Copyright
©The Author(s) 2016.
World J Gastroenterol. Oct 21, 2016; 22(39): 8698-8719
Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8698
Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8698
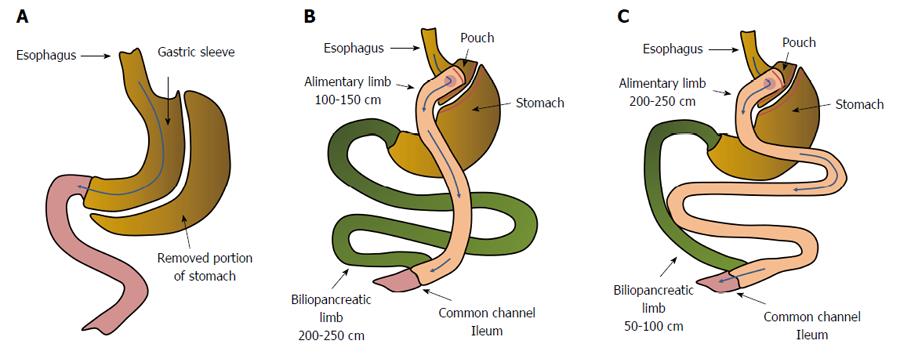
Figure 1 Anatomical changes in gastrointestinal tract resulting from: sleeve gastrectomy (A), Roux-en-Y gastric bypass - distal/scopinarized (B), Roux en Y gastric bypass - long limb (C).
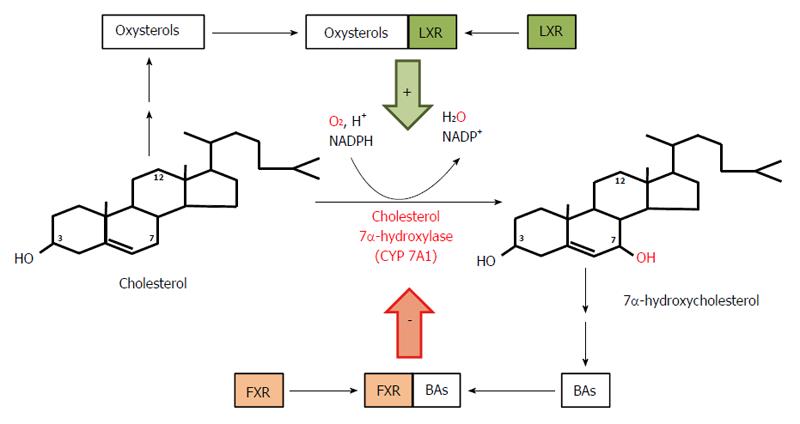
Figure 2 Course and regulation of reactions catalyzed by cholesterol 7α-hydroxylase - the rate limiting enzyme in bile acid biosynthesis.
Bile acids (BAs) formed from 7α-hydroxycholesterol bind to farnezoid X receptor (FXR) and inhibit expression of the gene coding for 7α-hydroxylase, subsequently diminishing the rate of BA biosynthesis in liver. Oxysterols formed from cholesterol bind to liver X receptor (LXR) and stimulate expression of the gene coding for 7α-hydroxylase and the subsequent conversion of cholesterol to 7α-hydroxycholesterol and to BAs.
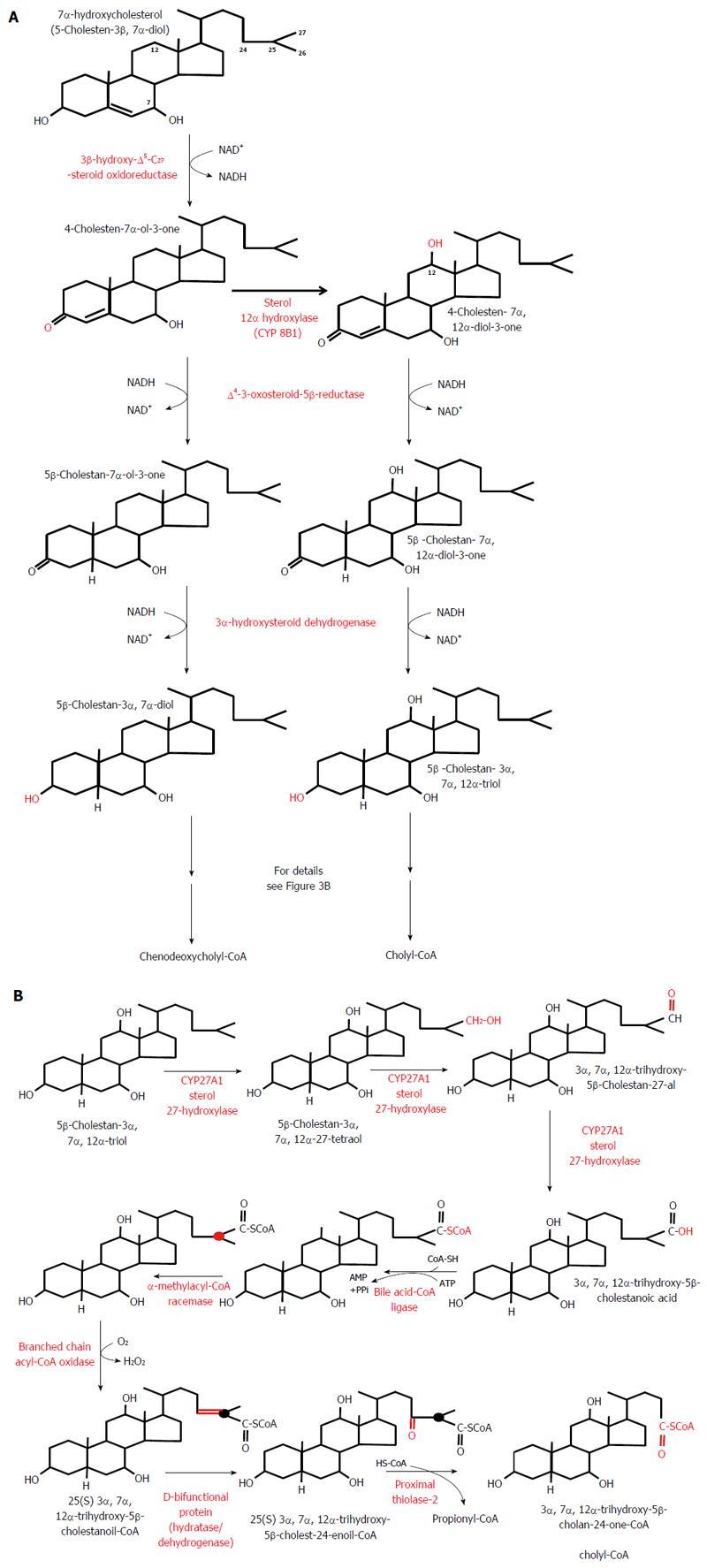
Figure 3 Classic pathway of bile acid biosynthesis in the liver.
A: Conversion of 7α-hydroxycholesterol to 5β-cholestan-3α, 7α-diol - a precursor of chenodeoxycholyl-CoA - and of 7α-hydroxycholesterol to 5β-cholestan-3α, 7α,12α-triol - a precursor of cholyl-CoA. B: Conversion of 5β-cholestan-3α, 7α, 12α-triol to cholyl-CoA. Note that the conversion of 5β-cholestan-3α,7α-diol to chenodeoxycholyl-CoA biosynthesis takes place in the same manner and it is catalyzed by the same liver enzymes (not shown).
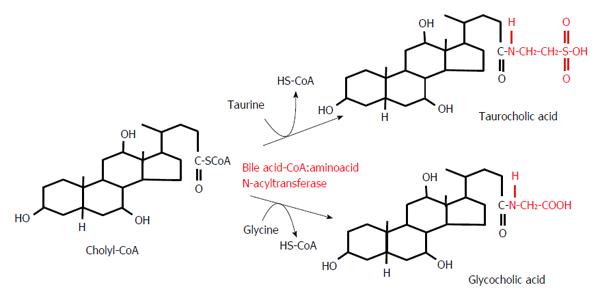
Figure 4 Conjugation reactions of cholyl-CoA with taurine or glycine.
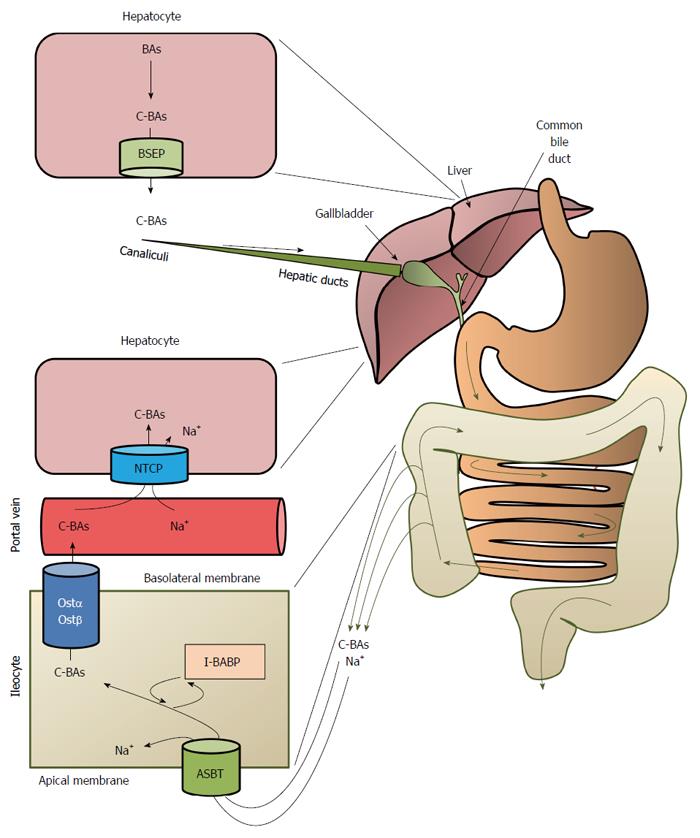
Figure 5 Overview of the enterohepatic circulation of bile acids.
BAs: Bile acids; C-BAs: Conjugated bile acids; BSEP: Bile salt export proteins; ASBT: Bile acid transporter; I-BABP: Ileocyte bile-acid binding protein; OSTα/OSTβ: Organic solute transporters α/β; NTCP: Na+-taurocholate cotransport peptide.
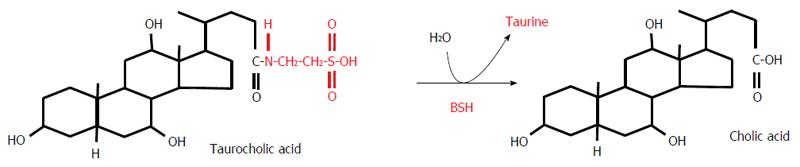
Figure 6 Deconjugation of taurocholic acid by bile salt hydrolase.
BSH: Bile salt hydrolase.
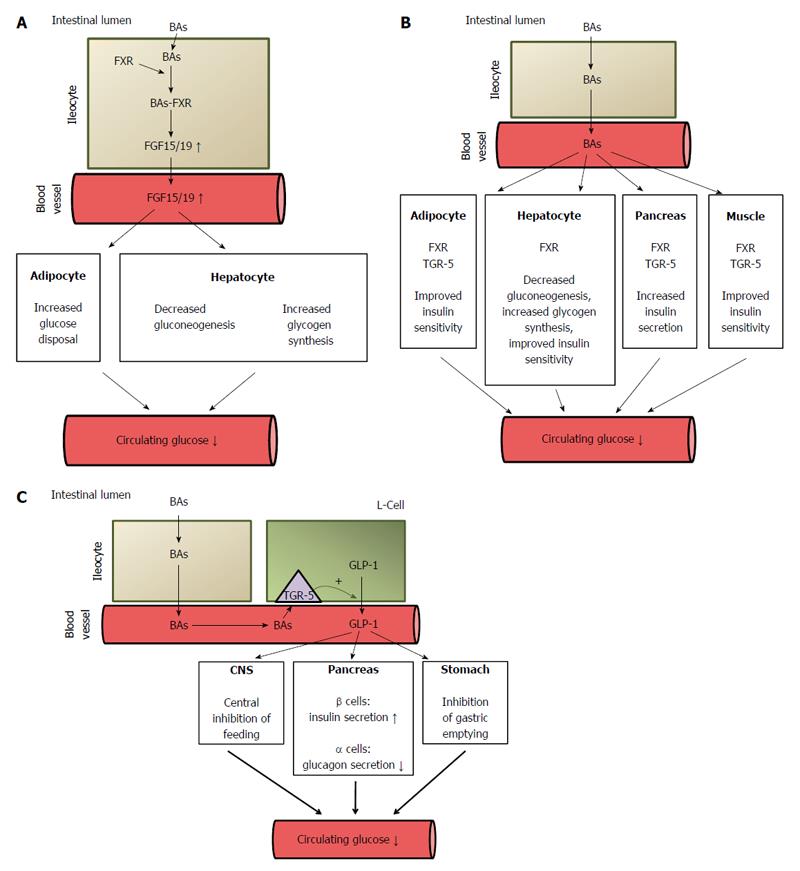
Figure 7 Potential mechanisms of bile acid mediated improvement of serum glucose concentration.
A: Stimulatory effect of bile acids on FGF15/19 synthesis in intestinal cells (ileocytes). The activation of FXR in ileocytes by BAs leads to increased synthesis (via regulation of gene expression) and the release of fibroblast growth factor 15/19 (FGF 15/19) which, through the activation of the FGF-R present in hepatocyte and adipocyte membranes, regulates carbohydrate metabolism, leading to a decrease in circulating glucose concentrations. FGF 15/19 stimulates glycogen synthesis and inhibits gluconeogenesis in the liver and glucose disposal in adipose tissue. ↓: Decrease; ↑: Increase; B: Decreasing effect of BAs on circulating glucose concentration. BAs, by activating FXR, downregulate (via regulation of gene expression) liver gluconeogenesis and stimulate glycogen synthesis. BAs, by binding to FXR or to TGR-5 in pancreatic β-cells, stimulate insulin secretion. BAs, by binding to FXR or TGR-5 in adipose tissue and skeletal muscle, improve insulin sensitivity. ↓: Decrease; C: Potential mechanisms of BA-mediated decrease in circulating glucose concentrations after bariatric surgery caused by the increased release of GLP-1 by intestinal L-cells. ↓: Decrease; ↑: Increase. FXR: Farnezoid X receptor; FGR: Fibroblast growth factor; GLP: Glucagon-like peptide-1; BAs: Bile acids.
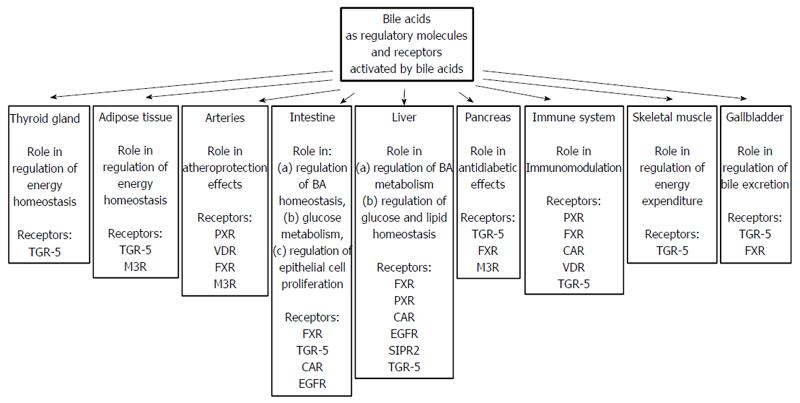
Figure 8 Bile acids as regulatory molecules and receptors activated by bile acids present in different organs.
- Citation: Kaska L, Sledzinski T, Chomiczewska A, Dettlaff-Pokora A, Swierczynski J. Improved glucose metabolism following bariatric surgery is associated with increased circulating bile acid concentrations and remodeling of the gut microbiome. World J Gastroenterol 2016; 22(39): 8698-8719
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8698.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8698