INTRODUCTION
Methotrexate(MTX), a folic acid analog, is a widely used antimetabolite in cancer chemotherapy. It inhibits dihydrofolate reductase, an enzyme necessary for purine and pyrimidine synthesis. However, MTX also acts on other rapidly dividing cells such as trophoblast cells, which comprise the implantation site of early gestation. Other toxic effects include depression of the bone marrow and damage to the epithelium of the gastrointestinal tract. Selective targeting of therapeutic and diagnostic agents may improve MTX’s efficacy and reduce its adverse effects. Existing methods for selective targeting are usually based upon chemical conjugation of agents to cell-specific targeting molecules (e.g. ligands, antibodies).
Receptor mediated endocytosis may provide a pathway by which exogenous molecules are transported into the interior of target cells. In recent years a number of receptors have been used for DNA delivery via receptor-mediated endocytosis, including asialoglycoprotein[1-4], transferrin[5-7], epidermal growth factor (EGF) peptides[8-10], as well as insulin receptor[11-13]. It has been shown that expression of insulin receptor is increased on a variety of malignant tumor cells[14-17]. Insulin can be internalized into nuclei of cells possessing insulin receptors[18-21]. These facts indicate that insulin may be an attractive candidate carrier for anticancer drugs in target therapy of carcinomas.
To reduce side effects and increase safety, we planned to delivery MTX via insulin receptor-mediated endocytosis. In the present study, we conjugated MTX to insulin and characterized the binding activity and cytotoxicity of insulin-MTX complex. Our results suggest that insulin is a suitable candidate carrier for anticancer drugs.
MATERIALS AND METHODS
Radioiodination of insulin
Porcine insulin was radioiodinated using the Ch-T method and purified by polyacrylamide gel electrophoresis. Radiochemical purity of the 125I-insulin was measured by thin layer chromatogram (TLC).
The radiochemical purity of 125I-insulin was 98% and remained over 95% 14 d after stored at -20 °C.
Preparation of insulin-MTX
Insulin-MTX was synthesized by the Department of Pharmacy, Sichuan University. Briefly, MTX (50 mg), EDC (100 mg) and HOBt (50 mg) were dissolved in 3 mL of phosphate-buffered saline (pH = 8.9) and mixed with 1 mL of phosphate-buffered saline (pH = 8.9) containing 25 mg of insulin. The mixture was stirred at 4 °C for 48 h. The precipitation was removed by filtration and pH of the solvent was adjusted to 5.5 with 0.5 mol/L HCL. The reaction mixture was kept at 4 °C overnight. Thereafter, the solution was filtered through a 0.25 μm membrane (Millipore, Germany) and the filtrate was evaporated under vacuum. The products were purified over a 3 cm × 45 cm Sephadex G-25 column eluted with 2% ammonium carbonate, and fractions which had the maximal absorbance at 276 nm were collected.
Isolated insulin-MTX was analyzed by analytical HPLC (Beckman) with a 4 mm × 200 mm ODS column. The mobile phase was 270 mL/L acetonitrile and 730 mL/L 0.1 mol/L NaH2PO4 (pH = 0.3) at a flow rate of 0.5 mL/min.
SDS-polyacrylamine gel electrophoresis (SDS-PAGE) was performed in 150 g/L polyacrylamine gel to calculate the molecular weight of insulin-MTX.
Preparation of cell membrane
Hepatocellular carcinoma specimens were obtained from six patients during surgery whose diagnoses were confirmed by histopathology and immediately frozen at -70 °C. Cell membrane fractions were prepared according to established techniques. Tissues were cut into pieces, put into Tris-HCL buffer (pH 7.5) and homogenized. The cell membrane fractions purified by centrifugation in a discontinuity sucrose density gradient were stored at -70 °C. The protein concentration was determined according to Lowrry method.
