Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.427
Revised: July 15, 2003
Accepted: July 24, 2003
Published online: February 1, 2004
AIM: The diagnosis of cholangiocarcinoma is often difficult, making management approaches problematic. A reliable serum marker for cholangiocarcinoma would be a useful diagnostic test. The aims of our study were to evaluate the usefulness of a serum CA19-9 determination in the diagnosis of cholangiocarcinoma.
METHODS: We prospectively measured serum CA19-9 and CEA concentrations in patients with cholangiocarcinoma (n = 35), benign biliary diseases (n = 92), and healthy individuals (n = 15). Serum CA19-9 and CEA concentrations were measured by an immunoradiometric assay without knowledge of the clinical diagnosis.
RESULTS: The sensitivity of a CA19-9 value > 37 KU·L-1 and a CEA value > 22 μg·L-1 in diagnosing cholangiocarcinoma were 77.14% and 68.57%, respectively. When compared with the benign biliary diseases group, the true negative rates of serum CA19-9 and CEA were 84.78% and 81.52%, respectively. The false positive rates of serum CA19-9 and CEA were 15.22% and 18.48%, whereas the accuracy of serum CA19-9 and CEA were 82.68% and 77.95%, respectively. Serum CA19-9 and CEA concentrations were significantly elevated (P < 0.001 and P < 0.05) in patients with cholangiocarcinoma (290.31 ± 5.34 KU·L-1 and 36.46 ± 18.03 μg·L-1) compared with patients with benign biliary diseases (13.38 ± 2.59 KU·L-1 and 13.84 ± 3.85 μg·L-1) and healthy individuals (12.78 ± 3.69 KU·L-1 and 11.48 ± 3.37 μg·L-1). In 15 patients undergoing curative resection of cholangiocarcinoma, the mean serum CA19-9 concentration was decreased from a preoperative level of 286.41 ± 4.36 KU·L-1 to a postoperative level of 62.01 ± 17.43 KU·L-1 (P < 0.001), and the mean serum CEA concentration from 39.41 ± 24.35 μg·L-1 to 28.69 ± 11.03 μg·L-1(P < 0.05). In patients with cholangiocarcinoma, however, no correlation was found between serum CEA and CA19-9 concentrations (r = 0.036).
CONCLUSION: These data suggest that the serum CA19-9 determination is a useful addition to the available tests for the differential diagnosis of cholangiocarcinoma. Serum CA19-9 is an effective tumor marker in diagnosing cholangiocarcinoma, deciding whether the tumor has been radically resected and monitoring effect of treatment.
- Citation: Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J Gastroenterol 2004; 10(3): 427-432
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.427
Cholangiocarcinoma is a malignant tumor arising from bile duct epithelium. Unlike most human cancers, a pathological diagnosis of cholangiocarcinoma is often extremely difficult because of its location, size, and desmoplastic characteristics[1-3]. Percutaneous fine needle aspiration is frequently not possible because many of these tumors are located in the liver hilum amid large vascular structures[4,5]. Furthermore, tumor masses are often not even identifiable by CT, ultrasound, or magnetic resonance imaging[6-8]. Endoscopic approaches are also of limited usefulness in tissue diagnosis because of the desmoplastic nature of these cancers. Indeed, bile cytology obtained at endoscopic retrograde cholangiography has a sensitivity of only 33% - 56%[1,9-11], endobiliary brush cytology of 50% - 68%[12-14], and endoscopic transpapillary biopsy of 53% - 86% for detecting cholangiocarcinoma[15-18]. Because of the problems in obtaining diagnostic tissues, treatment and management decisions for patients with biliary disease that may be malignant are problematic.
