Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1753
Revised: July 27, 2004
Accepted: December 28, 2004
Published online: March 28, 2005
AIM: To investigate the expression of matrix metallopr-oteinase-2 and tissue inhibitor of metalloproteinase-1 in hepatic fibrosis and the antifibrogenic role of exogenous interleukin-10 (IL-10).
METHODS: Hepatic fibrosis was induced by CCl4 administration and 60 male Sprague-Dawley rats were randomly divided into normal control group (group N, 8 rats), CCl4-induced group (group C, 28 rats) and IL-10-treated group (group I, 24 rats). At the beginning of the 7th and 11th wk, rats in each group were routinely perfused with pronase E and type IV collagenase through portal vein catheter and the suspension was centrifuged by 11% Nycodenz density gradient to isolate hepatic stellate cells (HSCs). RT-PCR was used to analyze mRNA of MMP-2 and TIMP-1 from freshly isolated cells. Densitometric data were standardized with β-actin signals. Immunocytochemistry was performed to detect MMP-2 and TIMP-1 expression in HSC cultured for 72 h.
RESULTS: Compared to group N in the 7th wk, MMP-2 and TIMP-1 mRNA increased in group C (P = 0.001/0.001) and group I (P = 0.001/0.009). The level of MMP-2 and TIMP-1 mRNA in group I was significantly lower than that in group C (P = 0.001/0.001). In the 11th wk, MMP-2 mRNA in group I was still lower than that in group C (P = 0.005), but both dropped compared with that in the 7th week (P = 0.001/0.004). TIMP-1 mRNA in group I was still lower than that in group C (P = 0.001), and increased in group C (P = 0.001) while decreased in group I (P = 0.042) compared with that in the 7th wk. Same results were found by immunocytochemistry.
CONCLUSION: Expression of MMP-2 and TIMP-1 is increased in hepatic fibrosis. IL-10 exhibits an antifibrogenic effect by suppressing MMP-2 and TIMP-1 expression.
- Citation: Zheng WD, Zhang LJ, Shi MN, Chen ZX, Chen YX, Huang YH, Wang XZ. Expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in hepatic stellate cells during rat hepatic fibrosis and its intervention by IL-10. World J Gastroenterol 2005; 11(12): 1753-1758
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1753
Hepatic fibrosis is a common pathological change resulted from various chronic hepatic injuries characterized by an increase of extracelluar matrix (ECM) deposition in the Disse’s space and the imbalance between synthesis and degeneration of ECM[1,2]. There is an evidence that liver fibrosis is a potentially reversible process involving effective ECM degradation[3,4].
Hepatic stellate cells (HSCs) play a central role in the pathogenesis of liver fibrosis, a key feature of which is their ability to regulate matrix degradation in the liver[5]. Following liver injury, these cells proliferate and are activated to a profibrogenic myofibroblastic phenotype. In addition to increasing matrix protein synthesis, HSCs express a wide range of matrix metalloproteinases (MMPs) and specific tissue inhibitor of metalloproteinases (TIMPs). MMPs and TIMPs may play a significant role in hepatic fibrosis[6].
In the present study, rat hepatic fibrosis model was established, HSCs were isolated and the expression of MMP-2 and TIMP-1 in HSC was determined to investigate their possible roles during CCl4-induced hepatic fibrogenesis in rats and the effect of interleukin-10 (IL-10) on this change in vivo.
Sixty clean male Sprague-Dawley rats weighing 200-300 g (provided by Shanghai Experimental Animal Center) were divided randomly into three groups. The control group (group N) included 8 rats, the CCl4 group (group C) included 28 rats and the IL-10 group (group I) included 24 rats, respectively. All the rats were bred under routine conditions (room temperature, 22±2 °C; humidity, 55±5%; light, 12 h per day; free access to water and food). The rats of group N were injected intraperitoneally with saline 2 mL/kg, twice a week. The rats of group C and group I were injected intraperitoneally with 50% CCl4 (dissolved in castor oil) 2 mL/kg, twice a week. From the third week, the rats of group I were injected intraperitoneally with IL-10 4 μg/kg (dissolved in saline) 20 min before they were injected with CCl4. All injections were given twice a week until the rats were killed with their body weight determined before each injection. At the beginning of the 7th and 11th wk, two rats from each group were selected randomly for histological examination, five rats from each group were selected randomly for isolating HSCs.
In the 7th and 11th wk, two rats in each group were killed to collect liver samples. The liver tissues were fixed in 40 g/L formaldehyde and embedded with paraffin. Sections were stained with HE and examined under a light microscope.
In the 7th and 11th wk, five rats in each group were used to isolate HSCs. Isolation and identification of HSC were described previously[7]. Briefly, the rat liver was perfused via portal vein with 0.02% pronase E (Merck) and 0.025% type IV collagenase (Sigma). Cell suspension was centrifuged by 11% Nycodenz (Sigma) density gradient to isolate HSC. The viability of HSC was determined by trypan blue exclusion staining. The purity of HSC was identified by the expression of desmin (human anti-rat monoclonal antibody, DAKO) using immunocytochemistry S-P method.
Preparations of HSC were seeded onto 96-well plastic tissue culture plates at 1×106 cells/mL in DMEM containing 20% fetal calf serum. HSCs were incubated at 37 °C in an atmosphere of 50 mL/L CO2, most of HSCs attached to the dishes 72 h after primary culture. Then the 96-well plates were washed twice with 0.1 mol/L PBS and fixed with poly-formaldehyde at 4 °C overnight. The following procedures were performed according to the instructions of streptavidin/peroxidase (S-P) kit (Beijing Zhongshan Company). The dilution of goat anti-rat MMP-2 and TIMP-1 monoclonal antibody (Beijing Zhongshan Company) was 1:300. Briefly, cells were washed with PBS, incubated with bovine serum albumin in PBS, reacted with primary antibody dissolved in PBS, washed again and incubated with peroxidase-conjugated second antibody, washed again and reacted for 20 min with S-P. A brown reaction product was developed by incubation with a buffer containing 3,3-diaminobenzidine tetrahydrochloride (DAB). In the negative controls, the primary antibody was replaced by PBS. The reactions were assessed according to their intensities and percentage of the positive cells.
Total RNA was extracted from freshly isolated HSC according to the RNA isolation kit (Jingmei Biotechnology Company of Shenzhen) instructions. Then its quantity and purity were assessed by measuring the optical density at 260 and 280 nm, the ratio of A260 and A280 ranged from 1.8 to 2.0. One microgram of total RNA was reverse transcribed into complementary DNA (cDNA) according to the instructions of first strand cDNA synthesis kit (Fermentas Life Sciences Jingmei Biotechnology Company of Shenzhen). Twenty microliters of reaction mixtures were transcribed at 42 °C for 60 min, at 99 °C for 5 min.
PCR system contained 2 μL cDNA, 5 μL 10×buffer, 5 μL 25 mmol/L MgCl2, 1 μL 10 mmol/L dNTP, 1 μL 20 pmol/μL target gene sense and anti-sense primer, 1 μL 20 pmol/μL β-actin primer pair, 3 U Taq DNA polymerase. PCR was carried out as follows: initial denaturation at 95 °C for 5 min, 30 amplification cycles (denaturation at 94 °C for 45 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min) for MMP-2 or 28 amplification cycles (denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 1 min) for TIMP-1, and final extension at 72 °C for 7 min.
Primer sequences were as follows: MMP-2: sense: 5’-GTGCTGAAGGACACCCTCAAGAAGA-3’, antisense: 5’-TTGCCGTCCTTCTCAAAGTTGTACG-3’; TIMP-1: sense: 5’-GCCATGGAGAGCCTCTGTGG-3’, antisense: 5’-GCAGGCAGGCAAAGTGATCG-3’; Primers for β-actin were used as the internal control: sense: 5’-GAGCTATGAGCTGCCTGACG-3’, antisense: 5’-AGCACTTGCGGTCCACGATG-3’.
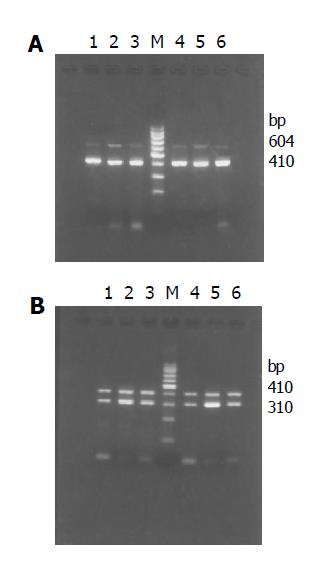
The PCR products were run on 2% agarose gel electrophoresis and visualized with ethidium bromide staining. The expected product sizes were 604 bp of MMP-2, 310 bp of TIMP-1 and 410 bp of β-actin. Bioimagine system was employed to detect the density of bands of PCR products. The values of MMP-2 and TIMP-1 expression were semi-quantified by scanning densitometry using the ratios of MMP-2/β-actin and TIMP-1/β-actin to assess the relative level. The detection was analyzed five times. SPSS10.0 was used to describe the difference between groups.
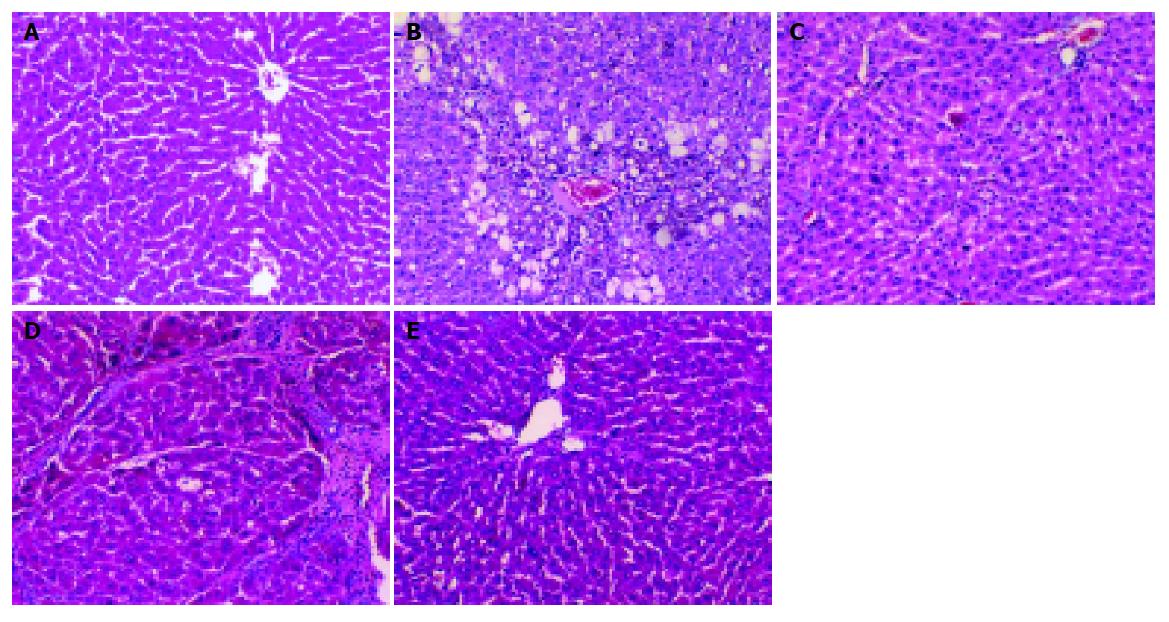
Specimens from group N showed normal structure of hepatic lobules (Figure 1A). Liver fibrosis became remarkable during the treatment with CCl4. In the 7th wk, specimens from group C showed that hepatic lobular structure was destroyed completely, steatosis and ballooning degeneration were obvious with lots of mononuclear cells and unusual neutrophils surrounding the centrilobular veins and fibrotic septa, the collagen fibers increased and began to extend to the parenchyma (Figure 1B). Only a few inflammatory cells infiltrated around centrilobular veins without evident changes of lobular structure in group I (Figure 1C). In the 11th wk, complete fibrous septa were seen and pseudolobular structures were also present occasionally in group C (Figure 1D). In group I, normal lobular structure was present and less fibrotic septa and inflammatory infiltrates were seen (Figure 1E). The results illustrated that fibrogenesis of group I was much less severe than that of group C.
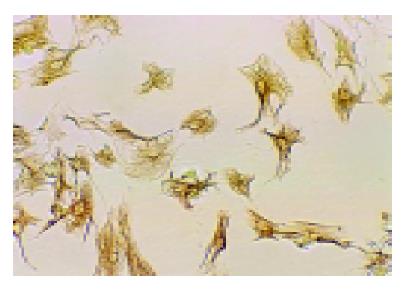
A total of 2-4.5×107 cells per rat were harvested. Cell vitality checked by trypan blue exclusion was higher than 95%. The mean purity of freshly isolated HSC was higher than 95% identified by the expression of desmin (Figure 2).
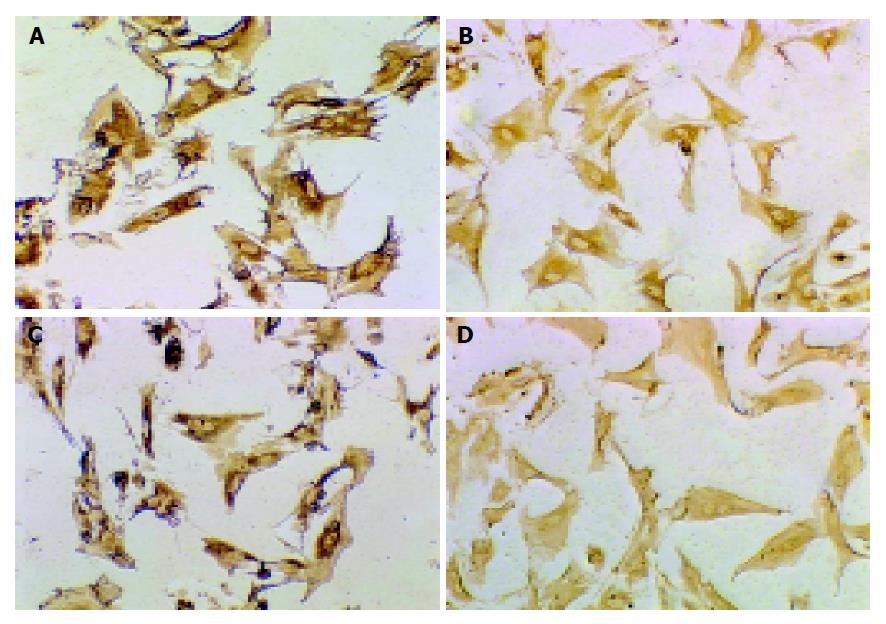
MMP-2 and TIMP-1 positive expressions were localized in cytoplasm and cell membrane of HSC in all groups by immunocytochemistry. In group N, the expression signals of MMP-2 and TIMP-1 were weak. In the 7th wk, the size of HSC in group C and group I was a little larger than that in group N. The number and length of pseudopodia, the expression signals of MMP-2 and TIMP-1 in group C were stronger than those in group I (Figure 3). In the 11th wk, cell phenotype in group I was a little smaller than that in the 7th wk. The expression signals of MMP-2 in group C decreased obviously compared to those in the 7th wk, while the expression signals of TIMP-1 in group C increased compared to those in the 7th wk. Though limited by the number of samples, the expression of TIMP-1 increased obviously with the development of hepatic fibrosis, and decreased after treatment with IL-10; however, the expression of MMP-2 increased in the earlier phase of hepatic fibrosis and decreased after treatment with IL-10.
In the 7th wk, MMP-2 and TIMP-1 mRNA increased obviously in group C compared with that in group N (P<0.01), and decreased significantly after treatment with IL-10 (P<0.01). In the 11th wk, MMP-2 mRNA in group I was still lower than that in group C (P<0.01), but both dropped compared with that in the 7th wk (P<0.01). TIMP-1 mRNA in group I was still lower than that in group C (P<0.01) and group I (P<0.05) compared with that in the 7th wk (Tables 1, 2 and Figure 4).
Liver fibrosis is traditionally considered as a progressive pathological process involving multiple cellular and molecular events that lead to deposition of excess matrix proteins in the extracellular space. When this process is combined with ineffective regeneration and repair, there is increasing distortion of the normal liver architecture, and the end result is cirrhosis[8,9].
Current evidence indicates that liver fibrosis is dynamic and can be bidirectional (involving progression and regression); this pathological process involves major changes in the regulation of matrix degradation[10].
In the extracellular space, matrix degradation occurs predominantly as a consequence of the action of a family of enzymes known as MMPs. MMPs are secreted from cells into the extracellular space as proenzymes, which are then activated by a number of specific, usually cell surface-associated cleavage mechanisms. The activated enzymes are in turn inhibited by a family of tissue inhibitors of metalloproteinases (TIMP-1 to -4). By this combination of mechanisms, ECM degradation is closely regulated, which prevents inadvertent tissue damage[11].
HSCs situated in the perisinusoidal Disse’s space play an essential role in liver fibrosis. In normal liver, HSCs are nonfibrogenic cells containing an abundant amount of vitamin A. After chronic liver injury, HSCs proliferate, lose their vitamin A, and transform to α-SMA-positive myofibroblastic cells that are the major source of collagens, glycoproteins, and proteoglycans accumulated in the fibrotic liver. A variety of growth factors and inflammatory cytokines produced by Kupffer cells, hepatocytes, and infiltrating leukocytes in injured livers induce HSC proliferation or matrix synthesis[12,13].
When cultured for several days on plates, HSCs from normal livers undergo activation remarkably similar to that occurring in vivo after liver injury[14].
IL-10 is an important immunoregulatory cytokine produced by many cell populations. Its main biological function is to limit and terminate inflammatory responses and regulate differentiation and proliferation of immune cells such as T cells, B cells, natural killer cells, and granulocytes[15-18]. IL-10 plays a role in inflammatory, malignant and autoimmune diseases and recombinant human IL-10 has been produced and tested in clinical trials, suggesting that IL-10 may become a new therapeutic target[19].
The knock-out experiments (IL-10/mice) indicated that endogenous IL-10 actually relieves CCl4-induced fibrosis[20,21]. Similar results have been reported by Nelson[22], but its mechanism remains obscure.
MMPs are able to degrade a wide variety ECM and play a pivotal role in regulating ECM composition. MMP-2 plays an important role in regulating basement membranes as it degrades several of its components including collagen IV, laminin, and fibronectin[23,24]. Our studies showed that compared with normal liver, expression of MMP-2 mRNA was increased several folds in CCl4-induced liver fibrosis in rats. The change promoted further degradation of the normal liver basement membranes, leading to increased activation and proliferation of HSC and synthesis of type I collagen. This positive feedback loop would theoretically promote the progression of liver fibrosis. The regulation of MMP-2 is not clear. Cytokine TGF-β1 has variable effects on MMP-2 expression and can promote synthesis and activation of MMP-2[25,26], and inflammatory cytokines upgrade the expression of MMP-2 during the earlier stage of liver fibrosis[27,28].
Furthermore, the present study showed that the level of MMP-2 decreased in the 11th wk compared with that in the 7th wk in hepatic fibrosis model group. Possibly, the metabolism of collagen could slow down in the later phase of hepatic fibrosis, and the negative feedback loop could degrade the expression of MMP-2. On the other hand, with the development of hepatic fibrosis, the increased TIMPs inhibit the secretion and activation of MMPs including MMP-2. In contrast to the other authors[29], our study showed weak expression in HSC isolated from normal group. Zhang et al, established CCl4-induced hepatic fibrosis model and IL-10 intervention model and reported that IL-10 can down-regulate the level of cytokines like TGF-β1, TNF-α and IL-6. Chen et al[30], investigated rat HSC cultured in vitro and found that IL-10 inhibits the expression of TGF-β1 and FGF, suggesting that IL-10 might inhibit the expression of MMP-2 indirectly by suppressing the expression of cytokines and growth factors.
The activities of MMPs are inhibited by TIMPs[31]. Four members of the TIMP family have been characterized so far, and designated as TIMP-1, TIMP-2, TIMP-3 and TIMP-4. TIMP-1 and TIMP-2 are capable of inhibiting the activities of all known MMPs and play a key role in maintaining the balance between ECM deposition and degradation in different physiological processes, including liver fibrosis development[32]. In the liver, TIMP-1 and TIMP-2 have been identified and TIMP-1 plays a more important role in the pathological process of liver fibrosis than TIMP-2[12,33,34].
In the present study, TIMP-1 expression in HSC was markedly up-regulated in group C and correlated with the histological degree of liver fibrosis. Because the expression of MMP-1 remains unchanged in liver at any stage of fibrosis[35], the strong expression of TIMP-1 inhibits the degeneration of collagen by MMP-1, thus promoting the deposition of ECM. The continuous deposition of collagen fibers in the liver finally results in hepatic fibrosis. The result suggests that TIMP-1 plays a pivotal role in liver fibrosis development. Whether TIMP-1 has other pathways to promote hepatic fibrosis is still unknown; further studies are needed to elucidate the mechanism.
Antifibrotics can be used to inhibit TIMP-1 expression. In the present study, we observed the effect of recombinant IL-10 on HSC expression of TIMP-1 in vivo. Our results indicate that IL-10 reduces collagen deposition, which may result from a decrease in TIMP-1 synthesis by HSCs. Because TIMP-1 and MMP-2 have a similar source, IL-10 might inhibit the expression of TIMP-1 indirectly also by suppressing the expression of cytokines and growth factors such as TGF-β1, TNF-α and IL-6.
In summary, MMP-2 expression increases in the early stage of hepatic fibrosis and TIMP-1 expression increases in the whole process of hepatic fibrosis. MMP-2 and TIMP-1 play an important role in liver fibrosis, IL-10 exhibits an antifibrogenic effect by suppressing MMP-2 and TIMP-1 expression.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Liu HL, Li XH, Wang DY, Yang SP. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 expression in fibrotic rat liver. World J Gastroenterol. 2000;6:881-884. [PubMed] [Cited in This Article: ] |
| 2. | Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017-7026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Hammel P, Couvelard A, O'Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 281] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 4. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 789] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 5. | McCrudden R, Iredale JP. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol Histopathol. 2000;15:1159-1168. [PubMed] [Cited in This Article: ] |
| 6. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] [Cited in This Article: ] |
| 7. | Zheng WD, Wang XZ, Zhang LJ, Shi MN. A simple in isolating rat hepatic stellate cells. Fujian Yike Daxue Xuebao. 2004;38:71-73. [Cited in This Article: ] |
| 8. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] [Cited in This Article: ] |
| 9. | Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Bode W, Fernandez-Catalan C, Grams F, Gomis-Rüth FX, Nagase H, Tschesche H, Maskos K. Insights into MMP-TIMP interactions. Ann N Y Acad Sci. 1999;878:73-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13 Suppl:S33-S38. [PubMed] [Cited in This Article: ] |
| 13. | Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39-S45. [PubMed] [Cited in This Article: ] |
| 14. | Burt AD. Pathobiology of hepatic stellate cells. J Gastroenterol. 1999;34:299-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58-S63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158:1033-1036. [PubMed] [Cited in This Article: ] |
| 17. | Wakkach A, Cottrez F, Groux H. Can interleukin-10 be used as a true immunoregulatory cytokine? Eur Cytokine Netw. 2000;11:153-160. [PubMed] [Cited in This Article: ] |
| 18. | Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20:281-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 665] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 202] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491-21494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3230] [Cited by in F6Publishing: 3096] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 24. | Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol. 2002;192:160-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Ma C, Chegini N. Regulation of matrix metalloproteinases (MMPs) and their tissue inhibitors in human myometrial smooth muscle cells by TGF-beta1. Mol Hum Reprod. 1999;5:950-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Martelli-Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD. Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol. 2003;74:296-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 207] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabró A, Ciancio G, Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528-537. [PubMed] [Cited in This Article: ] |
| 30. | Chen YX, Wang XZ, Weng SG, Chen ZX, Huang YH, Zhang LJ. Effects of IL-10 and PDGF on expression of TGF-β1 at hepatic stellate cells. Zhongxiyi Jiehe Ganbing Zazhi. 2002;12:343-345. [Cited in This Article: ] |
| 31. | Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111-122. [PubMed] [Cited in This Article: ] |
| 32. | Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Iredale JP, Goddard S, Murphy G, Benyon RC, Arthur MJ. Tissue inhibitor of metalloproteinase-I and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci (Lond). 1995;89:75-81. [PubMed] [Cited in This Article: ] |