Receptor binding assay
The conditions of the assay system were modified according to the method reported earlier. In saturation experiments, the cell membrane fractions (80 µg protein) were incubated with increasing concentrations(5 × 10 3-5 × 10 5cpm) of 125I-insulin in the absence (total binding) or presence of the same unlabeled insulin (4.3 nmol/L, nonspecific binding). In competition experiment, the cell membrane fractions (80 µg protein) were incubated with 5 nmol/L 125I-insulin in the absence (total binding) and presence of increasing concentrations (10-12-10-7 mol/L) of the unlabeled insulin or insulin-MTX. After incubation for 20 h at 4 °C, free ligands were removed by centrifugation at 2000 r/min for 10 min after addition of 0.1 mL of 3 g/L bovine γ -globulin and 0.8 mL of 15.8% PEG. Radioactivity of the membrane pellets was determined in a gamma counter for 1 min as the total binding. Specific bindings were obtained by subtracting nonspecific bindings from total bindings.
Cell culture
Human hepatoma BEL7402 cells and human hepatocyte HL7702 cells were grown at 37 °C in a humidified incubator with 50 mL/L CO2 atmosphere in RPMI1640 (GIBCO BRL) medium containing 100 mL/L of decomplemented FBS, penicillin(100 U/mL) and streptomycin (100 U/mL).
Cell treatment and MTT assay
BEL7402 cells and HL7702 cells were seeded at a density of 104 cells per well into 96-well plates. After overnight growth, the medium was removed and RPMI containing different concentrations of insulin-MTX or MTX was added .At given time points, 20 µL of MTT (50 mg/mL) was added to each well. After incubation for 4 h at 37 °C in a humidified 50 mL/L CO2 atmosphere, the medium was removed and 0.2 mL of DMSO was added. Then, the absorbency at 570 nm was read.
Data calculation and statistical analysis
Binding data were calculated with receptor binding analysis software. Values were presented as mean ± SD. Statistical comparisons between the means were made with paired t-test at a confidence level of 95%.
RESULTS
Analysis of insulin-MTX
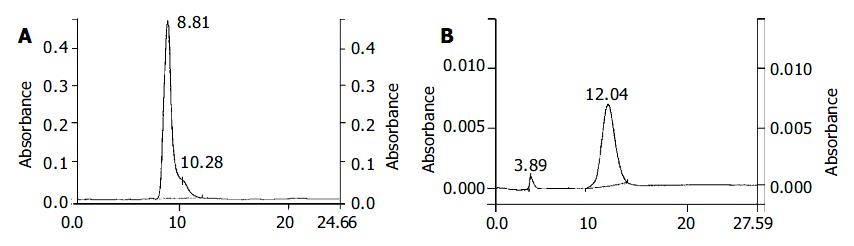
The recovery of insulin-MTX was around 71.5%. HPLC assay indicated that isolated insulin-MTX (retention time 12.08 min) yield was approximately 94% (Figure 1). The rest contained insulin (8.81 min) and MTX (4.16 min).
Figure 1 HPLC analysis of insulin and insulin-MTX.
The condition was described in MATERIALS AND METHODS. Retention time of insulin was 8.81 min. Retention time of insulin-MTX was 12.16 min. A: HPLC analysis of insulin. B: HPLC analysis of insulin-MTX.
The calculated molecular weight of insulin-MTX was 6779 u according to its relative position to the molecular mass marker in SDS-PAGE pattern.
Receptor binding
The binding of insulin receptors to unlabelled insulin and the insulin-MTX conjugate derivative were determined by their ability to compete with free radioactively labelled insulin for receptor sites on cell membranes of hepatocellular carcinoma. We first established the normal binding characteristics of 125I-insulin to cell membranes. Binding took place rapidly in the first 2 h, then slowed down and reached its maximum at approximately 18 h. For all subsequent binding experiments, the incubation time was 20 h at 4 °C. As the concentration of 125I-insulin increased, the binding mounted up and plateaued rapidly.
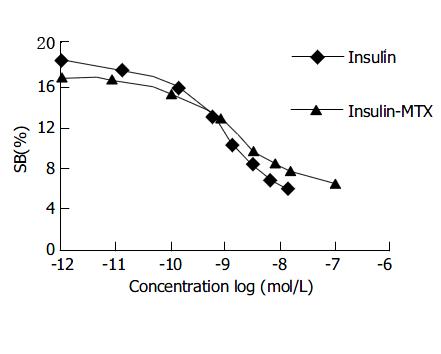
Similar to insulin, the conjugate competed for the binding sites with 125I-insulin on the insulin receptor in a dose-dependent manner (Figure 2). Binding of 125I-insulin (5 nmol/L) was reduced to 50% in the presence of 5.01 ± 1.24 nmol/L of unlabelled insulin or 93.82 ± 19.32 nmol/L of insulin-MTX, respectively. In contrast, MTX did20not compete for binding (data not shown).
Figure 2 Competitive binding curves of 125I-insulin against insulin-MTX or insulin.
The concentration of 125I-insulin was 5 nmol/L. The binding assay was described in the MATERI-ALS AND METHODS.
Inhibition of insulin-MTX complex on cell proliferation
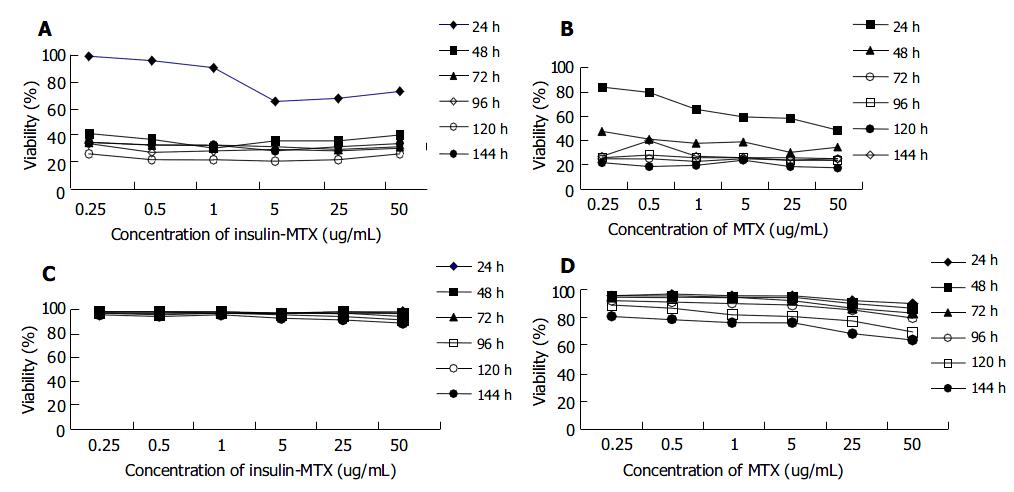
Dose-response curves of insulin-MTX and MTX on BEL7402 and HL7702 cells are shown in Figure 3. Viability was expressed as a percentage of control by dividing the absorbance of each treated well by the average of untreated control. BEL7402 cells were sensitive to both insulin-MTX and MTX. Cytoreductive effect of MTX and insulin-MTX on BEL7402 cells was concentration-dependent. As early as 24 h, 5.0 µg/mL of insulin-MTX inhibited BEL7402 cell growth by 34.3%, which was relatively lower as compared with 40.6% by 5.0 µg/mL of MTX. The effect reached its peak on the 5 th d, and BEL7402 cells were inhibited by 79% at a concentration of 5.0 µg/mL insulin-MTX and 82.1% at 5.0 µg/mL MTX, respectively. In contrast, treatment with 5.0 µg/mL of MTX and 5.0 µg/mL of insulin-MTX merely resulted in the inhibition of HL7702 cells by 31.5% and 7.8% on the 5th d.
Figure 3 Concentrations of MTX and insulin-MTX in BEL7402 and HL7702 cells.
BEL7402 cells and HL7702 cells having cultured overnight were exposed to insulin-MTX or MTX at the indicated concentration for 24 h, 48 h, 72 h, 96 h, 120 h and 144 h respectively. Cell viability was assessed using MTT assay. Viability was expressed as a percentage of control by dividing the absorbance of each treated well by the average of the untreated control. A: Viability of BEL7402 cells treated with insulin-MTX (mean ± SD, n = 3); B: Viability of BEL7402 cells treated with MTX (mean ± SD, n = 3); C: Viability of HL7702 cells treated with insulin-MTX (mean ± SD, n = 3); D: Viability of HL7702 cells treated with MTX (mean ± SD, n = 3).
DISCUSSION
The molecule of insulin has sufficient hydroxys and carboxyls that can be used for conjugation with other small molecules. Exploration of the efficiency and specificity of cell entry offered by insulin receptor mediated endocytosis has been proposed previously[22,23]. Here we demonstrated that cross-linking MTX to insulin produced a soluble molecule capable of both specific recognition of the insulin receptors and inhibition of growth of tumor cells, thereby providing a possible tool for target transporting anticancer drugs. The use of insulin as a carrier might offer two advantages. First, most of insulin-MTX could go to tumor cells which overexpress insulin receptors, thus rapidly dividing normal tissues would not be damaged by the drug. Second, tumor cells could develop MTX-resistance frequently via various mechanisms. One of them was due to decreased membrane transport[24-28]. The use of insulin as a carrier might offer an increased MTX transportation into drug-resistant tumor cells.
Since laboratory iodination of insulin with 125I could incur oxidation damage and brings about indiscriminate labelling of tyrosine residues leading to decreased biological activity including aberrant receptor binding, polyacrylamide gel electrophoresis was used to purify monoiodo [125I-TyrA14] insulin, which was fully active in receptor binding assays[29].Receptor binding analysis showed that insulin-MTX could specifically bind to insulin receptors on human hepatocellular carcinoma. The increased Kd indicated a decreased affinity for receptors, indicating that linking MTX to insulin molecule did interfere with the affinity of insulin-receptor binding. High affinity is one of the most important criteria of tumor-target agents. Decrease in affinity of insulin-MTX for insulin receptors may result from conjugation of MTX with A13 and B17 positions of insulin molecules. These positions are located in the hexamer-forming surface and on the opposite side of the classical binding site, and constitute a second domain of the molecule important for receptor binding[30]. Further studies to improve the affinity of insulin-MTX by modifying the method of conjugation would be needed.
Insulin receptor was considered to play a role in the regulation of hepatocellular carcinoma[31] and there have been reports showing increased insulin receptors expressed on these cells[29,32]. Therefore human hepatoma BEL7402 cell line was selected to measure the cytoreductive effect of insulin-MTX. Our studies showed that BEL7402 cells were sensitive to both insulin-MTX and MTX. However, the molecular weight of insulin-MTX complex was far greater than that of MTX. It was obsered that 0.375 nmoL (1.5 × 10 -6 mol/L × 0.25 mL) of MTX conjugated to insulin inhibited the growth of BEL7402 cells as effectively as 2.5 nmoL (1 × 10 -5 moL /L × 0.25 mL) of MTX. HL7702 cells appeared to be less sensitive to both insulin-MTX and MTX, especially to insulin-MTX, when compared with BEL7402 cells. Treatment with 5.0 µg/mL of insulin-MTX resulted in about 10-fold higher toxicity on BEL7402 cells than on HL7702 cells. This may result from the elevated proliferation and the increased expression of insulin receptors of BEL7402 cells. These results indicated that conjugation of insulin could enhance the cytotoxicity of MTX to hepatocarcinoma cells.
Our results showed that the new construct could bind specifically to insulin receptors, but its effect on glucose metabolism depended on the residues with which MTX molecule interacted. An unparalleled relationship of receptor binding and glucose uptake was observed in other insulin analogues and mutants[33,34]. Therefore, it is necessary to test the biological activity of insulin-MTX in glucose metabolism in order to understand its effects on plasma glucose levels. In our study, SDS-polyacrylamine agarose gel electrophoresis showed the molecular weight of insulin-MTX was 6779 u, which equaled the mass of one molecule of insulin (Mr = 5 800) and two molecules of MTX (Mr = 454.5). The ratio of insulin molarity to the MTX was estimated to be 1:2. On the basis of this molar ratio, only 20-200 µmoL insulin is required to obtain a high plasma concentration(10-100 µmol/L). Besides, the dose may be reduced because of the targeting ability of insulin-MTX. Even if the conjugates have partial bioactivity in glucose uptake, there is little probability that administration of such a low dose in therapy would result in adverse effects of glucopenia.
In the present study, we demonstrated that insulin-MTX complex could specifically bind to insulin receptors and efficiently inhibit the growth of human hepatoma BEL7402 cells, although the affinity needs to be improved. Receptor-mediated endocytosis has a tremendous potential for delivery of carcinoma target drugs because of the overexpression of insulin receptors on carcinoma tissues.