Of the two possible tumor markers available for detecting cholangiocarcinomas, carcinoembryonic antigen (CEA) is a glycoprotein tumor marker with the immunodeterminant present on the protein moiety of the molecule. The other, carbohydrate antigen 19-9 (CA19-9), is a mucin-type glycoprotein in serum with the immunodeterminant present on the carbohydrate moiety of the molecule. Both tumor markers have been investigated for the diagnosis of malignancies in the stomach, colon and pancreas[19-23] but have not been gained widespread use in bile duct. It has been reported that the sensitivity and specificity for CA19-9 value > 37 KU·L-1 for cholangiocarcinoma with primary sclerosing cholangitis (PSC) were 60% to 93% and 78% to 98%, respectively[9,16,21,24-27]. The corresponding indexes of CEA value > 22 μg·L-1 were 53% to 84% and 50% to 79%[25-30]. Although widely used as a tumor marker, the clinical value of serum CA19-9 determination in the diagnosis of cholangiocarcinoma in the absence of PSC is unknown. Thus, the objective of this study was to determine the clinical usefulness of CA19-9 value for cholangiocarcinoma.
From January 1995 to February 2003, we prospectively obtained serum samples from patients undergoing evaluation for benign and malignant biliary disease at the First Hospital of Xi’an Jiaotong University and Luoyang Central Hospital. Serum samples were also prospectively obtained from healthy individuals who served as the disease control group. Patients with the diagnosis of PSC were excluded from this study. Clinical information was obtained by a thorough review of the medical histories. This study included 35 patients with cholangiocarcinoma, 92 patients with benign biliary diseases, and 15 healthy individuals. Of the 35 patients with cholangiocarcinoma, the diagnosis was established by surgical biopsy in 25 patients, endoscopic biopsy and brushing in six patients, and fine needle aspiration in four patients. In patients with cholangiocarcinoma, the stage and resectability of the tumors were ascertained using information obtained by imaging studies or at the time of surgery. Unresectability was defined by Bismuth stage 4 cancer arising from the right and left hepatic ducts and extending intrahepatically. Patients with intra-and extrahepatic metastasis were also deemed unresectable.
The benign biliary diseases group consisted of 92 patients and included 26 patients with benign bile duct stricture, 36 patients with cholecystolithiasis, 20 patients with cholecystic polyp, and 10 patients with chronic cholecystitis. The other control group consisted of 15 healthy individuals without any disease.
Blood samples obtained from patients were stored at -20 °C until used. CA19-9 and CEA were assayed by means of an immunoradiometric method with a commercially available CA19-9 RIA diagnostic kit (ELISA-CA19-9, CIS Bio International, France) and CEA RIA diagnostic kit (CIS Bio Industries, Gif-Sur-Yvette, France). Cutoff values recommended for diagnostic purpose were 37 KU·L-1 for CA19-9 and 22 μg·L-1 for CEA. Values above the cutoff concentrations were considered positive in this study. Sensitivity in detecting each group was compared between CA19-9 and CEA.
The results were expressed as mean values ± standard deviation of the mean (mean ± SD). Statistical analysis was performed using a statistical program (SPSS, 11.0 Inc, Chicago, IL). Statistical significance in mean values was evaluated by the Student’s t test. The one-way analysis of variance (ANOVA) was used to compare the different groups, and the Mann-Whitney rank sum test was used for intergroup comparisons. The relationships between CA19-9 and CEA, total bilirubin, alkaline phosphatase, or AST were determined by linear regression analysis.
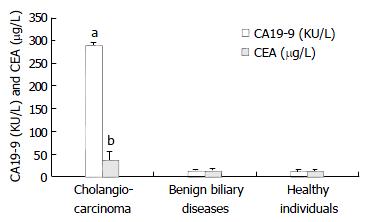
The Table 1 shows some of the characteristics of the different groups. Patients with cholangiocarcinoma were significantly older than either patients with benign biliary diseases or healthy individuals. However, the mean total serum bilirubin and serum alkaline phosphatase values were significantly higher in patients with cholangiocarcinoma, compared to the other two groups (P < 0.05). Thus, the patients with cholangiocarcinoma had a more marked cholestatic profile than the other two groups of patients.
| Characteristics | Cholangiocarcinoma | Benign biliary diseases | Healthy individuals |
| N | 35 | 92 | 15 |
| Age (yr) | 60.37 ± 11.2 | 49.78 ± 10.6 | 27.6 ± 4.7 |
| Sex (M/F) | 19/16 | 39/53 | 15/0 |
| Total bilirubin (umol/L) | 47.4 ± 2.1a | 8.3 ± 0.7 | 8.6 ± 0.9 |
| AST (U/L) | 79 ± 21.2a | 26 ± 3.5 | 21 ± 4.6 |
| ALP (U/L) | 193 ± 41.8a | 114 ± 50.6 | 90 ± 18.3 |
| CA19-9 (KU/L) | 290.31 ± 5.34bc | 13.38 ± 2.59 | 12.78 ± 3.69 |
| CEA (μg/L) | 36.46 ± 18.03a | 13.84 ± 3.85 | 11.48 ± 3.37 |
The mean CA19-9 and CEA concentrations were significantly greater in the cholangiocarcinoma group than those in the benign biliary diseases group or healthy individuals group (Figure 1). Table 1 shows the mean serum CA19-9 concentration in patients with cholangiocarcinoma was 290.31 ± 5.34 KU·L-1 in comparison with 13.38 ± 2.59 KU·L-1 in the benign biliary diseases group and 12.78 ± 3.69 KU·L-1 in the healthy individuals group (P < 0.001). Their corresponding mean serum CEA concentrations were 36.46 ± 18.03 μg·L-1, 13.84 ± 3.85 μg·L-1 and 11.48 ± 3.37 μg·L-1, respectively (P < 0.05).
Table 2 shows the distribution of serum CA19-9 and CEA values in patients with cholangiocarcinoma and benign biliary diseases. Of the 35 patients with cholangiocarcinoma, 27(77.14%) had a concentration exceeding 37 KU·L-1 in serum CA19-9. Using a CA19-9 concentration of 37 KU·L-1, the true negative rate of a CA19-9 for cholangiocarcinoma was 84.78% when assessed as the benign biliary diseases. The sensitivity (true positive) and specificity (true negative) of CEA were 68.57% and 81.52%, respectively (Table 2). Among the 92 patients with benign biliary diseases, the false positive rates of serum CA19-9 > 37 KU·L-1 and CEA > 22 μg·L-1 were 15.22% (14/92) and 18.48% (17/92), respectively. The combination of CA19-9 and CEA showed the highest sensitivity and specificity, they were 91.43% and 76.09% when CA19-9 or CEA was positive, and they were 62.86% and 86.96% when both CA19-9 and CEA were positive, respectively. If the cutoff value was increased from 37 KU·L-1 to 100 KU·L-1, the sensitivity and specificity of CA19-9 were 65.71% (23/35) and 88.04% (81/92), respectively
| CA19-9△ | CA19-9 | CEA | CA19-9 or CEA | CA19-9 and CEA | |
| Sensitivity(true positive)* | 65.71%(23/35) | 77.14%(27/35)a | 68.57%(24/35) | 91.43%(32/35) | 62.86%(22/35) |
| Specificity(true negative)** | 88.04%(81/92) | 84.78%(78/92) | 81.52%(75/92) | 76.09%(70/92) | 86.96%(80/92) |
| Positive predictive value | 67.65%(23/34) | 65.85%(27/41) | 58.54%(24/41) | 59.26%(32/54) | 64.71%(22/34) |
| Negative predictive value | 87.10%(81/93) | 90.70%(78/86) | 87.21%(75/86) | 95.89%(70/73) | 88.89%(80/90) |
| Accuracy | 81.89%(104/127) | 82.68%(105/127) | 77.95%(99/127) | 80.31%(102/127) | 80.31%(102/127) |
| False positive rate | 11.98%(11/92) | 15.22%(14/92) | 18.48%(17/92) | 23.91%(22/92) | 13.04%(12/92) |
In patients with cholangiocarcinoma, no correlation was found between CEA and CA19-9 concentration (r = 0.036). Likewise, no correlation was found among serum CA19-9, or ALP, AST, and total bilirubin (r = 0.015, r = 0.037 and r = 0.145, respectively). Thus, elevated serum CA19-9 value could not be attributed to either cholestasis or hepatocellular injury.
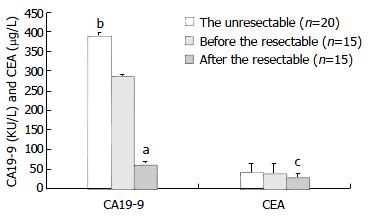
Of the 35 patients with cholangiocarcinoma, 15 patients underwent curative resection and 20 had unresectable cancer. The mean preoperative and postoperative serum CA19-9 levels in the respectable group were 286.41 ± 4.36 KU·L-1 and 62.01 ± 17.43 KU·L-1, respectively. The corresponding indexes of serum CEA were 39.41 ± 24.35 μg·L-1 and 28.69 ± 11.03 μg·L-1, respectively (Figure 2 and Table 3). Thus, patients after curative resection had significantly lower mean serum CA19-9 and CEA concentrations than those before operation (P < 0.001 and P < 0.05). The mean serum CA19-9 concentration in the respectable group was 286.41 ± 4.36 KU·L-1 as compared to 391.37 ± 5.76 KU·L-1 in the unresectable group (Figure 2 and Table 3). Thus, patients with unresectable disease had a significantly higher mean serum CA19-9 concentration than those with respectable disease (P < 0.05).
Perineural invasion by malignant cells was found in 23 of the 33 patients and more frequently encountered in cancer of the upper portion of extrahepatic bile ducts. Of the 35 tumors, lymph node metastasis was seen in 18 and venous invasion in 17. Histologically, 35 examples of cholangiocarcinoma were composed of 16 well-differentiated adenocarcinomas, 12 moderately-differentiated adenocarcinomas, 5 poorly-differentiated adenocarcinomas, 1 anaplastic carcinoma, and 1 adenosquamous carcinoma (Table 4). Table 4 shows statistically significant differences in serum CA19-9 and CEA between positive and negative lymph node metastases (P < 0.01, P < 0.05), perineural invasion (P < 0.01, P < 0.05), and venous invasion (P < 0.05). However, there appears to be no relationship between the degrees of differentiation of carcinomas and the values of serum CEA and CA19-9 diagnosis of pancreatic and biliary malignant diseases. Recently, its usefulness has been demonstrated in the staging, evaluation of resectability, and assessment of prognosis and recurrence, as well as in the initial diagnosis of malignant disorders, especially pancreatic cancer[31-37].
| No. of | CA19-9 | CEA | |
| patients | (KU/L) | (μg/L) | |
| Well-differentiated | 16 | 261.67 ± 6.31 | 34.37 ± 11.29 |
| adenocarcinoma | |||
| Moderately-differentiated | 12 | 270.56 ± 4.57 | 38.42 ± 19.23 |
| adenocarcinoma | |||
| Poorly-differentiated | 5 | 302.71 ± 5.81 | 48.23 ± 23.64 |
| adenocarcinoma | |||
| Anaplastic carcinoma | 1 | 468 | 64 |
| Adenosquamous carcinoma | 1 | 293 | 27 |
| Perineural infiltration | |||
| Positive | 23 | 310.12 ± 7.11a | 37.64 ± 10.35b |
| Negative | 10 | 195.16 ± 6.48 | 21.28 ± 13.15 |
| Venous invasion | |||
| Positive | 17 | 318.49 ± 3.27b | 39.78 ± 18.41b |
| Negative | 18 | 253.08 ± 5.37 | 29.67 ± 20.13 |
| Lymph node metastasis | |||
| Positive | 18 | 345.66 ± 4.23a | 46.37 ± 9.46b |
| Negative | 17 | 265.30 ± 4.58 | 31.89 ± 16.46 |
Since koprowski et al[19] discovered CA19-9 from the human colon cancer cell line, which has been commonly used in the diagnosis of pancreatic and biliary malignant diseases. Recently, its usefulness has been demonstrated in the staging, evaluation of resectability, and assessment of prognosis and recurrence, as well as in the initial diagnosis of malignant disorders, especially pancreatic cancer[31-37].
Elevated concentration of serum CA19-9 (> 37 KU·L-1) in bile duct cancers has been frequently reported. Torzilli et al[38] described a positive rate of 97% in patients with cholangiocarcinoma, whereas Hultcrantz et al[39] described a rate of 76%, and Caturelli et al[40] described a 68% rate. We have also found an elevation of CA19-9 in 77.14% of cholangiocarcinomas, which was comparable to the rates reported in other studies. Furthermore, as shown in Table 2, the application of CA19-9 for differentiating cancer from benign biliary diseases was inspiring in our study because of its high sensitivity and specificity. The sensitivity and specificity in our study were 77.14% and 84.78%, respectively, which were similar to 78.2% and 81.4% reported by kau et al[41]. However, the concentration of CA19-9 could raise in patients with benign inflammatory conditions as well as in malignant disease[42-45]. Indeed, Ahrendt et al[46] reported a moderate increase in CA19-9 concentration in 13.8% of patients with benign biliary tract diseases. The positive serum rate was 28% in patients with acute cholangitis, as reported by Ker et al[47], and 35.6% in patients with bile duct stones, as reported by Shiozawa et al[48]. In our present study, an increased concentration of CA19-9 was found in 15.22% of patients with benign biliary diseases. According to Ker et al[47], CA19-9 was synthesized by normal biliary ductal cells and malignant cells. If bile flow is blocked by biliary obstruction in benign conditions, such as choledocholithiasis, epithelial cells will be markedly impaired by inflammation and will proliferate concurrently. As a result, more CA19-9 may be secreted and leaked out into the bloodstream. An elevated CA19-9 level often returns to normal with appropriate decompression of the common duct or relief of acute cholangitis. However, the increment of CA19-9 in benign diseases is usually not significant, and a concentration of CA19-9 exceeding 480 KU·L-1 is rare. In our study, only 2(2.17%) patients with benign biliary diseases exhibited CA19-9 levels > 480 KU·L-1.
CEA is mainly secreted by digestive glandular cancers and their metastases. It has also been found in other types of cancer such as breast, lung, ovary, thyroid cancers. The sensitivity and specificity of serum CEA in our study were 68.57% and 81.52%, respectively, which were similar to 63.3% and 78.4% reported by Ramage et al[49]. The false positive rate of serum CEA was 18.48% (14/92). Serum CA19-9 was obviously superior to serum CEA in the diagnosis of cholangiocarcinoma and often considered the standard marker for pancreatic cancer and cholangiocarcinoma, with which other markers were compared[50-53]. Our data also showed a much higher sensitivity with serum CA19-9 than with serum CEA in detecting cholangiocarcinomas, but the sensitivity and specificity could be raised by combining these two tumor markers. Furthermore, no correlation between the levels of serum CEA and CA19-9 was found (r = 0.036).
Serum CA19-9 has been reported to be able to predict resectability of cholangiocarcinoma[54-57]. In our study, marked elevation of serum CA19-9 tended to associate with advanced and unresectable biliary cancers. Both serum CA19-9 and CEA levels had a positive correlation with tumor stage. This positive correlation is theoretically helpful in assessing the therapeutic effect and monitoring tumor recurrence after treatment. CA19-9 and CEA have been claimed to have a prognostic value in cholangiocarcinomas[7,21,24,30,58]. When applied to cholangiocarcinomas, both tumor markers could provide valuable prognostic information. In our study, serum CA19-9 and CEA concentrations were measured in 15 of 35 patients with cholangiocarcinoma after undergoing curative resection. Within two weeks after operation, the average concentration of serum CA19-9 and CEA was 62.01 ± 17.43KU·L-1 and 28.69 ± 11.03 μg·L-1, respectively, with an obvious decrease compared with those before operation (P < 0.001 and P < 0.05). Hence, serum CA19-9 could provide more important diagnostic and prognostic values than CEA in cholangiocarcinoma.
In summary, serum CA19-9 is an effective tumor marker in diagnosing cholangiocarcinoma, deciding whether the tumor has been radically resected and monitoring the effect of treatment. Serum CA19-9 determination is a useful adjunct in our diagnostic armamentarium for cholangiocarcinoma. However, serum CA19-9 has limitations in diagnosing cholangiocarcinoma. Indeed, our data clearly demonstrated that a negative test could not exclude cholangiocarcinoma. Moreover, for a patient suspected of having biliary cancer, consideration of the presence of acute cholangitis or cholestasis is suggested. If the patient has no evidence of acute cholangitis or cholestasis, a cutoff value of 37 KU·L-1 may be appropriate. However, if a patient shows symptoms and signs of acute cholangitis or cholestasis, application of CA19-9 should be delayed until after recovery from acute conditions or a cutoff value of 100 KU·L-1 should be used. Additional bile-or serum-based tests are needed in the diagnosis of cholangiocarcinoma. Ultimately, a profile of tests analogous to liver biochemistry measurement of serum or bile tumor markers and genetic analysis for cholangiocarcinoma-associated mutations will need to be developed to assist in the diagnosis of this disease.
This work was supported by the Department of Hepatobiliary Surgery, First Hospital of Xi’an Jiaotong University and Luoyang Central Hospital.
Edited by Zhang JZ and Wang XL
| 1. | Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Ueno N, Sano T, Kanamaru T, Tanaka K, Nishihara T, Idei Y, Yamamoto M, Okuno T, Kawaguchi K. Adenosquamous cell carcinoma arising from the papilla major. Oncol Rep. 2002;9:317-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Saito M, Hige S, Takeda H, Tomaru U, Shibata M, Asaka M. Combined hepatocellular carcinoma and cholangiocarcinoma growing into the common bile duct. J Gastroenterol. 2001;36:842-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Matsumoto A, Imamura M, Akagi Y, Kaibara A, Ohkita A, Mizobe T, Isomoto H, Aoyagi S. A case report of disseminated recurrence of inferior bile duct carcinoma in PTCD fistula. Kurume Med J. 2002;49:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, Abu-Elmaagd K, Madariaga JR, Slivka A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002;56:40-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385-391. [PubMed] [Cited in This Article: ] |
| 7. | Kinoshita H, Tanimura H, Uchiyama K, Tani M, Onishi H, Yamaue H. Prognostic factors of intrahepatic cholangiocarcinoma after surgical treatment. Oncol Rep. 2002;9:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Kijima H, Takeshita T, Suzuki H, Tanahashi T, Suto A, Izumika H, Miki H, Terasaki Y, Nakamura M, Watanabe H. Carcinosarcoma of the ampulla of Vater: a case report with immunohistochemical and ultrastructural studies. Am J Gastroenterol. 1999;94:3055-3059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Desa LA, Akosa AB, Lazzara S, Domizio P, Krausz T, Benjamin IS. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut. 1991;32:1188-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Davidson B, Varsamidakis N, Dooley J, Deery A, Dick R, Kurzawinski T, Hobbs K. Value of exfoliative cytology for investigating bile duct strictures. Gut. 1992;33:1408-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Shiota K, Taguchi J, Nakashima O, Nakashima M, Kojiro M. Clinicopathologic study on cholangiolocellular carcinoma. Oncol Rep. 2001;8:263-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Jiao W, Yakushiji H, Kitajima Y, Ogawa A, Miyazaki K. Establishment and characterization of human hilar bile duct carcinoma cell line and cell strain. J Hepatobiliary Pancreat Surg. 2000;7:417-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Kurzawinski T, Deery A, Dooley J, Dick R, Hobbs K, Davidson B. A prospective controlled study comparing brush and bile exfoliative cytology for diagnosing bile duct strictures. Gut. 1992;33:1675-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Ferrari Júnior AP, Lichtenstein DR, Slivka A, Chang C, Carr-Locke DL. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc. 1994;40:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465-467. [PubMed] [Cited in This Article: ] |
| 17. | Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Lindberg B, Arnelo U, Bergquist A, Thörne A, Hjerpe A, Granqvist S, Hansson LO, Tribukait B, Persson B, Broomé U. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 872] [Cited by in F6Publishing: 836] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Louhimo J, Finne P, Alfthan H, Stenman UH, Haglund C. Combination of HCGbeta, CA 19-9 and CEA with logistic regression improves accuracy in gastrointestinal malignancies. Anticancer Res. 2002;22:1759-1764. [PubMed] [Cited in This Article: ] |
| 21. | Nanashima A, Yamaguchi H, Nakagoe T, Matsuo S, Sumida Y, Tsuji T, Sawai T, Yamaguchi E, Yasutake T, Ayabe H. High serum concentrations of sialyl Tn antigen in carcinomas of the biliary tract and pancreas. J Hepatobiliary Pancreat Surg. 1999;6:391-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Safi F, Schlosser W, Falkenreck S, Beger HG. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253-259. [PubMed] [Cited in This Article: ] |
| 23. | Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431-434. [PubMed] [Cited in This Article: ] |
| 24. | Hyman J, Wilczynski SP, Schwarz RE. Extrahepatic bile duct stricture and elevated CA 19-9: malignant or benign. South Med J. 2003;96:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology. 2002;49:616-620. [PubMed] [Cited in This Article: ] |
| 26. | Kitagawa Y, Iwai M, Muramatsu A, Tanaka S, Mori T, Harada Y, Okanoue T, Kashima K. Immunohistochemical localization of CEA, CA19-9 and DU-PAN-2 in hepatitis C virus-infected liver tissues. Histopathology. 2002;40:472-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Qin XL, Shi JS, Shi L, Wang ZR, Wang L. Clinical value of CA19-9 determination in patients with bile duct carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:814-815. [Cited in This Article: ] |
| 28. | Giannini E, Borro P, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Risso D, Lantieri PB. Cholestasis is the main determinant of abnormal CA 19-9 levels in patients with liver cirrhosis. Int J Biol Markers. 2000;15:226-230. [PubMed] [Cited in This Article: ] |
| 29. | Watanabe M, Chigusa M, Takahashi H, Nakamura J, Tanaka H, Ohno T. High level of CA19-9, CA50, and CEA-producible human cholangiocarcinoma cell line changes in the secretion ratios in vitro or in vivo. In Vitro Cell Dev Biol Anim. 2000;36:104-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 30. | Qin XL, Li ZQ, Shi JS, Zhang L, Wang ZR, Wang L. Value of bile and serum CA19-9 and CEA in diagnosing biliary tract Carcinoma. Chin J Bases Clin Genered Surg. 2000;7:161-163. [Cited in This Article: ] |
| 31. | Haglund C, Ylätupa S, Mertaniemi P, Partanen P. Cellular fibronectin concentration in the plasma of patients with malignant and benign diseases: a comparison with CA 19-9 and CEA. Br J Cancer. 1997;76:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Böttger TC, Junginger T. Treatment of tumors of the pancreatic head with suspected but unproved malignancy: is a nihilistic approach justified. World J Surg. 1999;23:158-162; discussion 162-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Ogawa T, Yokoi H, Kawarada Y. A case of inflammatory pseudotumor of the liver causing elevated serum CA19-9 levels. Am J Gastroenterol. 1998;93:2551-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Montgomery RC, Hoffma JP, Ross EA, Riley LB, Ridge JA, Eisenberg BL. Biliary CA 19-9 values correlate with the risk of hepatic metastases in patients with adenocarcinoma of the pancreas. J Gastrointest Surg. 1998;2:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Goetz M, Steen PD. False elevation of CA 19-9 levels in a patient with a history of pancreatic cancer. Am J Gastroenterol. 1997;92:1390-1391. [PubMed] [Cited in This Article: ] |
| 36. | Brown RW, Campagna LB, Dunn JK, Cagle PT. Immunohistochemical identification of tumor markers in metastatic adenocarcinoma. A diagnostic adjunct in the determination of primary site. Am J Clin Pathol. 1997;107:12-19. [PubMed] [Cited in This Article: ] |
| 37. | Zhao XY, Yu SY, Da SP, Bai L, Guo XZ, Dai XJ, Wang YM. A clinical evaluation of serological diagnosis for pancreatic cancer. World J Gastroenterol. 1998;4:147-149. [PubMed] [Cited in This Article: ] |
| 38. | Torzilli G, Makuuchi M, Ferrero A, Takayama T, Hui AM, Abe H, Inoue K, Nakahara K. Accuracy of the preoperative determination of tumor markers in the differentiation of liver mass lesions in surgical patients. Hepatogastroenterology. 2002;49:740-745. [PubMed] [Cited in This Article: ] |
| 39. | Hultcrantz R, Olsson R, Danielsson A, Järnerot G, Lööf L, Ryden BO, Wahren B, Broomé U. A 3-year prospective study on serum tumor markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 1999;30:669-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Caturelli E, Bisceglia M, Villani MR, de Maio G, Siena DA. CA 19-9 production by a cystadenoma with mesenchymal stroma of the common hepatic duct: a case report. Liver. 1998;18:221-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Kau SY, Shyr YM, Su CH, Wu CW, Lui WY. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg. 1999;188:415-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Minato H, Nakanuma Y, Terada T. Expression of blood group-related antigens in cholangiocarcinoma in relation to non-neoplastic bile ducts. Histopathology. 1996;28:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Björnsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999;19:501-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Lin CL, Changchien CS, Chen YS. Mirizzi's syndrome with a high CA19-9 level mimicking cholangiocarcinoma. Am J Gastroenterol. 1997;92:2309-2310. [PubMed] [Cited in This Article: ] |
| 45. | Horsmans Y, Laka A, van Beers BE, Descamps C, Gigot JF, Geubel AP. Hepatobiliary cystadenocarcinoma without ovarian stroma and normal CA 19-9 levels. Unusually prolonged evolution. Dig Dis Sci. 1997;42:1406-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ahrendt SA, Pitt HA, Nakeeb A, Klein AS, Lillemoe KD, Kalloo AN, Cameron JL. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3:357-367; discussion 367-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Ker CG, Chen JS, Lee KT, Sheen PC, Wu CC. Assessment of serum and bile levels of CA19-9 and CA125 in cholangitis and bile duct carcinoma. J Gastroenterol Hepatol. 1991;6:505-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Shiozawa K, Ishii K, Mori T, Takamura N, Ikehara T, Shinohara M, Kawafune T, Sumino Y, Nonaka H. Heterochronous devel-opment of intrahepatic cholangiocellular carcinoma following hepatocellular carcinoma in a hepatitis B virus carrier. Intern Med. 2001;40:624-630. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Haglund C, Lundin J, Kuusela P, Roberts PJ. CA 242, a new tumour marker for pancreatic cancer: a comparison with CA 19-9, CA 50 and CEA. Br J Cancer. 1994;70:487-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Tsuji M, Kashihara T, Terada N, Mori H. An immunohistochemi-cal study of hepatic atypical adenomatous hyperplasia, hepato-cellular carcinoma, and cholangiocarcinoma with alpha-fetoprotein, carcinoembryonic antigen, CA19-9, epithelial mem-brane antigen, and cytokeratins 18 and 19. Pathol Int. 1999;49:310-317. [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Dorandeu A, Raoul JL, Siriser F, Leclercq-Rioux N, Gosselin M, Martin ED, Ramée MP, Launois B. Carcinoma of the ampulla of Vater: prognostic factors after curative surgery: a series of 45 cases. Gut. 1997;40:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Forsmark CE, Lambiase L, Vogel SB. Diagnosis of pancreatic cancer and prediction of unresectability using the tumor-associated antigen CA19-9. Pancreas. 1994;9:731-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, Kurimoto K, Ohashi Y. Prognostic values of preoperative and postoperative CEA and CA19.9 levels in pancreatic cancer. Pancreas. 1994;9:735-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Nakamura S, Suzuki S, Sakaguchi T, Konno H, Baba S, Kosugi I, Muro H. Second cancer during long-term survival after resection of biliary tract carcinoma. J Gastroenterol. 1996;31:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 58. | Su WC, Chan KK, Lin XZ, Lin PW, Chow NH, Shin JS, Chen CY, Tsao CJ. A clinical study of 130 patients with biliary tract cancers and periampullary tumors. Oncology. 1996;53:488-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |