Published online Jan 15, 2004. doi: 10.3748/wjg.v10.i2.190
Revised: July 23, 2003
Accepted: July 30, 2003
Published online: January 15, 2004
AIM: To evaluate the effects of p53 on apoptosis and proliferation of hepatocellular carcinoma (HCC) cells treated with transcatheter arterial chemoembolization (TACE).
METHODS: A total of 136 patients with HCC received TACE and other management before surgery were divided into TACE group and non-TACE group. TACE group included 79 patients who had 1-5 courses of TACE before surgery, of them, 11 patients had 1-4 courses of chemotherapy (group A), 33 patients had 1-5 courses of chemotherapy combined with iodized oil (group B), 23 patients had 1-3 courses of chemotherapy, iodized oil and gelatin sponge (group C), 12 patients had 1-3 courses of chemotherapy combined with iodized oil, ethanol and gelatin sponge (group D). Non-TACE group included the remaining 57 patients who had surgery only. The extent of apoptosis was analyzed by transferase mediated dUTP nick end labeling (TUNEL) staining. The expressions of p53, Bcl-2, Bax, Ki-67 and PCNA protein were detected by immunohistochemical method.
RESULTS: p53 protein expressions in trabecular and clear cells in HCC specimens were significantly lower than that in pseudoglandar, solid, poorly differentiated or undifferentiated and sclerosis HCC (P < 0.05). Expression of p53 protein in HCC cells increased with the increase of pathological grades (P < 0.05), and correlated positively with expressions of Ki-67 and PCNA protein, and negatively with Bcl-2 to Bax protein expression rate and AI (P < 0.05). Expression of p53 protein was significantly higher in group A than in groups B, C, D and the non-TACE group, and was higher in group B than in groups C and D, and lower in group D than in the non-TACE group (P < 0.05).
CONCLUSION: Expression of p53 protein can enhance proliferation of HCC cells and suppress apoptosis of HCC cells after TACE.
- Citation: Xiao EH, Li JQ, Huang JF. Effects of p53 on apoptosis and proliferation of hepatocellular carcinoma cells treated with transcatheter arterial chemoembolization. World J Gastroenterol 2004; 10(2): 190-194
- URL: https://www.wjgnet.com/1007-9327/full/v10/i2/190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i2.190
Hepatocellular carcinoma (HCC) is one of the most common malignancies. Surgical resection has been recognized as the most effective method for the treatment of HCC[1,2], but it is only indicated for small number of HCC patients. Transcatheter arterial chemoembolization (TACE) has become one of the most popular and effective palliative methods for HCC. Various mixtures of anticancer drugs, Lipiodol and gelatin sponge have been used as TACE agents. There have been a few reports on comparison of the efficacy of different TACE regimens on HCC patients[3].
Cellular homeostasis in tissue depends on the balance between apoptosis and cell proliferation. Wild-type p53 protein inhibits the growth of tumor by arrest of cell proliferation and induction of apoptosis[4]. Bcl-2 and Bax protein are important regulators of apoptosis[5]. Bcl-2 proteins can prolong cell survival by suppressing apoptosis, and Bax proteins can enhance apoptosis[6]. PCNA and Ki-67 protein are useful markers for proliferative activity[7]. PCNA functions as a cofactor of DNA-polymerase and an important mark for evaluating the proliferation of colon cancer[8], gastric adenocarcinoma[9], H pylori associated gastric epithelial lesions[10], lung cancer[11], ovarian cancer[12],large intestine polyps[13] and HCC[14,15] .
As far as we know, the effect of p53 on apoptosis and proliferation of HCC cells treated with different TACE regimens has not been investigated yet. In particular, it is unclear whether p53 can affect apoptosis and proliferation of HCC cells treated with TACE by modulating the expressions of Bcl-2, Bax, PCNA and Ki-67 proteins. In the present study, we examined the effects of p53 on apoptosis and proliferation of HCC cells treated with TACE alone or in combination with others.
From February 1992 to February 2001, 136 patients with HCC were referred to our hospital for surgery, including 122 men and 14 women with a mean age of 45 years (range 20 to 70 years). Preoperative ultrasound (US), computed tomography (CT), magnetic resonance (MRI), digital subtraction angiography (DSA) and plasma AFP levels were used to diagnose the conditions and the diagnosis was finally confirmed with pathological biopsy.
The patients were divided into TACE or non-TACE group. In TACE group, 79 patients underwent 1-5 courses chemoembolization prior to liver resection. Of them, 11 patients had 1-4 courses of chemotherapy only (group A), 33 patients had 1-5 courses of chemotherapy combined with iodized oil (group B), 23 patients had 1-3 courses of chemotherapy, iodized oil and gelatin sponge (group C), 12 patients had 1-3 courses of chemotherapy combined with iodized oil, ethanol and gelatin - sponge (group D). Considering the course of TACE, 50 patients underwent one course, 19 patients underwent two courses, 10 patients underwent three or more courses. The interval from the last TACE to the surgery was 52.8 ± 12.2 days (-x±s), 25 patients had 1 month or less, 29 patients had 2 months or less, 16 patients had 3 months or less, 9 patients had over 3 months. In non-TACE group, 57 patients had surgery without preoperative TACE. The types of hepatectomy were dependent on the location of tumor, the severity of concomitant hepatic cirrhosis and preoperative hepatic function.
Transferase-mediated dUTP nick end labeling (TUNEL) staining was used to examine apoptosis. Positive control slides were treated with DNAse-1 and negative controls were stained in the absence of terminal deoxynucleotidyl transferase enzyme. Dark brown nuclei with nuclear condensation in stained cells were considered as TUNEL positive. Apoptotic index was the ratio of the number of positively stained tumor cells to the total number of tumor cells.
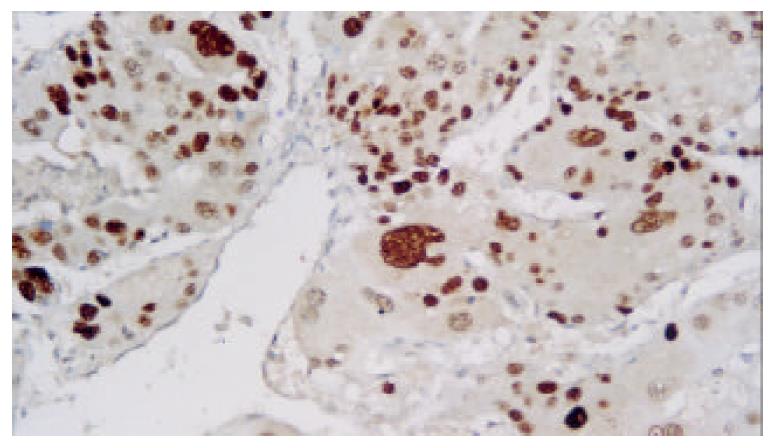
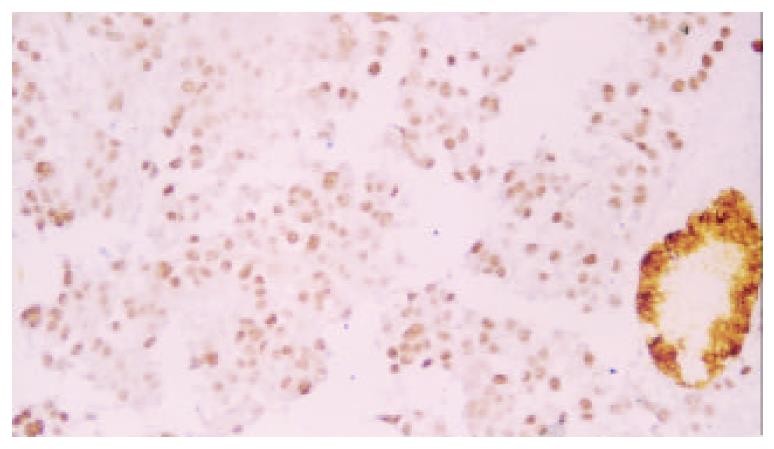
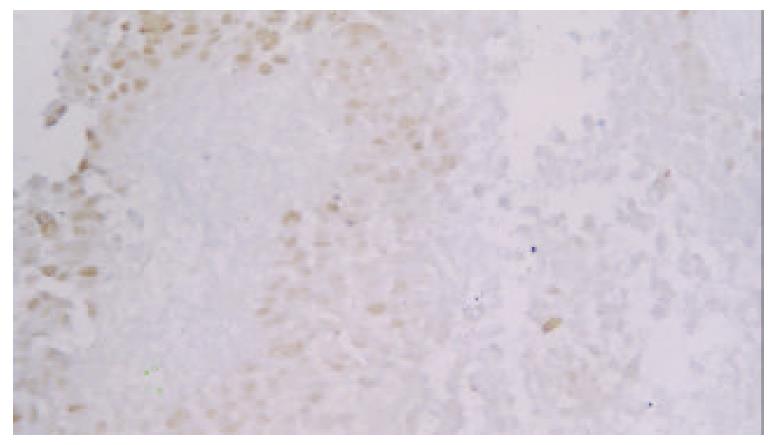
The formalin-fixed, paraffin-embedded specimens were examined immunohistochemically using respective antibodies to p53 M7001 (dilution:1:100), Bcl-2 MO887(dilution:1:60), Bax A3533 (dilution:1:200), Ki-67 M7187(dilution:1:50) and PCNA M0879 (dilution:1:200) (LSAB kit Dako). Positive controls were selected cases known to be positive for the primary antibody, such as laryngeal carcinoma or normal lymph nodes. Negative controls were stained with a nonspecific Ig G (normal rabbit Ig G) and Tris-buffered saline. Brown-yellow staining in nuclei of cancer was found in p53, Ki-67 and PCNA positive cells, while brown-yellow staining in cytoplasm and/or cell membrane was observed in Bcl-2 and Bax positive cells. All slides were reviewed and scored by two independent observers in blind. A few cases with discrepant scoring were reevaluated to reach a final agreement.
Data were expressed as -x±s and analyzed by means of SPSS 10.0 software package (SPSS, Chicago, IL, USA, 1999). The Student t test, the Crosstabs (chi-square and Fisher,s exact probability test), K independent samples and Pearson rank correlation coefficient test were used to test the correlation between parameters. A P value < 0.05 was considered statistically significant.
P53 protein expression in trabecular and clear cells of HCC was significantly lower than that in pseudoglandar, solid, poorly differentiated or undifferentiated and sclerosis HCC (P < 0.05, Table 1).
| Groups | Histological types | |||||
| Trabecular | Pseudoglandar | Solid | Clear cell | Poorly differentiated | Sclerosis | |
| Non-TACE | 63.66 ± 19.96 | 72.29 ± 12.47 | 70.83 ± 24.45 | 68.27 ± 19.22 | 74.79 ± 16.18 | 72.11 ± 0.00 |
| TACE | 56.90 ± 17.12 | 72.35 ± 13.70 | 74.93 ± 8.76 | 62.15 ± 11.78 | 73.66 ± 8.54 | |
| P | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
Pathological grades were divided into four groups. Expression of p53 protein in HCC cells increased as the increase of pathological grade in non-TACE or TACE group (P < 0.05). p53 protein expression in grade II specimens in TACE group was significantly lower than that in non-TACE group (P < 0.05, Table 2).
| Treat groups | I | II | III | IV |
| Non-TACE | 60.99 ± 30.58 | 67.22 ± 15.53 | 72.98 ± 20.60 | 93.47 ± 0.00 |
| TACE | 32.59 ± 11.68 | 60.02 ± 14.67 | 74.69 ± 8.65 | 82.64 ± 1.11 |
| P | > 0.05 | < 0.05 | > 0.05 | > 0.05 |
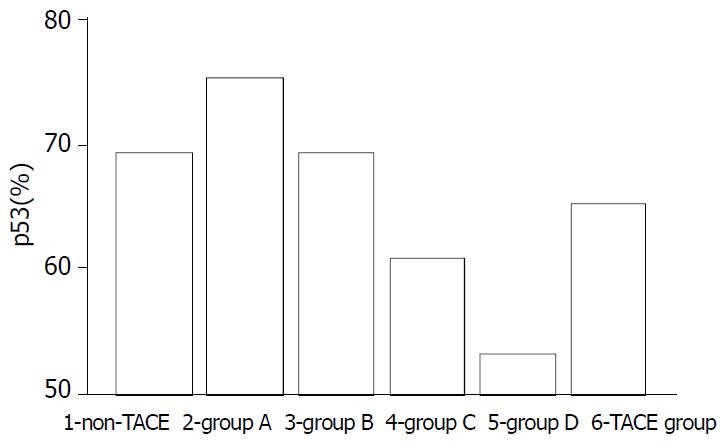
Expression of p53 protein in HCC cells was 69.37% ± 18.81% in the non-TACE group, 65.09% ± 15.71% in TACE group, 75.34% ± 5.36% in group A, 69.34% ±12.59% in group B, 60.94% ± 17.24% in group C, and was 53.41% ± 18.13% in group D. Expression of p53 protein was significantly higher in group A than in groups B, C, D and non-TACE group, and was higher in group B than in groups C and D, and lower in group D than in non-TACE group (P < 0.05, Figure 1, Figure 2, Figure 3 and Figure 4).
Expression of p53 protein in HCC cells was 69.37% ± 18.81% in non-TACE group, 65.76% ± 13.96% in one-course of TACE group, 64.09% ± 19.81% in two-courses of TACE group, and 65.19% ± 17.80% in three or more courses of TACE group. No statistical difference was found among groups (P > 0.05).
Considering the interval from the last TACE to the operation, expression of p53 protein was 69.37% ± 18.81% in non-TACE group, 63.54% ± 13.72% in TACE group with an interval ≤ 1 month, 61.17% ± 18.32% in TACE group with a 1-2 months interval, 70.87% ± 15.06% in TACE group with a 2-3 months interval, and 73.67% ± 5.87% in TACE group with an interval > 3 months. p53 protein expression was significantly lower in patients with an interval ≤ 2 months than that in patients with an interval > 3 months (P < 0.05).
In non-TCAE group, p53 expression (69.37% ± 18.81%) had a positive correlation with expressions of Ki-67 protein (44.43% ± 20.70%, P < 0.05), PCNA (62.92% ± 17.21%, P < 0.05), and Bax (44.29% ± 23.73%, P > 0.05), and a negative correlation with the ratio of Bcl-2 to Bax (0.48 ± 0.64, P < 0.05), AI (5.71 ± 1.38, P > 0.05) and Bcl-2 (12.72% ± 4.92%, P > 0.05) (Table 3).
In TACE group, expression of p53 protein (65.09% ± 15.71%) had a positive correlation with expression of Ki-67 (40.24% ± 16.59%, P < 0.05), PCNA (59.95% ± 17.75%, P < 0.05), and Bcl-2 (7.47% ± 6.41%, P > 0.05), Bcl-2 to Bax ratio (0.21 ± 0.29, P > 0.05), and a negative correlation with AI(14.69% ± 6.29%, P < 0.05) and Bax (54.59% ± 23.63%, P > 0.05) (Table 3).
HCC is one of the most common malignant neoplasms. Most HCC patients are treated palliatively to improve the resectable rate and prolong survival. The best therapeutic method for HCC with tumor thrombi in portal vein (PVTT) has been regional hepatic TACE treatment after hepatic resection with removal of tumor thrombi[16]. TACE has become one of the most common and effective palliative approaches. The prognosis of patients treated with TACE was dependent on both the effect of TACE and tumor factors[17].
To our knowledge, few data regarding the molecular mechanism of TACE treatment for HCC are available, the current study is the first report to describe the correlations between p53 expression and different TACE regimens.
Our study showed that the frequency of p53 expression was higher in group A than in group B, non-TACE group and TACE group, the lowest in group C and D (P < 0.05). Our previous study showed that multidrug resistant gene product-Pgp protein was significantly increased in chemotherapy group alone[18], suggesting that p53 expression increased after chemotherapy with the development of chemoresistance. p53 status might be an important determinant of tumor response to chemotherapy. Tumors with p53 (-) expression might respond to chemotherapy. Inversely, chemotherapy was not effective in patients with p53 (+) expression[19]. This study demonstrated that p53 protein expression had no significant difference between the TACE and non-TACE groups[20].
The reverse correlation between AI and p53 protein expression of HCC cells was found in both TACE and non-TACE groups in this study. Most p53 protein measured was the mutant-type. AI was significantly positively correlated with wild-type p53 which enhanced apoptosis by activating proapoptotic Bax and by down-regulating antiapoptotic Bcl-2[21].
The current study demonstrated that p53 expression was positively related to Ki-67 and PCNA protein expression in the non-TACE and TACE groups, suggesting that the mutated p53 protein could enhance the growth of HCC. Our previous study showed both PCNA and p53 expressions had a significantly parallel correlation, the overexpression of mutated p53 resulted in cancer immortalization, high tumor recurrence risk, more aggressive growth and poor survival[22] and Kieser et al[23] found that a mutated p53 gene could provide an advantage for tumor proliferation. Igarashi et al[24] reported that expression of p53 protein like Ki-67 labeling index was a useful indicator for high proliferative activity. A mutated p53 gene provided an advantage for tumor proliferation not only by allowing escape from apoptosis, but also by leading to formation of a vascular-rich microenvironment[25]. However, it has been known that wild-type p53 could inhibit the expression of PCNA[26].
Wild-type p53 is a positive transcriptional activator for human Bax gene and a negative transcriptional activator for human Bcl-2 gene. The activation of p53 pathway could lead to the down-regulation of Bcl-2 and up-regulation of Bax[27]. The current study demonstrated that p53 protein expression was positively related to Bax expression, and negatively to Bcl-2 expression in the non-TACE group. Bcl-2 expression had an inverse correlation with mutant p53, and wild-type p53 and majority of mutant p53 proteins could down-regulate Bcl-2 expression and up-regulate Bax expression in vitro and in vivo[28]. Moreover, Bcl-2 and p53 could cooperate in regulating pathogenetic pathways in the processes of tumor invasion and metastasis[29].
The current study demonstrated that p53 protein expression was negatively related to Bax expression, and positively to Bcl-2 expression in the TACE group, which was in accordance with the previous report that Bax expression was significantly increased in tumors with wild-type p53 gene after chemotherapy but did not increase in tumors with mutatant p53 gene[21].
This study demonstrated that discrepancy of p53 existed in different types of HCC. p53 protein expression was significantly lower in trabecular and clear cells of HCC than that in solid and poorly differentiated or undifferentiated HCC, in both non-TACE and TACE groups, Zhao et al[30] reported that p53 gene mutation varied in histological types and the mutated rate was 10.5% in trabecular cells, 37.5% in pseudoglandar cells, 60.0% in solid cells and 33.3% in sclerosis (P < 0.05). Our previous study showed that trabecular and clear cells were more sensitive to TACE than solid, poorly or undifferentiated, and small cells of HCC[31]. Wang et al[32] also found that the clear cells of HCC were more sensitive to TAE than small cells of HCC and poorly differentiated or undifferentiated HCCs. Yamashita et al[33] found that most HCCs responsed to TACE well. These suggest that p53 protein expression would influence the effects of TACE on HCC. Further study is needed to clarify the correlation between p53 protein expression and tumor necrosis and patient survival.
This study also demonstrated that p53 protein expression increased as grades increased in both non-TACE and TACE groups (P < 0.05), which was inconsistent with the study of Zhao et al[30].
p53 protein expression of grade II HCC was significantly lower in TACE group than in non-TACE group, suggesting that influences of the molecular markers were very complicated and discrepancy in therapeutic effect of TACE on different histopathological types of HCC was existed. It would be the best choice to use TACE in an individualizated mode.
The best interval of treatment for repeated TACE or second stage resection is controversial. In this study, we found that p53 protein expression of HCC cells was significantly lower in the group with a 2 months or less interval than in the group with an over 3 month treatment interval (P < 0.05). We found that the best interval between treatment with TACE or second stage resection was 2-3 months.
In conclusion, discrepancy of histological types and pathological grades in p53 protein expression is existed in HCC. p53 protein expression can enhance proliferation of HCC cells and suppress apoptosis of HCC cells after TACE. p53 protein expression would influence effects of TACE on HCC. We need further study to clarify the correlation between p53 protein expression, tumor necrosis and patient survival.
Edited by Ren SY and Wang XL
| 1. | Parks RW, Garden OJ. Liver resection for cancer. World J Gastroenterol. 2001;7:766-771. [PubMed] [Cited in This Article: ] |
| 2. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] [Cited in This Article: ] |
| 3. | Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 4. | Xie X, Clausen OP, De Angelis P, Boysen M. The prognostic value of spontaneous apoptosis, Bax, Bcl-2, and p53 in oral squamous cell carcinoma of the tongue. Cancer. 1999;86:913-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4399] [Cited by in F6Publishing: 4433] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 6. | Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721-724. [PubMed] [Cited in This Article: ] |
| 7. | Perry A, Jenkins RB, O'Fallon JR, Schaefer PL, Kimmel DM, Mahoney MR, Scheithauer BW, Smith SM, Hill EM, Sebo TJ. Clinicopatho-logic study of 85 similarly treated patients with anaplastic astro-cytic tumors: An analysis of DNA content (ploidy), cellular proliferation, and p53 expression. Cancer. 1999;86:672-683. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 8. | Kanazawa Y, Onda M, Tanaka N, Seya T. Proliferating cell nuclear antigen and p53 protein expression in submucosal invasive colorectal carcinoma. J Nippon Med Sch. 2000;67:242-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Elpek GO, Gelen T, Aksoy NH, Karpuzoglu T, Keles N. Microvessel count, proliferating cell nuclear antigen and Ki-67 indices in gastric adenocarcinoma. Pathol Oncol Res. 2000;6:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] [Cited in This Article: ] |
| 11. | Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Expression of proliferating cell nuclear antigen and CD44 variant isoforms in the primary and metastatic sites of nonsmall cell lung carcinoma with intrapulmonary metastases. Cancer. 1999;86:1174-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 12. | Wu X, Zhang Z, Cai S. [Proliferating cell nuclear antigen (PCNA) in ovarian carcinoma and its relation to lymph node metastasis and prognosis]. Zhonghua Zhong Liu Za Zhi. 1998;20:68-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Luo YQ, Ma LS, Zhao YL, Wu KC, Pan BR, Zhang XY. Expression of proliferating cell nuclear antigen in polyps from large intestine. World J Gastroenterol. 1999;5:160-164. [PubMed] [Cited in This Article: ] |
| 14. | Wu WY, Xu Q, Shi LC, Zhang WB. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol. 2000;6:216-219. [PubMed] [Cited in This Article: ] |
| 15. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] [Cited in This Article: ] |
| 16. | Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28-32. [PubMed] [Cited in This Article: ] |
| 17. | Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Xiao EH, Hu GD, Liu PC, Hu DY, Liu SC, Hao CY. The effect of different interventional treatment on P-glycoprotein in different histopathological types of primary hepatocellular carcinoma. Zhonghua Fangshexue Zazhi. 1999;33:150-152. [Cited in This Article: ] |
| 19. | Okumura H, Natsugoe S, Nakashima S, Matsumoto M, Sakita H, Nakano S, Kusano C, Baba M, Takao S, Furukawa T. Apoptosis and cell proliferation in esophageal sqamous cell carcinoma treated by chemotherapy. Cancer Lett. 2000;158:211-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl). 2000;113:446-448. [PubMed] [Cited in This Article: ] |
| 21. | Sato S, Kigawa J, Minagawa Y, Okada M, Shimada M, Takahashi M, Kamazawa S, Terakawa N. Chemosensitivity and p53-dependent apoptosis in epithelial ovarian carcinoma. Cancer. 1999;86:1307-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 22. | Li JQ, Zhang CQ, Feng KT. PCNA,p53 protein and prognosis in primary liver cancer. China Natl J New Gastroenterol. 1996;2:220-222. [Cited in This Article: ] |
| 23. | Kieser A, Weich HA, Brandner G, Marmé D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994;9:963-969. [PubMed] [Cited in This Article: ] |
| 24. | Igarashi N, Takahashi M, Ohkubo H, Omata K, Iida R, Fujimoto S. Predictive value of Ki-67, p53 protein, and DNA content in the diagnosis of gastric carcinoma. Cancer. 1999;86:1449-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Matsuura T, Fukuda Y, Fujitaka T, Nishisaka T, Sakatani T, Ito H. Preoperative treatment with tegafur suppositories enhances apoptosis and reduces the intratumoral microvessel density of human colorectal carcinoma. Cancer. 2000;88:1007-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 26. | Deb S, Jackson CT, Subler MA, Martin DW. Modulation of cellular and viral promoters by mutant human p53 proteins found in tumor cells. J Virol. 1992;66:6164-6170. [PubMed] [Cited in This Article: ] |
| 27. | Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805. [PubMed] [Cited in This Article: ] |
| 28. | Ioachim EE, Malamou-Mitsi V, Kamina SA, Goussia AC, Agnantis NJ. Immunohistochemical expression of Bcl-2 protein in breast lesions: correlation with Bax, p53, Rb, C-erbB-2, EGFR and proliferation indices. Anticancer Res. 2000;20:4221-4225. [PubMed] [Cited in This Article: ] |
| 29. | Giatromanolaki A, Stathopoulos GP, Tsiompanou E, Papadimitriou C, Georgoulias V, Gatter KC, Harris AL, Koukourakis MI. Combined role of tumor angiogenesis, bcl-2, and p53 expression in the prognosis of patients with colorectal carcinoma. Cancer. 1999;86:1421-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 30. | Zhao P. [A molecular pathological study of p53 gene in hepatocellular carcinoma]. Zhonghua Bing Li Xue Za Zhi. 1993;22:16-18. [PubMed] [Cited in This Article: ] |
| 31. | Xiao EH, Hu GD, Li JQ, Zhang YQ, Chen MS, Guo YP, Lin XG, Li SP, Shi M. The effect of transcatheter arterial chemoembolization on the different histopathological types of hepatocellular carcinoma. Zhonghua Gandanwaikexue Zazhi. 2001;7:411-414. [Cited in This Article: ] |
| 32. | Wang YP, Zhang JS, Gao YA. Therapeutic efficacy of transcatheter arterial embolization of primary hepatocellular carcinoma: dis-crepancy in different histopathological types of HCC. Zhonghua Fangshexue Zazhi. 1997;31:586-591. [Cited in This Article: ] |
| 33. | Yamashita Y, Matsukawa T, Arakawa A, Hatanaka Y, Urata J, Takahashi M. US-guided liver biopsy: predicting the effect of interventional treatment of hepatocellular carcinoma. Radiology. 1995;196:799-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |