions of bilateral or homonymous 1st-4th cervical dorsal root ganglia and their corresponding anterior horns (layers VIII–IX) of the spinal cord, ventromedial division of the facial nucleus and the accessory facial nucleus. Based on the anatomical structure of Fengch’ih acupoint, it is assumed that HRP is carried through the branches of the 1st-4th cervical nerve to dorsal root ganglia directly, or straight to the moton-eurons of the anterior horn of the 1st-4th spinal cord. After HRP was injected into the sternocleidomastoid and trapezius muscles, all labeling were found ipsilaterally; retrogradely labeled cells were located in the C2-C4 dorsal root ganglia, and transganglionic labelings in the C1 to the rostral C6 spinal segments and in the medulla oblongata. Labeled terminal fields were the lamina VI, the central cervical nucleus and the ventral horn in the cervical spinal cord[6]. Injections of WGA-HRP or free HRP into rostral cervical dorsal root ganglia and HRP application to C2 and C3 dorsal rami produced labeling in dorsal and ventral horns at the level of entrance[7]. Injections of WGA-HRP into the second cervical dorsal root ganglion produced labeling in the dorsal and ventral horns. Injections of aqueous HRP into the suboccipital muscles produced heavy transganglionic label within the central cervical nucleus[8]. After injection of HRP into ganglia (C3) without involvement of the ventral roots and spinal nerves, a few ipsilateral spinal ventral horn neurons (C3) were retrogradely labeled with HRP. Subsequent to an HRP and wheat germ agglutinin WGA-HRP-mixture injection into the dorsal neck or suboccipital muscles, many spinal motoneurons (C3) were labeled retrogradely with an HRP mixture. These findings strongly suggest that some spinal motoneurons send their axon collaterals to the dorsal root ganglia, in which the terminals of the axon collaterals directly synapse with the dorsal root ganglion cells[9].
The neurons of the ventromedial division of facial nucleus were labeled retrogradely with an HRP through the posterior auricular nerve of the facial nerve. The facial nerve were six main branches which were the zygomatico-orbital, cervical, posterior auricular, anterior auricular, superior labial, and inferior labial branches[10]. The facial nucleus of a variety of species was divided cytoarchitectonically into the ventral, medial, intermediate, dorsal and lateral divisions. When horseradish peroxidase (HRP) was applied to the inferior labial, cervical or posterior auricular branch of the facial nerve, HRP-labeled neurons were seen in the lateral, ventral or medial division of the facial nucleus, respectively. After applying HRP to the anterior auricular-zygomatico-orbital branch, labeled neurons were observed mainly in the intermediate and dorsal divisions. HRP applied to the superior labial branch labeled neurons within the dorsal and lateral divisions[11-14]. The neurons of the accessory facial nucleus were labeled retrogradely with an HRP through the nerve supplying the posterior belly of the digastrics muscle in the accessory facial nucleus[15]. These results are consistent with those of ours.
The absence of HRP in the posterior horn of spinal cord, the cuneate nucleus, the gracile nucleus, the ambiguous nucleus, the nucleus of solitary tract, and the trigeminal nerve nucleus indicates that Fengch’ih functions without these nuclei. It is still uncertain whether the 1st-4th cervical nerve is directly related to facial nucleus and accessory facial nucleus, or to the anterior horn of spinal cord, whether facial nucleus and accessory facial nucleus are related to the anterior horn of the 1st-4th cervical spinal cord, which deserves further investigation.
This study would not have been possible without the Scientific Research Fund supported by the Health Department of Hubei Province. We gratefully thank accessory director physician, Yan WQ, for purchasing chemical reagent and accessory professor, Zhang XJ, for improving the paper. Lastly, we would like to express our gratitude to the help of all doctors and nurses of the Department of Neurology of Taihe Hospital Affiliated to Yunyang Medical College.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Sun X, Li XW. Acupuncture manipulation of the acupoint “Fengch’ih”. Beijing Zhongyiyao Daxue Xuebao. 1999;22:75-76. [Cited in This Article: ] |
| 2. | Gu YM, Chen YC, Ye LM. A new highly sensitive HRP-TMB method using sodium tungstate as a stabilizer, I Light microscopic study. Shenjing Jiepouxue Zazhi. 1990;6:121-127. [Cited in This Article: ] |
| 3. | Xi GM, Wang HQ, Tang TY, Zhou LH, Yuan YW, Fan HY, Ye TX. Development of the excitability amino acid neurons in hippocampal formation of postnatal rat. Shenjing Jiepouxue Zazhi. 1999;2:165-170. [Cited in This Article: ] |
| 4. | Wu ZS. Investigation on form and structure of the acupoint “ShangTian Zhu and Fengch’ih”. Shanghai Zhenjiu Zazhi. 1987;6:28-30. [Cited in This Article: ] |
| 5. | Li WD, Du Z, Fang ZQ, Wang LH. Investigation on anatomical structure and safety acupuncture. Zhongguo Zhenjiu. 1997;17:505-506. [Cited in This Article: ] |
| 6. | Ishii Y. Central afferent projections from the rat sternocleidomastoid and trapezius muscles. A study using transganglionic transport of horseradish peroxidase. Osaka Daigaku Shigaku Zasshi. 1989;34:193-212. [PubMed] [Cited in This Article: ] |
| 7. | Neuhuber WL, Zenker W. Central distribution of cervical primary afferents in the rat, with emphasis on proprioceptive projections to vestibular, perihypoglossal, and upper thoracic spinal nuclei. J Comp Neurol. 1989;280:231-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Prihoda M, Hiller MS, Mayr R. Central projections of cervical primary afferent fibers in the guinea pig: an HRP and WGA/HRP tracer study. J Comp Neurol. 1991;308:418-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kayahara T. Synaptic connections between spinal motoneurons and dorsal root ganglion cells in the cat. Brain Res. 1986;376:299-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Uemura-Sumi M, Manabe Y, Matsushima R, Mizuno N. Correlation of the main peripheral branches of the facial nerve with the cytoarchitectonic subdivisions of the facial nucleus in the guinea pig. Anat Embryol (Berl). 1986;174:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Hinrichsen CF, Watson CD. The facial nucleus of the rat: representation of facial muscles revealed by retrograde transport of horseradish peroxidase. Anat Rec. 1984;209:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Populin LC, Yin TC. Topographical organization of the motoneuron pools that innervate the muscles of the pinna of the cat. J Comp Neurol. 1995;363:600-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Satoda T, Takahashi O, Tashiro T, Matsushima R, Uemura-Sumi M, Mizuno N. Somatotopic organization of facial nucleus of rabbit. With particular reference to intranuclear representation of perioral branches of the facial nerve. Anat Anz. 1988;165:83-90. [PubMed] [Cited in This Article: ] |
| 14. | Watson CR, Sakai S, Armstrong W. Organization of the facial nucleus in the rat. Brain Behav Evol. 1982;20:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Terashima T, Kishimoto Y, Ochiishi T. Musculotopic organization of the facial nucleus of the reeler mutant mouse. Brain Res. 1993;617:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |