Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2894
Revised: March 27, 2004
Accepted: April 5, 2004
Published online: October 1, 2004
AIM: To investigate the effects and molecular mechanisms of recombinant human growth hormone (rhGH) on protecting liver function and alleviating portal hypertension of liver cirrhotic rats.
METHODS: Liver cirrhosis of male Sprague-Dawley rats was induced by administration of thioacetamide. The rats with or without liver cirrhosis were randomly divided into four groups. Group A consisted of the normal rats was treated with normal saline (NS), group B consisted of the normal rats was treated with rhGH, group C consisted of cirrhotic rats was treated with NS, and group D consisted of cirrhotic rats was treated with rhGH. The rats of different groups were subcutaneously injected with 0.5 mL of NS or 333 ng/kg of rhGH daily for 7 d. After treatments, the following parameters were examined, including GH-binding capacity (RT) by 125I-hGH binding, growth hormone receptor mRNA(GHR mRNA) expression by RT-PCR, relative content of collagen (RCC) by histomorphomertry, and level of malon-dialdehyde (MDA) and superoxide dismutase (SOD) in liver tissue by thiobarbituric acid reaction and pyrogallic acid self-oxidation, respectively. Serum albumin (ALB), alanine transaminase (ALT) and portal vein pressure (PVP) were also examined.
RESULTS: rhGH up-regulated both the GH-binding capacity (RT) and the expression of GHR mRNA in vivo. RT in group A (72 ± 12 fmol/mg protein) was significantly higher than that in group C (31 ± 4 fmol/mg protein) (P < 0.05). RT in group B (80 ± 9 fmol/mg protein) increased markedly compared to group A (P < 0.05). RT in group D (40 ± 7 fmol/mg protein) raised remarkably compared with group C (P < 0.05), but less than that in group A, and there was no significant GH binding affinity contrast (Kd) change. The GHR mRNA level (iOD, pixel) in group A (29 ± 3) was significantly higher than that in group C (23 ± 3) (P < 0.05). GHR mRNA levels were significantly raised in group B (56 ± 4) and group D (42 ± 8) compared with groups A and C (29 ± 3 and 23 ± 3, respectively) (P < 0.05). Compared with the normal liver, MDA level was higher and SOD level was lower in cirrhotic livers. After rhGH treatment, MDA level was significantly declined to 12.0 ± 2.2 nmol/mg protein and SOD was raised to 1029 ± 76 U/mg protein in group D (P < 0.05). ALB levels in groups B and D (42 ± 7 g/L and 37 ± 7 g/L, respectively) were significantly raised compared with those in groups A and C (35 ± 5 g/L and 29 ± 4 g/L, respectively) (P < 0.05). ALT level was markedly lower in group D (69 ± 7 U/L) compared to group C (89 ± 15 U/L) (P < 0.05), and close to group A (61 ± 10 U/L). RCC in group C (22.30% ± 3.86%) was significantly higher than that in group A (1.14% ± 0.21%) and group D (14.70% ± 2.07%) (P < 0.05). In addition, rhGH markedly alleviated portal hypertension in liver cirrhotic rats (group D vs C, 9.3 ± 1.5 cmH2O vs 14.4 ± 2.0 cmH2O) (P < 0.05).
CONCLUSION: Pharmacological doses of rhGH can increase RT and GHR mRNA expression, ameliorate liver functions, repress fibrosis and decline portal hypertension, suggesting it has potentially clinical usage as a hepatotropic factor.
- Citation: Chen S, Wang HT, Yang B, Fu YR, Ou QJ. Protective effects of recombinant human growth hormone on cirrhotic rats. World J Gastroenterol 2004; 10(19): 2894-2897
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2894
Liver cirrhosis is a common pathway of a variety of chronic liver diseases[1], and is associated with high protein catabolism, low anabolism and negative nitrogen balance[2], resulting in hypoproteinemia which contributes to ascites, dysfunction of coagulation and suppression of immune response[3]. Early reports showed that cirrhotic patients undergone emergency abdominal surgery exhibited a higher mortality[4]. In retrospective studies of liver transplant recipients, protein-calorie malnutrition has been associated with adverse outcomes in patients with end-stage liver diseases[5,6]. A prospective study showed that cirrhotic patients with hypermetabolism and emaciation had a much higher mortality rate after liver transplantation than those with normal metabolism[7]. It is critical for patients with hepatic cirrhosis to correct malnutrition. To date, studies have shown that nutritional support is always not effective enough to prevent protein loss and optimize the care of these patients in severe catabolic illnesses, including cirrhosis[8,9].
Growth hormone (GH) is essential for body growth in children. In adults, GH continues to stimulate many anabolic processes. GH secreted by the pituitary gland is pulsatile, and its action depends on its binding to growth hormone receptor on cell membrane[10-13] . New insights have initiated new applications and a growing potential for GH replacement therapy in adults. Recombinant human growth hormone (rhGH) has been clinically used in many states, such as after abdominal operation[14], organ transplantation[15], major trauma[16] and severe burns[17], to enable the patients to survive an aggressive surgery[18]. After treatment with rhGH, donor site healing rate in children with severe burns was enhanced and hospitalization time was decreased[19-21]. It significantly enhanced cell-mediated immunity and decreased wound infection rates and length of hospitalization in a large group of postoperative patients[22]. Some clinical trials reported that growth hormone enhanced nitrogen retention of patients with chronic obstructive lung diseases[23], severe sepsis[24,25] and emaciated AIDS[26,27], in addition to fasted adult volunteers. Although there are many controversies[28-32], it has been confirmed that rhGH is an effective drug to accelerate protein anabolism[33] and plays a central role in metabolic intervention with a significant cost-effect benefit[34].
In this study, we investigated the effects and molecular mechanism of pharmacological doses of recombinant human growth hormone (rhGH) on expression of growth hormone receptor (GHR) in liver tissue, liver function and portal vein pressure in a rat cirrhotic model with portal hypertension.
Male SD rats were purchased from Medical Animal Center of Sun Yat-Sen University. Rat liver cirrhosis was induced by daily intraperitoneal injection of 30 g/L thioacetamide (TAA, 50 mg/kg for 9 to 12 wk). Twenty normal rats (body mass 200-300 g) were randomly divided into two groups: group A (n = 10) was treated with normal saline (NS), and group B (n = 10) was treated with rhGH. Twenty cirrhotic rats (body mass, 200-300 g) were randomly divided into two groups: group C (n = 10) was treated with NS and group D (n = 10) was treated with rhGH. The rats were injected subcutaneously with 0.5 mL of NS or 333 ng/kg of rhGH daily for 7 d.
Rats were anesthetized with pentobarbital (30 mg/kg, subcutaneous injection), weighed, antisepsised. Then peritoneum was incised to explore the liver.
Measurement of portal vein pressure (PVP) After the portal vein was punctured, PVP was measured directly.
Estimation of liver function Blood samples from inferior vena cava were collected to measure serum albumin (ALB) and alanine transaminase (ALT) levels by biochemical autoanalyzers.
Tissue sampling Partial liver tissue samples were frozen in liquid nitrogen immediately, then stored at -80 °C for use, The rest part was fixed in 100 g/L formaldehyde solution and stained with Masson trichrome stain for regular pathological examination.
GH binding capacity (RT) analysis One hundred µL (approximately 20000 cpm) of 125I-hGH (NEN inc, USA) with a specific activity of about 108 µCi/µg, 100 µL of unlabelled hGH with various concentrations (0-3 nmol/L was divided into 7-9 concentration gradients, standard samples were bought from Northern Biological Technical Company), and 100 µL of liver membrance microsomes (preparation with gradient centrifugation technique) were mixed and incubated at 4 °C overnight. Dissociated ligands were eliminated by filtration. The precipitates were subjected to a radioactive counter, and then 125I-GH binding capacity (RT, fmol/mg protein) and binding affinity contrast (kd, nmol/L) were calculated by Scatchard analysis.
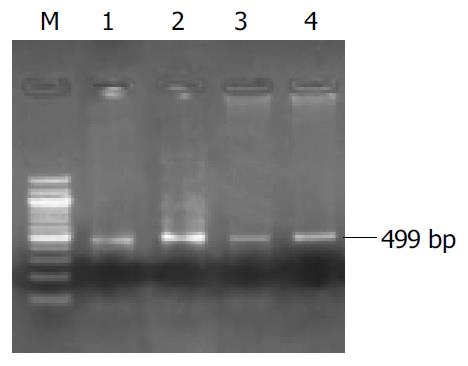
Expression of GHR mRNA in liver tissue Self-designed primers were as follows: forward, 5’-ATGTGAGATCCAGACAACG-3’, and reverse, 5’-ATGTCAGGGTCATAACAGC-3’. The amplification segment containing introns was supposed to be 499 bp in length. Total RNA of the rats’ liver tissues was extracted with Trizol following the manufacturer’s instructions. RT-PCR was performed with RT-PCR kits (Epicedure inc. USA) as previously described[35]. After thirty amplification cycles were performed, the PCR products were detected by gel electrophoresis. The level of GHR mRNA was expressed as iOD (pixel, the integral optical density of amplification segment).
Level of malon-dialdehyde (MDA) and superoxide dismutase (SOD) Thiobarbituric acid reaction and pyrogallic acid self oxidation method were adopted to measure the amount of MDA (the degradation products of peroxide lipid in liver) and the activity of SOD, respectively.
Measurement of relative content of collagen (RCC) in liver Liver fibrosis levels was expressed as RCC. After stained with Masson’s trichrome stain, liver collagen content was calculated by histomorphometric measurement. Three random areas were chosen and analyzed by computers with Kontron IBAS2.5 system (Germany) for digital image analysis. The total area and the fibrotic area with positive staining were automatically selected, outlined and evaluated by planimetry. RCC was calculated as a percentage of positive staining area in the total area.
The data were processed with duplex factor χ2 analysis by software statistica 5.0. Least significant difference (LSD) was adopted to compare the inter-group variance. Values were expressed as mean ± SD. P < 0.05 was considered statistically significant.
As shown in Table 1, RT in group A was significantly higher than that in group C (P < 0.05). RT in group B increased markedly compared with group A (P < 0.05). It significantly increased in group D compared with group C (P < 0.05), but was lower than that in group A. There was no significant difference in Kd.
| Group | RT (fmol/mg | GHR mRNA | ALB(iOD) | ALT | MDA (nmol/mg | SOD | RCC | PVP |
| protein) | (iOD) | (g/L) | (U/L) | protein) | (U/mg) | (%) | (cmH2o) | |
| A | 72 ± 12 | 29 ± 3 | 35 ± 5 | 61 ± 10 | 10.2 ± 1.4 | 1078 ± 185 | 1.14 ± 0.21 | 5.6 ± 0.7 |
| B | 80 ± 9c | 56 ± 4c | 42 ± 7c | 55 ± 11 | 9.4 ± 1.2 | 1057 ± 159 | 1.13 ± 0.18 | 5.8 ± 0.7 |
| C | 31 ± 4a | 23 ± 3a | 29 ± 4a | 89 ± 15a | 18.7 ± 3.2a | 824 ± 108a | 22.30 ± 3.86a | 14.4 ± 2.0a |
| D | 40 ± 7ac | 42 ± 8ac | 37 ± 7c | 69 ± 7ac | 12.0 ± 2.2ac | 1029 ± 76c | 14.70 ± 2.07ac | 9.3 ± 1.5ac |
As shown in Table 1 and Figure 1, the expression of GHR mRNA (iOD, pixel) in group A was significantly higher than that in group C (P < 0.05). It was significantly raised in groups B and D after rhGH treatment, which was 1.9 and 1.8 times higher than that in groups A and C, respectively (P < 0.05). It was higher in group D than in group A.
Without rhGH treatment, ALB level in cirrhotic rats (group C) was significantly lower than that in normal rats (group A), and ALT in cirrhotic rats was markedly higher than that in normal rats (P < 0.05) (Table 1). After rhGH administration, ALB in groups B and D increased significantly (P < 0.05), ALT in group D decreased remarkably (P < 0.05), which was close to normal rats (Table 1).
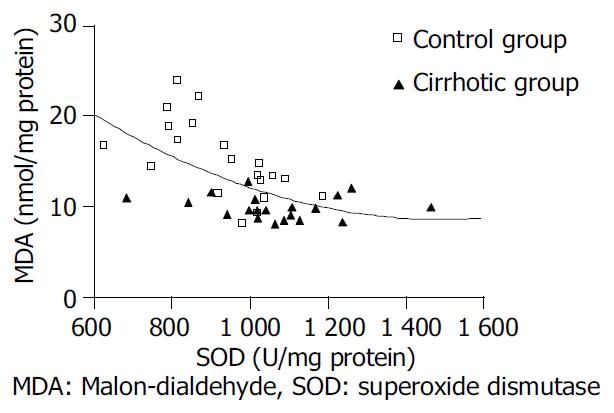
Before the treatment with rhGH, the amount of MDA in cirrhotic rats (group C) was significantly higher than that in normal rats (group A) (P < 0.05), and SOD in cirrhotic rats was significantly lower than that in normal rats (P < 0.05). After rhGH administration, MDA and SOD in group B were not obviously changed, but in group D, MDA was significantly lower than that in group C (P < 0.05), while SOD was significantly higher than that in group C (P < 0.05). The curve correlation analysis manifested a significant correlation between SOD and MDA (P < 0.01). (Table 1, Figure 2).
As shown in Table 1, RCC in group C was significantly higher than that in group A (P < 0.05). Compared with group C, it was significantly lower in group D (P < 0.05).
As shown in Table 1, PVP in cirrhotic rats (group C) was significantly higher than that in normal rats (group A) (P < 0.05). After rhGH administration, no significant change was found in normal rats, however PVP in cirrhotic rats (group D) was significantly lower than that in group C (P < 0.05).
In cirrhotic patients, nutritional status was an important predictor of morbidity, mortality, and survival after transplantation[36]. The poor status of these patients was associated with the state of acquired GH resistance[37,38], which is common in conditions associated with malnutrition and protein catabolism, trauma or surgery, organ failure and critical illness. Much has been done regarding the expression of GHR and signal transduction[10-13], but the expression of GHR and GHR mRNA in some pathological states such as cirrhotic hepatocytes, malignant cells, remains to be established. Chang et al[39] reported that 125I-rhGH binding activity in 6 cases of hepatocellular carcinoma and adjacent cirrhotic liver tissues could not be detected and they believed that GHR in cirrhotic hepatic tissues disappeared although the study only examined one aspect of the GHR and GH binding. Another study[40] showed that specific binding of 125I-hGH in liver tissues from liver transplant of 17 patients with end-stage liver diseases was lower than that in normal controls, but only in 3 cirrhotic livers Scatchard analysis was performed for calculation of GH binding capacity and affinity. In this setting of tissue-based GH binding assay, there was still a controversy about the expression of GHR on cirrhotic liver cells.
Our study showed that rhGH could significantly increase serum ALB and SOD levels, decrease ALT and MAD to nearly the normal level in liver cirrhotic rats. In addition, after rhGH treatment, both the liver fibrosis level and PVP were remarkably decreased.
In our study, the expression of GHR mRNA in cirrhotic liver tissue was lower than that in normal liver tissue, suggesting that liver cirrhosis could down-regulate GHR gene transcription and result in decrease of GHR, which might be an important reason of malnutrition in liver cirrhosis.
Simultaneously, we found that rhGH up-regulated GHR and its mRNA in both cirrhotic and normal liver tissues. in normal liver, the changes before and after rhGH treatment were not obvious. We hypothesized that the up-regulation of GHR and its mRNA in cirrhotic rats could improve liver function, and decrease liver fibrosis levels and PVP. This implied that the effects of rhGH on the expression of GHR and GHR mRNA in cirrhotic liver tissues might play an important role in ameliorating the sensibility of cirrhotic liver tissues to rhGH, thereby exerting a therapeutic effect on liver cirrhosis.
In conclusion, rhGH can up-regulate the expression of GHR and its mRNA in livers, particularly in cirrhotic livers, which can increase the sensibility of cirrhotic liver tissue to growth hormones. Thus, rhGH can protect liver function, repress fibrosis, alleviate portal hypertension of cirrhotic livers.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Strong RW. Liver transplantation: current status and future prospects. J R Coll Surg Edinb. 2001;46:1-8. [PubMed] [Cited in This Article: ] |
| 2. | Dichi JB, Dichi I, Maio R, Correa CR, Angeleli AY, Bicudo MH, Rezende TA, Burini RC. Whole-body protein turnover in malnourished patients with child class B and C cirrhosis on diets low to high in protein energy. Nutrition. 2001;17:239-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Sobhonslidsuk A, Roongpisuthipong C, Nantiruj K, Kulapongse S, Songchitsomboon S, Sumalnop K, Bussagorn N. Impact of liver cirrhosis on nutritional and immunological status. J Med Assoc Thai. 2001;84:982-988. [PubMed] [Cited in This Article: ] |
| 4. | Maull KI, Turnage B. Trauma in the cirrhotic patient. South Med J. 2001;94:205-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Moukarzel AA, Najm I, Vargas J, McDiarmid SV, Busuttil RW, Ament ME. Effect of nutritional status on outcome of orthotopic liver transplantation in pediatric patients. Transplant Proc. 1990;22:1560-1563. [PubMed] [Cited in This Article: ] |
| 6. | Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Müller MJ, Lautz HU, Plogmann B, Bürger M, Körber J, Schmidt FW. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 207] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27:262-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 364] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69:1364-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Carter-Su C, Schwartz J, Smit LS. Molecular mechanism of growth hormone action. Annu Rev Physiol. 1996;58:187-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 227] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Hocquette JF, Postel-Vinay MC, Kayser C, de Hemptinne B, Amar-Costesec A. The human liver growth hormone receptor. Endocrinology. 1989;125:2167-2174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Husman B, Andersson G, Norstedt G, Gustafsson JA. Characterization and subcellular distribution of somatogenic receptor in rat liver. Endocrinology. 1985;116:2605-2611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | van Neste L, Husman B, Möller C, Andersson G, Norstedt G. Cellular distribution of somatogenic receptors and insulin-like growth factor-I mRNA in the rat liver. J Endocrinol. 1988;119:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wong WK, Soo KC, Nambiar R, Tan YS, Yo SL, Tan IK. The effect of recombinant growth hormone on nitrogen balance in malnourished patients after major abdominal surgery. Aust N Z J Surg. 1995;65:109-113. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Rodeck B, Kardorff R, Melter M, Ehrich JH. Improvement of growth after growth hormone treatment in children who undergo liver transplantation. J Pediatr Gastroenterol Nutr. 2000;31:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Petersen SR, Holaday NJ, Jeevanandam M. Enhancement of protein synthesis efficiency in parenterally fed trauma victims by adjuvant recombinant human growth hormone. J Trauma. 1994;36:726-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Singh KP, Prasad R, Chari PS, Dash RJ. Effect of growth hormone therapy in burn patients on conservative treatment. Burns. 1998;24:733-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Iglesias P, Díez JJ. Clinical applications of recombinant human growth hormone in adults. Expert Opin Pharmacother. 1999;1:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg. 1995;221:649-656; discussion 656-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Aili Low JF, Barrow RE, Mittendorfer B, Jeschke MG, Chinkes DL, Herndon DN. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns. 2001;27:447-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, Chinkes DL, Wolf SE. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Vara-Thorbeck R, Guerrero JA, Rosell J, Ruiz-Requena E, Capitán JM. Exogenous growth hormone: effects on the catabolic response to surgically produced acute stress and on postoperative immune function. World J Surg. 1993;17:530-537; discussion 530-537;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Pape GS, Friedman M, Underwood LE, Clemmons DR. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest. 1991;99:1495-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Koea JB, Breier BH, Douglas RG, Gluckman PD, Shaw JH. Anabolic and cardiovascular effects of recombinant human growth hormone in surgical patients with sepsis. Br J Surg. 1996;83:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Li W, Li J, Xu B, Yin L, Wang L, Gu J, Ren J, Quan Z. [Effect of recombinant human growth hormone with total parenteral nutrition on albumin synthesis in patients with peritoneal sepsis]. Zhonghua Waike Zazhi. 1998;36:643-645. [PubMed] [Cited in This Article: ] |
| 26. | Schambelan M, Mulligan K, Grunfeld C, Daar ES, LaMarca A, Kotler DP, Wang J, Bozzette SA, Breitmeyer JB. Recombinant human growth hormone in patients with HIV-associated wasting. A randomized, placebo-controlled trial. Serostim Study Group. Ann Intern Med. 1996;125:873-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 254] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Waters D, Danska J, Hardy K, Koster F, Qualls C, Nickell D, Nightingale S, Gesundheit N, Watson D, Schade D. Recombinant human growth hormone, insulin-like growth factor 1, and combination therapy in AIDS-associated wasting. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | O'Leary MJ, Ferguson CN, Rennie M, Hinds CJ, Coakley JH, Preedy VR. Effect of growth hormone on muscle and liver protein synthesis in septic rats receiving glutamine-enriched parenteral nutrition. Crit Care Med. 2002;30:1099-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Losada F, García-Luna PP, Gómez-Cía T, Garrido M, Pereira JL, Marín F, Astorga R. Effects of human recombinant growth hormone on donor-site healing in burned adults. World J Surg. 2002;26:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Carroll PV, Van den Berghe G. Safety aspects of pharmacological GH therapy in adults. Growth Horm IGF Res. 2001;11:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 666] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 32. | Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Raguso CA, Genton L, Kyle U, Pichard C. Management of catabolism in metabolically stressed patients: a literature survey about growth hormone application. Curr Opin Clin Nutr Metab Care. 2001;4:313-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Wilmore DW. Postoperative protein sparing. World J Surg. 1999;23:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Zhao J, van Tol HT, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. The effect of growth hormone on rat pre-antral follicles in vitro. Zygote. 2000;8:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Mendenhall CL, Tosch T, Weesner RE, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorell M, Tamburro C, Zetterman R. VA cooperative study on alcoholic hepatitis. II: Prognostic significance of protein-calorie malnutrition. Am J Clin Nutr. 1986;43:213-218. [PubMed] [Cited in This Article: ] |
| 37. | Møller S, Becker U, Grønbaek M, Juul A, Winkler K, Skakkebaek NE. Short-term effect of recombinant human growth hormone in patients with alcoholic cirrhosis. J Hepatol. 1994;21:710-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Donaghy A, Ross R, Gimson A, Hughes SC, Holly J, Williams R. Growth hormone, insulinlike growth factor-1, and insulinlike growth factor binding proteins 1 and 3 in chronic liver disease. Hepatology. 1995;21:680-688. [PubMed] [Cited in This Article: ] |
| 39. | Chang TC, Lin JJ, Yu SC, Chang TJ. Absence of growth-hormone receptor in hepatocellular carcinoma and cirrhotic liver. Hepatology. 1990;11:123-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Shen XY, Holt RI, Miell JP, Justice S, Portmann B, Postel-Vinay MC, Ross RJ. Cirrhotic liver expresses low levels of the full-length and truncated growth hormone receptors. J Clin Endocrinol Metab. 1998;83:2532-2538. [PubMed] [DOI] [Cited in This Article: ] |