Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.104
Revised: May 28, 2004
Accepted: June 25, 2004
Published online: January 7, 2005
AIM: To examine the effect of eradication of Helicobacter pylori prior to usage of NSAIDs, by investigating gastric inflammatory activity, myeloperoxidase (MPO) activity, prostaglandin (PG) E2 synthesis in H pylori-infected, and H pylori-eradicated gerbils followed by administration of indomethacin and rofecoxib.
METHODS: Six-week-old male gerbils were orally inoculated with H pylori. Seven weeks later, anti-H pylori triple therapy and vehicle were given to gerbils respectively and followed by oral indomethacin (2 mg/kg.d) or rofecoxib (10 mg/kg.d) for 2 wk. We examined the area of lesions, gastric inflammatory activity, PGE2 synthesis and MPO activity in the stomach.
RESULTS: In indomethacin and rofecoxib-treated gerbils, the following results were obtained in H pylori-infected group vs H pylori-eradicated group respectively: hyperplasia area of the stomach (mm2): 82.4±9.2 vs 13.9±3.5 (P<0.05), 30.5±5.1 vs 1.3±0.6 (P<0.05); erosion and ulcer area (mm2): 14.4±4.9 vs 0.86±0.5 (P<0.05), 1.3±0.6 vs 0.4±0.3 (P<0.05); score of gastritis: 7.0±0.0 vs 3.6±0.5 (P<0.05), 7.0±0.0 vs 2.7±0.5 (P<0.05); MPO activity (μmol H2O2/min/g tissue): 104.7±9.2 vs 9.0±2.3 (P<0.05), 133.5±15.0 vs 2.9±0.7 (P<0.05); PGE2 synthesis (pg/mg wet weight/min): 299.2±81.5 vs 102.8±26.2 (P<0.05), 321.4±30.3 vs 11.9±4.8 (P<0.05).
CONCLUSION: Eradication of H pylori reduced gastric damage of NSAID-treated Mongolian gerbils. Rofecoxib caused less severe gastric damage than indomethacin in H pylori-eradicated gerbils.
-
Citation: Chang CC, Chen SH, Lien GS, Lou HY, Hsieh CR, Fang CL, Pan S. Eradication of
Helicobacter pylori significantly reduced gastric damage in nonsteroidal anti-inflammatory drug-treated Mongolian gerbils. World J Gastroenterol 2005; 11(1): 104-108 - URL: https://www.wjgnet.com/1007-9327/full/v11/i1/104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.104
Helicobacter pylori is considered a major cause of acute and chronic gastritis, peptic ulcer disease, and is highly associated with the development of gastric mucosa associated lymphoid tissue lymphoma and gastric cancer[1-3]. Both NIH and IARC issued statements on the importance of H pylori eradication and carcinogenic risks in patients with H pylori infection[4,5]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are probably the most common cause of gastroduodenal injury in the United States of America today. Approximately half the patients who regularly take NSAIDs have gastric erosions, and 15-30% reveal ulcers on endoscopy examination[6]. In addition, severe side effects including gastrointestinal bleeding and perforation can be encountered after usage of aspirin or NSAIDs[7-11]. Both H pylori and NSAIDs are the major causes of peptic ulcer. However, their interaction is complex and controversial, with studies showing inconsistent results. Hawkey et al[12] reported that, in long-term NSAID users with past or current peptic ulcers or troublesome dyspepsia, eradication of H pylori led to impaired healing of gastric ulcers, and did not affect the rate of development of peptic ulcers or dyspepsia over 6 mo. However, Chan et al[13,14] reported that eradication of H pylori before use of NSAIDs lowered the occurrence of NSAID-induced ulcers in patient without peptic ulcer or previous exposure to NSAIDs. Because the gerbil model is quite suitable for in vivo study of the pathogenesis of H pylori-induced gastric diseases, it exhibits pathological features that mimic those of human patients with H pylori[15,16]. To examine the effect of eradication of H pylori prior to usage of NSAIDs, we investigated the gastric inflammatory activity, myeloperoxidase (MPO) activity and prostaglandin E2 (PG E2) production in H pylori-infected, H pylori-eradicated and H pylori-uninfected gerbils which were followed by giving indomethacin or rofecoxib.
Male Mongolian gerbils (6-wk-old, 40-50 g) were purchased from Seac Yoshitomi (Fukuoka, Japan). They were kept in an isolated clean room at a regulated temperature (20-22 °C) and humidity (50-55%) in a 12/12 h light-dark cycle. Gerbils were fasted for 24 h before and 4 h after H pylori inoculation. Four hours after H pylori inoculation, they were afforded water and food.
H pylori strains (ATCC 43504) were incubated in a brain-heart infusion broth containing 10% fetal bovine serum at 37 °C overnight under a microaerophilic atmosphere and allowed to grow to a density of 2.0×108 colony-forming units (CFU)/mL. The culture broth was orally inoculated into gerbils at a dosage of 1.0 mL/animal. Normal animals received 1 mL of the medium only.
A ‘triple therapy’ was performed. Amoxicillin, clarithromycin and lansoprazole were suspended in 0.5% w/w carboxymethyl cellulose (CMC) sodium salt solution and administered orally twice a day for four days at doses of 3, 30, 10 mg/kg body weight, respectively. CMC as a vehicle was administrated to H pylori- infected and H pylori-uninfected gerbils. The success rate of H pylori eradication is around 85.9%.
Indomethacin (non-selective COX inhibitor) and rofecoxib (selective COX-2 inhibitor) were orally given to two different groups of gerbils at does of 2 mg/kg and 10 mg/kg body weight once a day for 2 wk, respectively.
We performed H pylori culture and rapid urease test to confirm the existence of H pylori. The gerbils were sacrificed with ether anesthesia, and then their half-stomachs (right side) were excised. After approximately 50-100 g of the stomach was punched out for MPO activity examination, the rest part of the stomach was homogenized in 10 mL phosphate buffered saline with a Polytron, followed by dilution with the same buffer. Aliquots (100 μL) of the dilutions were applied to Brucella agar plates containing 10% horse blood (Nippon Bio-Test Laboratories, Tokyo, Japan), 2.5 μg/mL amphotericin B, 9 μg/mL vancomycin, 0.32 μg/mL polymyxin B, 5 μg/mL trimethoprim and 50 μg/mL 2, 3, 5-triphenyltetrazolium chloride. The plates were incubated at 37 °C under microaerophilic atmosphere (N2 850 mL/L, CO2 10 mL/L, O2 5%) for seven days. H pylori was identified as gold colonies in spiral shape under a microscope and positive for rapid urease test.
Gross observation The stomachs were opened along the greater curvature and washed with phosphate buffered saline, spread gently and fixed with pins on a cork board. All the stomachs were determined by an experienced researcher who was unaware of the treatment of the animals. Under a dissecting microscope, variable sizes and types of gastric lesions were checked and recorded with a square grid.
Microscopic observation Half of each stomach (left side) was fixed in 10% formalin and embedded with paraffin. Four μm thick sections were prepared and were stained with hematoxylin and eosin. The diagnosis of gastritis was made according to the modified criteria reported by Ohta et al[17] which was modified slightly from Rauwas et al[18]. The parameters of chronic active gastritis were as follows: lymphocyte infiltration (0: none, 1: mild infiltration to lamina propria, 2: moderate infiltration to lamina propria, 3: severe infiltration and lymphoid follicle formation), polymorphonuclear leukocyte infiltration (0: none, 1: the number of cells in lamina propria was <30 in the field of magnification ×400, 2: 30-100/field, 3 >100/field), superficial erosions (0: none, 1:deletion of surface epithelial cells). The total score of these variables varied from 0 to 7, and was used as a measure of the activity of gastritis. Microscopic gastritis was classified as non-gastritis (score: 0), mild gastritis (scores: 1-3); moderate gastritis (scores: 4-5) and severe gastritis (scores: 6-7). An experienced pathologist determined the gastric pathology without awareness of the prior treatment.
PGE2 production in gastric mucosae of the gerbils was determined according to the method of Lee and Feldman[19]. We punched out about 50-100 mg gastric specimen from the border of gastric antrum and corpus (right half side) in each gerbil. The specimen was placed in 50 mmol/L Tris HCl (pH 8.4) buffer and then minced with a pair of scissors. After the tissue samples were washed and resuspended in 2 mL of buffer, each sample was subjected to vortex mixing at room temperature for 1 min to stimulate PGE2 production, followed by centrifugation at 10000 g for 15 s. The PGE2 levels in the resulting supernatants were determined by means of an enzyme immunoassay (PGE2 EIA kit; Cayman Chemicals, Ann Arbor, Michigan, USA). PGE2 production was expressed as picograms of PGE2 per minute per milligram of tissue.
After removal of supernatants for PGE2 analysis, the MPO activity in the remaining tissue was determined by the method described by Takahashi and Keto[20,21]. Each sample (approximately 50-100 mg) was first homogenized with a Polytron in 1.0 mL of 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltri-methyl-ammonium bromide (Sigama), and then subjected to three sessions of freezing-thawing. Subsequently, the homogenates were centrifuged at 1600 g for 10 min at 4 °CAfter 5 μL aliquots of each supernatant was mixed with 145 μL of phosphate buffer (pH 6.0) containing 0.167 mg/mL o-dianisidine dihydrochloride (Sigama) and 0.0005% H2O2, the change in the rate of absorbance at 450 nm was measured with a microplate reader (Thermo Max; Molecular Devices, Sunnyvale, CA). The MPO activity was expressed as the degradation of H2O2 μmol/min/g tissue.
Data was presented as mean±SE from 5 to 7 animals per group. Student’s t-test and Fisher’s exact test were used for comparison between two groups. P<0.05 was considered statistically significant.
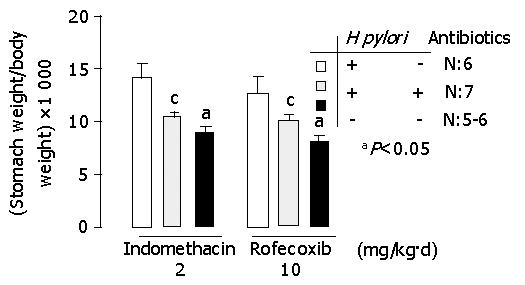
In indomethacin-treated gerbils, the ratio of stomach weight to body weight (×103) was 14.1±1.3, 10.5±0.3 and 9.0±0.3 in H pylori infected, H pylori-eradicated and H pylori-uninfected groups respectively (Figure 1). In rofecoxib treated gerbils, the ratio of stomach weight to body weight (×103) was 12.7±0.4, 10.1±0.3 and 8.1±0.4 respectively (Figure 1). Among gerbils treated with indomethacin or rofecoxib, the ratio of stomach weight to body weight increased significantly in H pylori-infected group than in H pylori-eradicated or H pylori-uninfected group (P<0.05). This data suggested that the ratio of stomach weight to body weight was decreased after H pylori eradication. Since the tissue weight was usually proportional to the inflammation, the stomach became less oedemata after H pylori eradication.
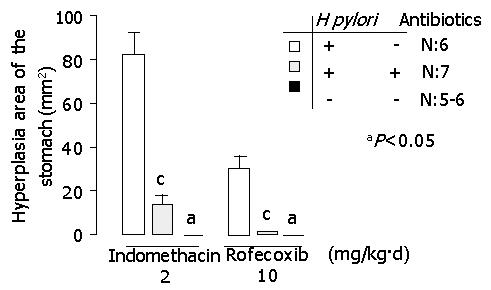
In indomethacin-treated gerbils, hyperplastic area of the stomach (mm2) was 82.4±9.2, 13.8±3.5 and 0 in H pylori-infected, H pylori-eradicated and H pylori-uninfected groups, respectively. In rofecoxib treated gerbils, hyperplasia area of the stomach (mm2) was 30.5±5.1, 1.3±0.6 and 0 respectively (P<0.05, H pylori infected group vs H pylori-eradicated or H pylori- uninfected group) (Figure 2).
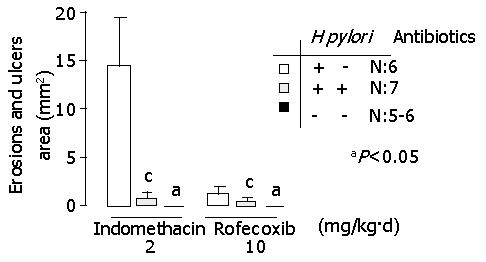
In indomethacin-treated gerbils, gastric erosion and ulcer areas of the stomach (mm2) were 14.4±4.9, 0.86±0.46 and 0 in H pylori infected, H pylori-eradicated and H pylori-uninfected groups, respectively (Figure 3). In rofecoxib-treated gerbils, those were 30.5±5.1, 1.3±0.6 and 0 in H pylori infected, H pylori-eradicated and H pylori-uninfected groups, respectively (Figure 3). Either treated with indomethacin or rofecoxib, more severe inflammation of the stomach was found in H pylori-infected group than in H pylori-eradicated or H pylori-uninfected group (P<0.05).
In addition, upper gastrointestinal bleeding was found in two out of seven gerbils (28.6%) in H pylori-infected and indomethacin treated groups. However, none of the gerbils suffered from upper gastrointestinal bleeding in other groups.
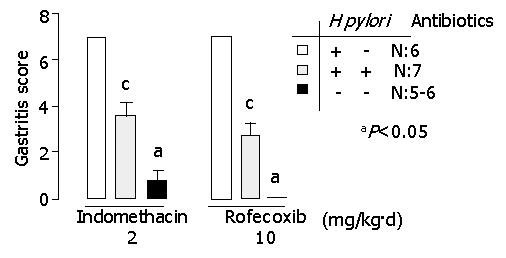
There was a more significant improvement of chronic active gastritis in H pylori-eradicated gerbils than in H pylori-infected gerbils, treated either with indomethacin or with rofecoxib (P<0.05) (Figure 4). The score of chronic active gastritis was shown in Table 1. No gastritis was noted in H pylori-uninfected gerbils treated with rofecoxib. Mild gastritis was found in H pylori-eradicated gerbils treated with rofecoxib and in some of H pylori-uninfected gerbils treated with indomethacin. Moderated gastritis was observed in H pylori-eradicated gerbils treated with indomethacin. Severe gastritis was observed in H pylori-infected gerbils.
| H pylori-infected | H pylori-eradicated | H pylori-uninfected | P | |
| Stomach weight ratio (×1000) | 14.1±1.3 | 10.5±0.3 | 9.0±0.3 | <0.05a |
| <0.05c | ||||
| Hyperplasia area (mm2) | 82.4±9.2 | 13.9±3.5 | 0 | <0.05a |
| <0.05c | ||||
| Erosion and ulcer area (mm2) | 14.4±4.9 | 0.9±0.5 | 0 | <0.05a |
| <0.05c | ||||
| Score of gastritis | 7.0±0.0 | 3.6±0.5 | 0.8±0.4 | <0.05a |
| <0.05c | ||||
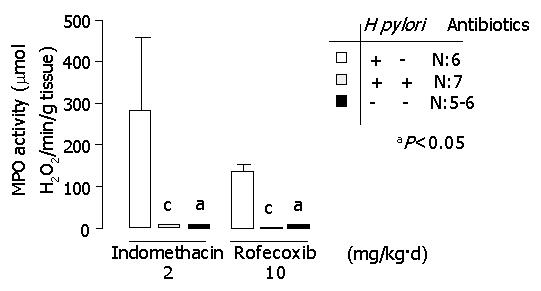
| MPO activity (mmoL H2O2/min/g tissue) | 104.7±9.2 | 8.9±2.3 | 7.8±2.5 | <0.05a |
| <0.05c | ||||
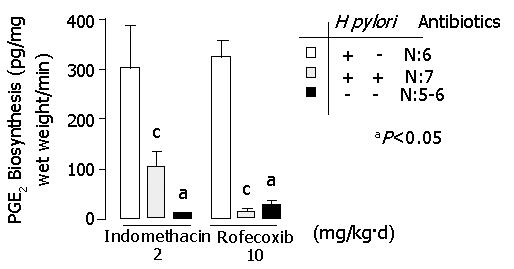
| PGE2 synthesis (pg/mg wet weight/min) | 299.2±81.5 | 102.8±26.2 | 9.6±1.3 | <0.05a |
| <0.05c |
MPO activity is demonstrated in Figure 5. A significantly lower activity of MPO was found in H pylori-uninfected or H pylori-eradicated groups compared to H pylori-infected groups (P<0.05). The data of each group are shown in Tables 1 and 2.
| H pylori-infected | H pylori-eradicated | H pylori-uninfected | P | |
| Stomach weight ratio (×1000) | 12.8±0.4 | 10.1±0.3 | 8.1±0.4 | <0.05a |
| <0.05c | ||||
| Hyperplasia area (mm2) | 30.5±5.1 | 1.3±0.6 | 0 | <0.05a |
| <0.05c | ||||
| Erosion and ulcer area (mm2) | 1.3±0.6 | 0.4±0.3 | 0 | <0.05a |
| <0.05c | ||||
| Score of gastritis | 7.0±0.0 | 2.7±0.5 | 0 | <0.05a |
| <0.05c | ||||
| MPO activity (mmoL H2O2/min/g tissue) | 133.5±15.0 | 2.9±0.7 | 6.6±1.7 | <0.05a |
| <0.05c | ||||
| PGE2 synthesis (pg/mg wet weight/min) | 321.4±30.3 | 11.9±4.8 | 25.9±15.9 | <0.05a |
| <0.05c |
Figure 6 demonstrates PGE2 synthesis in indomethacin- or rofecoxib-treated gerbils. PGE2 synthesis in H pylori-eradicated gerbils was decreased significantly in comparison with that in H pylori-infected gerbils (P<0.05). The data of each group are shown in Tables 1 and 2.
H pylori infection Indomethacin-treated gerbils had more severe gastric mucosa damages than rofecoxib-treated gerbils on the hyperplastic area, erosion and ulcer area of the stomach (P<0.05). There was no statistically significant difference between indomethacin-treated and rofecoxib-treated gerbils in the score of chronic active gastritis, MPO activity and PGE2 synthesis of the stomach (P>0.05).
H pylori eradication Rofecoxib was significantly superior to indomethacin on the less severe gastric mucosa damage in H pylori-eradicated gerbils (P<0.05). There was also less severity on the erosion and ulcer area of the stomach and score of chronic active gastritis in rofecoxib-treated gerbils than in indomethacin-treated gerbils, although there was no statistically significant difference (P>0.05).
This study clearly demonstrates that eradication of H pylori could significantly reduce gastric mucosa damage in Mongolian gerbils treated with indomethacin or rofecoxib.
Clinically, it has not been clear whether H pylori infection aggravates gastric mucosa injury in long-term NSAID users. Santucci et al[22] found that more severe gastroduodenal mucosa lesions developed after a four-week NSAID medication in H pylori-infected patients than in H pylori-uninfected patients. However, other studies showed no evidence of remarkable injury when H pylori infection coexisted with NSAID use. Graham et al[23] reported gastric erosions in 34% and bleeding in 32% of patients who also had H pylori infection, representing a lower incidence than in patients without H pylori infection (57% and 61%, respectively). Recently, several investigators reported more severe gastric damage in H pylori-infected and NSAIDs-treated gerbils. Takahashi et al[24] suggested that NSAIDs synergistically aggravated gastric lesions in moderate H pylori infection, but not in severe gastritis. In addition, Yoshida et al[25] reported that H pylori infection potentiated aspirin-induced gastric mucosal injury in Mongolian gerbils.
What most physicians are concerned about is the necessity for patients to receive anti-H pylori therapy before using NSAIDs. It remains still controversial. Two important clinical studies gave conflicting results. Hawkey and colleagues[12] reported that, in long-term NSAID users with past or current peptic ulcers or troublesome dyspepsia, eradication of H pylori led to impaired healing of gastric ulcers, and did not affect the rate of development of peptic ulcers or dyspepsia in over 6 mo. However, Chan and colleagues[14] reported that eradication of H pylori before usage of NSAIDs lowered the occurrence of NSAID-induced ulcers in patients without peptic ulcer or previous exposure to NSAIDs. In our study, we used the gerbil model which was consecutively treated with NSAIDs (indomethacin or rofecoxib) to investigate the severity of gastric inflammation among H pylori-eradicated, H pylori-infected and H pylori-uninfected groups.
From gross observation, the most severe gastric mucosa damage was found in H pylori-infected and indomethacin treated groups. Around 28.6% of them suffered from gastric ulcer with bleeding. However, there was no gastric ulcer with bleeding in H pylori-infected and rofecoxib treated groups. Less severe damage of gastric mucosa caused by rofecoxib might be due to the selective COX-2 inhibition. The most important finding in our study was that eradication of H pylori prior to administration of NSAIDs (indomethacin or rofecoxib) could significantly reduce gastric damage from macroscopic and/or microscopic observations.
MPO activity in gastric mucosa is an indicator of infiltration of neutrophils into mucosae. Keto and colleagues[26] reported that MPO activity was lower in indomethacin-treated gerbils than in H pylori infected and indomethacin-treated gerbils. It seems that H pylori infection may aggravate gastric mucosa damage in gerbils treated with NSAIDs. Perhaps, eradication of H pylori may lead to improvement of mucosal inflammation. Keto et al[21] proved that MPO activity was lower in H pylori- eradicated gerbils than in controls. However, we found a significant reduction of MPO activity in H pylori-eradicated gerbils treated with NSAIDs (indomethacin or rofecoxib).
PGE2 might increase blood flow, secretion of mucus and bicarbonate, inhibit acid secretion, and directly protect gastric cells against toxic stimuli[27]. In response to H pylori infection, COX-2 expression is absent in normal mucosa, but is profoundly induces in H pylori-positive gastritis. COX-2 expression was higher in H pylori infected gerbils than in normal controls. The COX-2 level was higher in gerbils infected with H pylori for 12 wk than in those are infected with H pylori for 12 wk[24]. The production of PGE2 in the stomach of gerbils was significantly higher in H pylori-infected groups than in normal controls[24]. In our study, we observed a significant reduction of PGE2 production in gastric mucosa of gerbils after consecutive treatment with NSAIDs (indomethacin or rofecoxib) in H pylori-eradicated group in comparison with that in H pylori-infected group.
Selective COX-2 inhibitors are believed to cause less severe gastroduodenal mucosa damage than non-selective COX inhibitors. Our results have shown that selective COX-2 inhibitors are partially superior to conventional NSAIDs in H pylori- infected and H pylori-eradicated gerbils.
It is very important to elucidate the interaction between H pylori and NSAIDs on the injury of gastric mucosa and the necessity of H pylori eradication before using NSAIDs. In conclusion, H pylori infection profoundly aggravates gastric mucosa damage following the use of NSAIDs (indomethacin or rofecoxib). Eradication of H pylori can significantly reduce gastric mucosa damage in consecutive NSAIDs-treated Mongolian gerbils.
The authors thank Mr. Kenji Nakjima, Miss Keiko Kanatani, Dr. Kikuko Amagase and Professor Susumu Okabe for their instructions and help.
Edited by Wang XL
| 1. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] [Cited in This Article: ] |
| 2. | Hentschel E, Brandstätter G, Dragosics B, Hirschl AM, Nemec H, Schütze K, Taufer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 524] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2805] [Cited by in F6Publishing: 2668] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 4. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 752] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | IARC monographs on the evaluation of carcinogenic risks to humancarcinogenic risks to human. Schistomomes, liver flukes and Helicobacter pylori: Geneva: WHO, International agency for research on cancer. Monography. 1994;61:177-220. [Cited in This Article: ] |
| 6. | Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 390] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832-1847. [PubMed] [Cited in This Article: ] |
| 9. | García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 655] [Cited by in F6Publishing: 614] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992;327:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 718] [Cited by in F6Publishing: 660] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Laine L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am. 1996;6:489-504. [PubMed] [Cited in This Article: ] |
| 12. | Hawkey CJ, Tulassay Z, Szczepanski L, van Rensburg CJ, Filipowicz-Sosnowska A, Lanas A, Wason CM, Peacock RA, Gillon KR. Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Helicobacter Eradication for Lesion Prevention. Lancet. 1998;352:1016-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Chan FK, Chung SC, Suen BY, Lee YT, Leung WK, Leung VK, Wu JC, Lau JY, Hui Y, Lai MS. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 2001;344:967-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 480] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Chan FK, To KF, Wu JC, Yung MY, Leung WK, Kwok T, Hui Y, Chan HL, Chan CS, Hui E. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 2002;359:9-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31:24-28. [Cited in This Article: ] |
| 17. | Ohta T, Shibata H, Kawamori T, Iimuro M, Sugimura T, Wakabayashi K. Marked reduction of Helicobacter pylori-induced gastritis by urease inhibitors, acetohydroxamic acid and flurofamide, in Mongolian gerbils. Biochem Biophys Res Commun. 2001;285:728-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94:33-40. [PubMed] [Cited in This Article: ] |
| 19. | Lee M, Feldman M. Age-related reductions in gastric mucosal prostaglandin levels increase susceptibility to aspirin-induced injury in rats. Gastroenterology. 1994;107:1746-1750. [PubMed] [Cited in This Article: ] |
| 20. | Takahashi S, Keto Y, Fujita H, Muramatsu H, Nishino T, Okabe S. Pathological changes in the formation of Helicobacter pylori-induced gastric lesions in Mongolian gerbils. Dig Dis Sci. 1998;43:754-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Keto Y, Takahashi S, Okabe S. Healing of Helicobacter pylori-induced gastric ulcers in Mongolian gerbils: combined treatment with omeprazole and clarithromycin. Dig Dis Sci. 1999;44:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Santucci L, Fiorucci S, Patoia L, Di Matteo FM, Brunori PM, Morelli A. Severe gastric mucosal damage induced by NSAIDs in healthy subjects is associated with Helicobacter pylori infection and high levels of serum pepsinogens. Dig Dis Sci. 1995;40:2074-2080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Graham DY, Lidsky MD, Cox AM, Evans DJ, Evans DG, Alpert L, Klein PD, Sessoms SL, Michaletz PA, Saeed ZA. Long-term nonsteroidal antiinflammatory drug use and Helicobacter pylori infection. Gastroenterology. 1991;100:1653-1657. [PubMed] [Cited in This Article: ] |
| 24. | Takahashi S, Fujita T, Yamamoto A. Nonsteroidal anti-inflammatory drug-induced acute gastric injury in Helicobacter pylori gastritis in Mongolian gerbils. Eur J Pharmacol. 2000;406:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Yoshida N, Sugimoto N, Hirayama F, Nakamura Y, Ichikawa H, Naito Y, Yoshikawa T. Helicobacter pylori infection potentiates aspirin induced gastric mucosal injury in Mongolian gerbils. Gut. 2002;50:594-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Keto Y, Ebata M, Tomita K, Okabe S. Influence of Helicobacter pylori infection on healing and relapse of acetic acid ulcers in Mongolian gerbils. Dig Dis Sci. 2002;47:837-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |