Published online Jan 6, 2023. doi: 10.37126/aige.v4.i1.1
Peer-review started: September 14, 2022
First decision: September 29, 2022
Revised: October 18, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: January 6, 2023
Processing time: 113 Days and 13.5 Hours
Chronic gastritis (CG) is a widespread and frequent disease, mainly caused by Helicobacter pylori infection, which is associated with an increased risk of gastric cancer. Virtual chromoendoscopy improves the endoscopic diagnostic efficacy, which is essential to establish the most appropriate therapy and to enable cancer prevention. Artificial intelligence provides algorithms for the diagnosis of gastritis and, in particular, early gastric cancer, but it is not yet used in practice. Thus, technological innovation, through image resolution and processing, optimizes the diagnosis and management of CG and gastric cancer. The endoscopic Kyoto classification of gastritis improves the diagnosis and management of this disease, but through the analysis of the most recent literature, new algorithms can be proposed.
Core Tip: Advances in virtual chromoendoscopy have improved the knowledge and management of chronic gastritis and led to the Kyoto classification. Artificial intelligence promotes progression in the management of gastric cancer and the diffusion of innovation, allowing new diagnostic algorithms that include both active inflammation and dysplasia as evolutionary steps towards cancer.
- Citation: Panarese A, Saito Y, Zagari RM. Kyoto classification of gastritis, virtual chromoendoscopy and artificial intelligence: Where are we going? What do we need? Artif Intell Gastrointest Endosc 2023; 4(1): 1-11
- URL: https://www.wjgnet.com/2689-7164/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.37126/aige.v4.i1.1
Chronic gastritis (CG) is a widespread and frequent disease, often undiagnosed even after a gastroscopy. Chronic gastritis is relevant to the symptoms that it causes, for the absorption defects that it can entail, but, above all, for the oncological risk with which it is associated[1]. The correct diagnosis improves the management of the disease and, on the endoscopic side, the identification of dysplasia and early gastric cancer is fundamental, possible due to advances in endoscopic technology, which allows the observation of the gastric mucosa in detail[2]. The appropriate classification of CG, defining the etiology, severity, extension, and, in particular, the oncological risk, allows the correct management of the disease and contributes to the results of artificial intelligence, capable of developing algorithms for the diagnosis of CG and gastric cancer[3-6].
Objectively, considering that gastric cancer is still a major cancer, the diagnosis of dysplasia is a relevant outcome[3,7,8], and endoscopic surveillance, important for detecting dysplasia and gastric cancer, is efficient in the high-risk population[9,10]. Early-stage gastric cancer can be cured via endoscopic submucosal dissection, which is curative and less invasive than surgery if carried out according to the guidelines[11-13].
Helicobacter pylori (H. pylori) infection is the main cause of CG and gastric cancer[14]. H. pylori is a type I carcinogen for gastric cancer[15]. However, the risk of gastric cancer in H. pylori-positive subjects is related to the development of precancerous conditions, i.e., mucosal atrophy (MA) and intestinal metaplasia (IM)[14,16-18]. Indeed, gastric cancer morbidity and mortality are declining due to H. pylori eradication therapy[19]. Therefore, the accurate assessment of H. pylori infection status is important[20-22]. The 13C-urea breath test, stool antigen test, and serology are non-invasive tests for the diagnosis of H. pylori infection[5,22].
The Kyoto classification of gastritis, introduced in 2013 in Kyoto, during the 85th Congress of the Japan Gastroenterological Endoscopy Society, was developed with the aim of endoscopically diagnosing H. pylori infection and assessing the risk factors for gastric cancer[23].
This review is an up-to-date study on the Kyoto classification of gastritis[3,23] and highlights advances and future prospects in the endoscopic diagnosis of gastritis and gastric cancer to help improve endoscopic practice.
The Kyoto classification of gastritis accurately considers the endoscopic characteristics of gastritis associated with H. pylori infection and identifies gastric cancer risk factors[23].
In the Kyoto classification of gastritis, there are 19 characterized endoscopic findings that are related to the presence/absence of H. pylori infection, gastritis, and the risk of gastric cancer (Table 1). Among them, MA, IM, enlarged folds, nodularity, and diffuse redness are accounted for in the Kyoto classification score, which is the sum of their scores. The Kyoto score ranges from 0 to 8 (Table 2) and a high score reflects a higher risk of current H. pylori infection and gastric cancer[23].
| Kyoto classification | |
| Related to gastric cancer risk | Mucosal atrophy |
| Intestinal metaplasia | |
| Enlarged folds | |
| Nodularity | |
| Related to Helicobacter pylori infection status | Diffuse redness |
| Others | Regular arrangement of collecting venules |
| Map like redness | |
| Foveolar hyperplastic polyp | |
| Xanthoma | |
| Mucosal swelling | |
| Patchy redness | |
| Depressed erosion | |
| Sticky mucus | |
| Hematin | |
| Red streak | |
| Spotty redncess | |
| Multiple white and flat elevated lesions | |
| Fundic gland polyp | |
| Raised erosion | |
| Endoscopic finding | Score | ||
| Mucosal atrophy | 0 | None | C0-CI according to Kimura Takemoto classification |
| 1 | Mild | CII-CIII | |
| 2 | Severe | OI-OIII | |
| Intestinal metaplasia | 0 | None | None |
| 1 | Mild | Within the antrum | |
| 2 | Severe | Up to the corpus | |
| Enlarged folds | 0 | Negative | < 5 mm gastric fold width |
| 1 | Positive | ≥ 5 mm gastric fold width | |
| Nodularity | 0 | Negative | None |
| 1 | Positive | Small nodules in the antrum | |
| Diffuse redness | 0 | None | None |
| 1 | Mild | Mild translucency of collecting venules in the body | |
| 2 | Severe | Severe translucency of collecting venules in the body | |
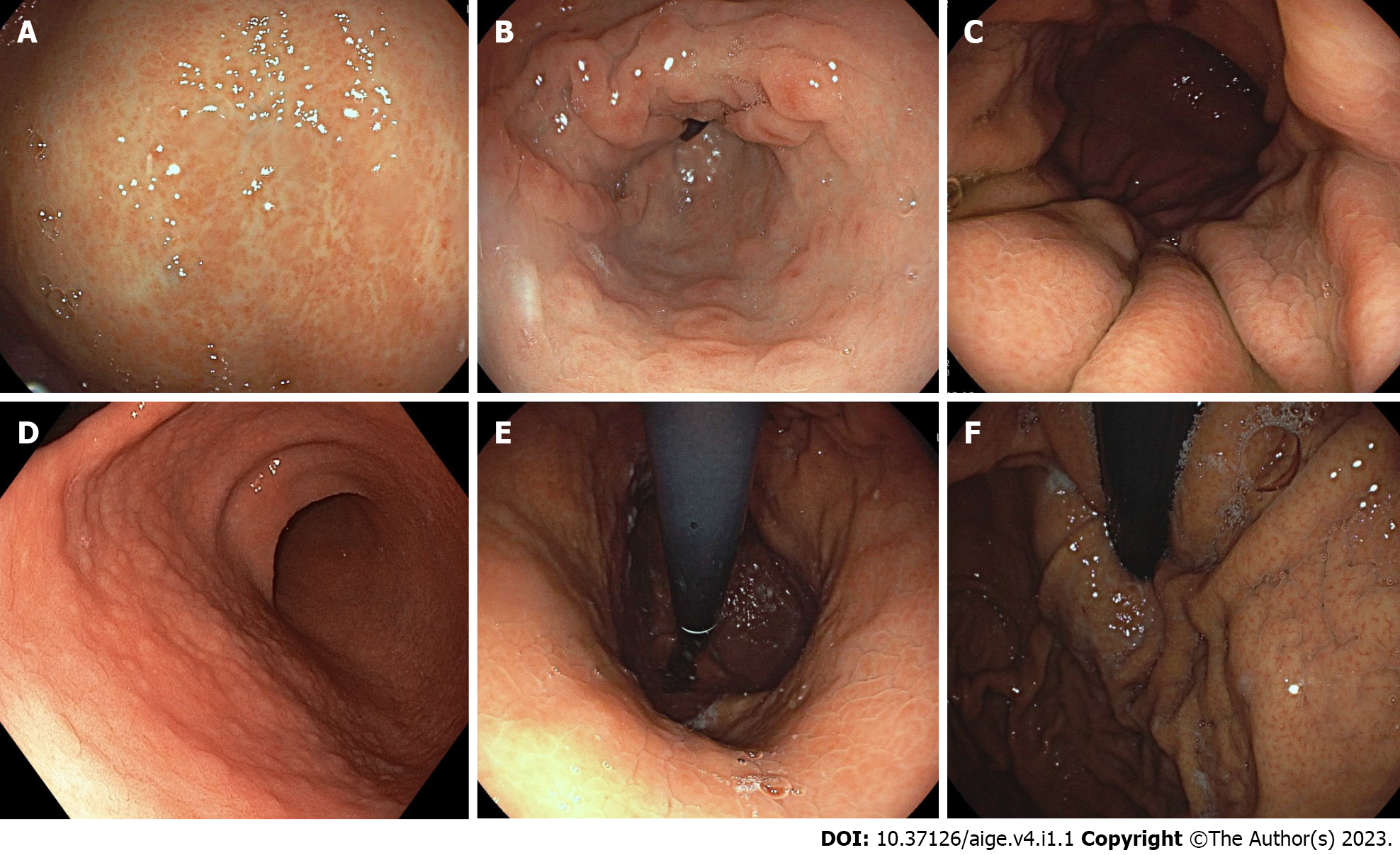
The endoscopic MA (Figure 1A) consists of discolored/pale mucosa with a visible capillary network, and it is classified according to the Kimura and Takemoto classification[24], widely used to diagnose atrophic borders using white light imaging (WLI). In Japan, this classification correlates with histological findings and serum pepsinogen levels observed in the case of MA[24,25], while, in Western countries, WLI cannot correctly diagnose MA and IM according to guidelines, which recommend gastric biopsies for the confirmation of MA, as for IM[26].
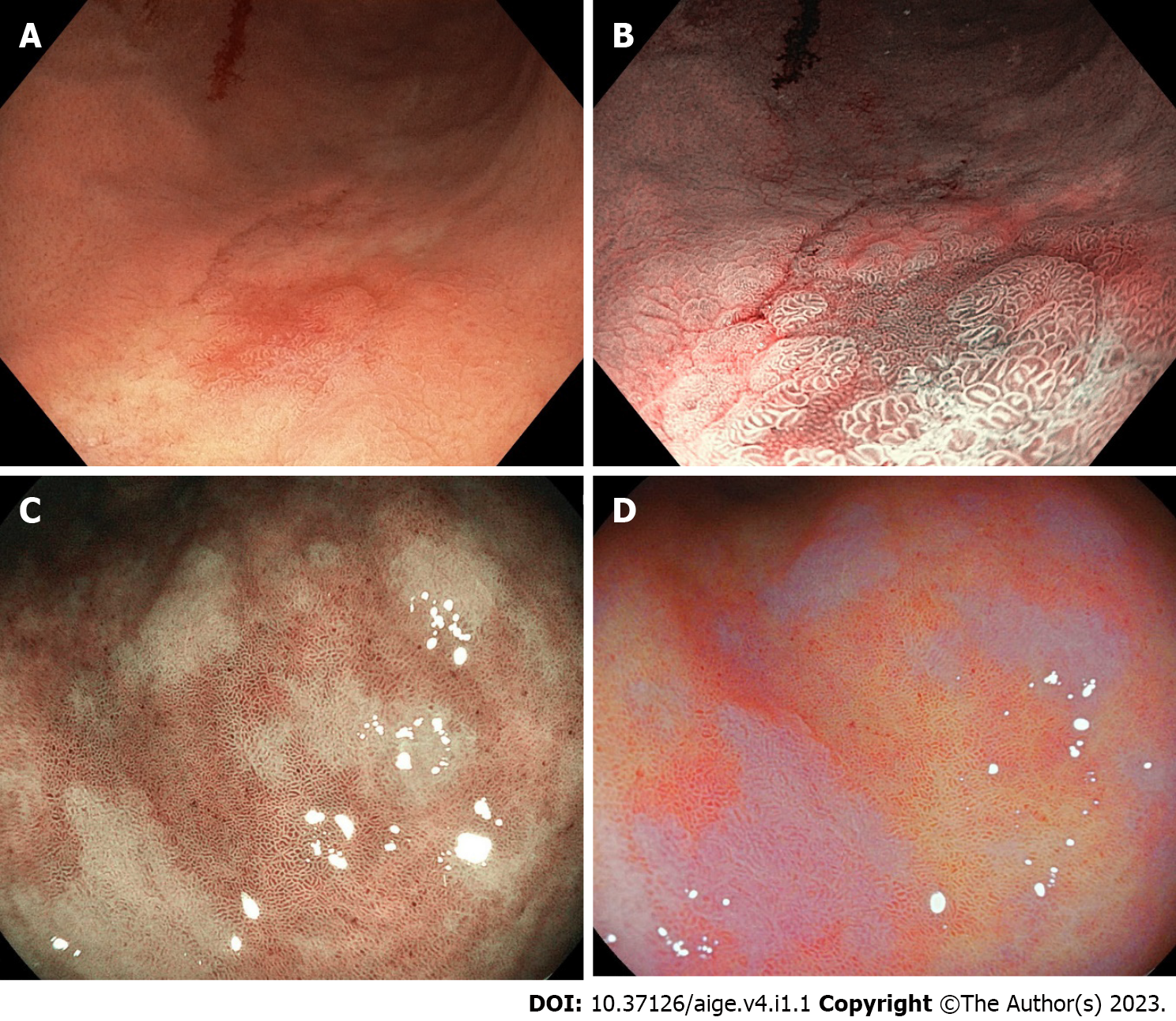
The endoscopic IM (Figure 1B) appears as an irregular surface with slightly elevated/flat/depressed grayish-white plaques surrounded by mixed patchy pink and pale areas of the mucosa. Useful indicators for the endoscopic diagnosis of IM are villous patterns, whitish colors, and rough surfaces, distinctly visible with virtual chromoendoscopy (Figure 2). WLI presents low sensitivity for the diagnosis of IM compared to that of the pathological diagnosis[16,25].
Enlarged folds (Figure 1C) are folds of the body with a width ≥ 5 mm, caused by increased mucosal thickness due to foveolar hyperplasia and the massive infiltration of inflammatory cells. Insufflation does not flatten them or does so partially. The thickness of the folds normalizes after the eradication of H. pylori[10,23].
Nodularity (Figure 1D), i.e., nodular gastritis, is localized mainly in the antrum and consists of a nodular or micronodular diffuse pattern of the mucosa, similar to "goosebumps". Nodular antral gastritis pathologically consists of prominent lymphoid follicles with the infiltration of mononuclear cells. It can be observed more frequently in the stomachs of children than in those of adults, suggesting that it is a characteristic of the early stage of H. pylori infection[27-29].
Diffuse redness (Figure 1E) is the uniform redness of the non-atrophic fundic mucosa, an expression of the congestion and dilation of the subepithelial capillary network by inflammation, with the infiltration of neutrophils and mononuclear cells[30].
Regular arrangement of collecting venules (Figure 1F), in the corpus, from a distance, appears as numerous dots and, more closely, as a regular pattern of starfish-like shapes.
The effectiveness of the Kyoto classification of gastritis is proven by testing scores in patients with CG, gastritis associated with H. pylori, and gastric cancer.
The Kyoto classification proposes the endoscopic diagnosis of H. pylori infection status, although the diagnostic confirmation comes from other investigations[5,17]. Endoscopic diagnosis of H. pylori infection by WLI has low sensitivity (18%-75%) and poor inter-observer agreement[31,32], while magnified image-enhanced endoscopy (M-IEE) is more accurate[9-10,26].
Regarding active H. pylori infection related-gastritis, enlarged folds, nodularity, and diffuse redness have low sensitivity but good specificity, particularly for nodularity (sensitivity: 6.4%-32.1%, specificity: 95.8%-98.8%). On the other hand, regular arrangement of collecting venules has high sensitivity for non-infection (86.7%-100%). Furthermore, for the diagnosis of past H. pylori infection, endoscopic MA has lower specificity (75.5%) compared to IM and map-like redness (92.6% and 98.0%, respectively)[33].
Regarding the total Kyoto classification score and serum H. pylori antibody titer, Kyoto scores increase in line with the H. pylori antibody titer[34,35].
A Kyoto score of 0, 1, and ≥ 2, and no history of H. pylori eradication therapy, corresponds to H. pylori infection rates of 1.5%, 45%, and 82%. However, an active H. pylori infection is not always present in the case of a high Kyoto score, due to a spontaneous negative conversion, IM, or unintentional eradication, after the treatment of other infectious diseases with antibiotics[35,36].
It has been reported that there is good agreement between endoscopic findings for MA and IM and histopathological diagnosis[36]. Endoscopic determination of IM in the corpus is useful because when endoscopic IM is present in the corpus (i.e., the IM 2 score of the Kyoto classification), the pathological IM is significantly associated with a higher risk of gastric cancer[38]. Furthermore, corpus-predominant activity, i.e., the presence of neutrophil activity, has a higher risk of leading to gastric cancer than antral predominant activity[38]. Severe endoscopic MA, enlarged folds, and nodularity correspond to higher neutrophil activity in the corpus than that in the antrum[37].
The topographic distribution of neutrophil activity and IM is strongly associated with gastric cancer risk and corresponds to the separate assessment of pathological gastritis in the corpus and antrum[38].
Regarding gastric cancer as assessed by the Kyoto classification of gastritis, its incidence increases, as %/year, for mild (0.04-0.10), moderate (0.12-0.34), and severe (0.31-1.60) atrophy[38-40]. In detail, over a period of 10 years, the incidence was extremely high (16.0% of patients with severe atrophy)[16] and increased according to the extent of the MA. The prevalence of O-II/O-III-type atrophy according to the Kimura–Takemoto classification is significantly higher in patients with gastric cancer than in subjects with gastritis alone (45.1% vs 12.7%, P < 0.001)[10,39], and Kyoto gastritis scores of MA and IM are significantly higher in the H. pylori-positive cancer group than in subjects with gastritis alone (P < 0.001). Furthermore, endoscopic IM is associated with intestinal-type early gastric cancer, with an OR of 5.0; enlarged folds and nodularity are associated with diffuse-type gastric cancer, with an OR of 5.0 and 13.9, respectively[10].
A cross-sectional study suggests that a Kyoto classification score of ≥ 4 might indicate gastric cancer risk, considering that the Kyoto classification score is 4.8 and 3.8, respectively, for patients with and without gastric cancer[10].
Virtual chromoendoscopy is necessary for a correct evaluation of CG because it remarkably improves the accuracy in the diagnosis of premalignant lesions and early gastric cancer, increasing the visibility of endoscopic findings[26]. Image-enhanced endoscopy (narrow-band imaging, NBI, linked color imaging, LCI, blue laser imaging, BLI, texture and color enhancement imaging, TXI, and autofluorescence imaging) achieves significantly better sensitivity and specificity than WLI, due to the examination of the glandular epithelium by observing the microvascular architecture and structure of the microsurface[31,32,41,42]. However, M-IEE requires skills and experience[43].
Narrow-band imaging, through the use of narrower-band light via blue and green filters, enhances the visualization of the vascular, rather than the surface, structure of the mucosa[44]. Magnified narrow-band imaging can accurately diagnose inflammation and premalignant conditions with sensitivity higher that that of WLI[31,41,42]. Yellowish-white nodules are a predictive marker of nodular gastritis because they have high specificity for the histological finding of lymphoid follicles in the H. pylori-positive stomach[42]. The presence of a fine blue-white line on the crests of the epithelial surface, a light blue crest, and a white opaque substance an accumulation of lipid micro-droplets in the superficial area of certain IM are highly accurate signs of IM[31,41]. The correspondence between histology and magnified narrow-band imaging has been verified regarding the diagnosis of IM and MA[31,41].
Autofluorescence imaging detects the natural fluorescence of some components of the gastric mucosa in real time during endoscopy to differentiate between non-atrophic mucosa, in purple, and atrophic mucosa infected with H. pylori, in green[45]. However, current endoscopic systems do not have the autofluorescence imaging function. Otherwise, texture and color enhancement imaging clearly define subtle tissue differences due to the enhancement of three image factors in WLI (texture, brightness, and color). This is available on the new-generation Olympus instruments[46].
Blue laser imaging-bright and linked color imaging use narrow-band, short-wavelength light because they separately correct blue, green, and red color information. Blue laser imaging, as in narrow-band imaging, produces red-colored, high-intensity, contrast-enhanced images, through blue and green color information, allowing the superior visualization of microvascular and microsurface patterns. In blue laser imaging-bright, a spotty pattern is correlated with an active H. pylori infection, while a cracked pattern corresponds to a post-inflammatory change after the eradication of H. pylori and the mottled pattern to IM[46-47]. Linked color imaging uses the information of all three colors and returns images with color enhancement in its own color range (e.g., red is changed to vivid red and white to clear white) via unique image processing. Regardless of H. pylori infection status, linked color imaging, compared to WLI, increases the color difference around the atrophic border. Linked color imaging identifies diffuse redness of the fundus as a crimson red color, and, compared to WLI, it offers significantly higher overall diagnostic accuracy in patients with an H. pylori-positive stomach and those with an H. pylori-negative stomach after eradication. On the contrary, regarding the presence of the regular arrangement of collecting venules or diffuse redness, WLI, linked color imaging, and magnifying endoscopy with WLI have similar diagnostic accuracy for H. pylori infection. Linked color imaging identifies IM as a lavender color and the diagnostic accuracy is significantly higher than in WLI. Moreover, it increases the visibility of diffuse redness, spotty redness, map-like redness, patchy redness, red streaks, and atrophic borders. Bright-blue laser imaging improves the IM visibility[48].
Magnified-IEE allows the correct endoscopic diagnosis of gastritis with the application of the Kyoto classification of gastritis, and it guarantees the accuracy and reproducibility of endoscopic diagnosis for premalignant lesions related to H. pylori infection throughout the stomach, during active infection and after the eradication of H. pylori[49].
Artificial intelligence, based on deep learning which has allowed considerable progress in various fields through the convolutional neural network (CNN), a method for image recognition can be trained with endoscopic images and could detect gastric cancer accurately[4,50]. Artificial intelligence autonomously extracts and learns the discriminative features of the images and analyzes their complex features, including shapes, colors, and textures. Thus far, several artificial intelligence-assisted CNN computer-aided diagnosis systems have been built, whose diagnostic accuracy in detecting CG and gastric cancer is based on WLI and/or IEE[4,51-53], thus allowing better performance among endoscopists. However, since their introduction in this field occurred recently, the results of most studies need to be further validated, considering all the aspects of endoscopy and formulating increasingly advanced algorithms.
Prospective studies suggest that the deep-learning-based real-time video monitoring diagnostic model works better than endoscopists in the diagnosis of CG and gastric cancer[6,52] and that computer-aided diagnosis systems based on deep learning algorithms have significantly higher accuracy for IEE than for WLI[54]. Computer-aided diagnosis has been studied with the Kyoto classification of gastritis for the endoscopic diagnosis of H. pylori infection, demonstrating accuracy similar to that of experienced endoscopists, being superior with IEE rather than using WLI. Real-time analyses are validating, in prospective studies, the accuracy of computer-aided diagnosis with IEE, using the Kyoto classification of gastritis, for the diagnosis of H. pylori infection and the evaluation of the gastric cancer risk[55].
Technological developments in IEE allow high diagnostic performance to identify CG and determine the risk of gastric cancer according to the Kyoto classification of gastritis[33]. Magnified narrow-band imaging identifies MA and IM due to its detailed examination of the gastric mucosal pattern, e.g., a light blue crest and white opaque substance[26,31]. At a distant view, non-magnified narrow-band imaging, blue laser imaging-bright, and autofluorescence imaging are useful in evaluating MA and IM, and linked color imaging can be used to identify MA, IM, diffuse redness, and a regular arrangement of collecting venules, compared with WLI. Linked color imaging allows the highest visibility among the findings of the Kyoto classification of gastritis and early gastric cancer after H. pylori eradication. Blue laser imaging has the highest visibility for the microvascular pattern, microsurface pattern, and demarcation line in magnifying observations[41,42,47,48]. Matsumura et al[49] suggest that the best methods for the detection and early diagnosis of gastric cancer after H. pylori eradication are linked color imaging observation of the stomach and magnifying blue laser imaging, respectively[33].
Furthermore, in the evaluation of gastric cancer risk, the total score of the grading system is useful in patients with active H. pylori gastritis[10,39], but after H. pylori eradication, it may not be accurate due to the disappearance of diffuse redness, enlarged folds, and nodularity. Moreover, the absence of a regular arrangement of collecting venules is identified as an independent risk factor for gastric cancer after H. pylori eradication[56,57]. Therefore, studies with IEE, applying the Kyoto classification of gastritis, require prospective confirmation and a new grading system, which includes the findings of MA, IM, and regular arrangement of collecting venules to assess the risk of gastric cancer in patients with past H. pylori infection.
Secondly, GC is a disease with two important etiologies, H. pylori infection and autoimmunity, worthy of different treatments and surveillance to reduce the risk of gastric cancer, which could remain a frequent cancer, precisely because the prevalence of H. pylori infection will decrease but the incidence of autoimmune gastritis will increase[21,58]. Definitive endoscopic diagnostic criteria should be established for autoimmune gastritis, in addition to the dosage of antibodies, and they should be considered as endoscopic findings[3,59]. A new endoscopic classification that considers H. pylori gastritis as well as autoimmune gastritis is desirable. Regarding H. pylori-related gastritis, the endoscopic diagnosis of H. pylori infection must be confirmed by an additional test for H. pylori, including histology or a 13C-urea breath test. Endoscopic diagnosis is not sufficient to prescribe antibiotic therapy, even if, due to recent advances in IEE, the diagnostic accuracy is improved and the Kyoto classification of gastritis unifies the endoscopic diagnostic criteria for gastritis, allowing an association between gastritis and H. pylori infection. Eradication therapy for H. pylori is prescribed if positivity is confirmed by the 13C-urea breath test, histological examination, or a fecal test[21]. In conclusion, currently, it is not possible to obtain a conclusive diagnosis of active H. pylori infection only endoscopically.
Thirdly, MA, enlarged folds, nodularity, diffuse redness, and regular arrangement of collecting venules have been considered to assess the correlation between endoscopic findings and histopathology[33], with the limitation that only the combination of different endoscopic findings can improve the diagnostic accuracy, because no single endoscopic feature is highly specific for histological MA and inflammation[23]. However, endoscopic MA and IM are associated with pathological atrophy and IM, respectively, according to several studies[37-59], and the Kyoto classification of gastritis score correlates with the activity and distribution of neutrophils, which are related to the risk of cancer[60]. It is necessary to point out, however, that studies on the consistency between the Kyoto classification of gastritis and histology based on the updated Sydney system are few.
Fourthly, the endoscopy-based Kyoto classification of gastritis score, which predicts the risk of gastric cancer, changes after H. pylori eradication. Toyoshima et al[61], in a retrospective study, concluded that the Kyoto classification score decreases after H. pylori eradication for enlarged folds, nodularity, and diffuse redness. It is probably necessary to calculate the score after the eradication of H. pylori infection to better define the cancer risk and the need for surveillance. As for other preneoplastic conditions of the digestive tract, e.g., Barrett’s esophagus, it is necessary to estimate the score when the inflammation is not active. High-resolution WLI, in association with narrow-band imaging, increases low-grade dysplasia detection on visible lesions after the regression of active H. pylori-induced chronic gastritis. Extensive gastric mapping is required in patients with an overlap between autoimmune atrophic gastritis and H. pylori-induced gastritis[40,49,61,62].
In a future consensus setting, a new algorithm should be published[3]. Considering that H. pylori is oncogenous, we should screen for H. pylori infection, treat positives, and then perform gastroscopy when the infection is no longer active and the possibility of detecting dysplasia is greater. Indeed, dysplasia is the real condition that increases the risk for gastric cancer[63,64]. Current technologies make it possible to detect dysplasia[64], which must be included in the final score as a new finding. Of course, we must perform high-quality gastroscopy, and, currently, biopsies must be carried out with M-IEE, for an accurate histological assessment of gastritis[26]. The accuracy of M-IEE in trained hands further increases the yield of targeted biopsies, necessary for correct risk stratification with OLGA – OLGIM histological staging systems (Operative Link for Gastritis Assessment, Operative Link for Gastric Intestinal Metaplasia Assessment)[65]. Patients in OLGA and OLGIM stage III or IV have a higher gastric cancer risk, and a surveillance endoscopy should be offered to these patients. In this regard, considering the technological advances that make dysplasia visible, it may be the case that the updated Sydney system undergoes further evolutions and, ultimately, also the OLGA-OLGIM system.
Considering all the above, computer-aided diagnosis CNN systems using IEE and the Kyoto score should be refined to confirm the diagnosis of H. pylori infection and to accurately estimate the risk of gastric cancer. Artificial intelligence, through IEE and future advances, will allow us to overcome the problem of subjectivity related to the training and experience of operators[66].
Given that the assessment of gastritis can be considered complete only when a gastroscopy with M-IEE is performed by an experienced operator, who determines the Kyoto score and performs gastric biopsies, which are evaluated by a pathologist, who establishes the OLGA-OLGIM stage, it may be the case that the artificial intelligence allows some steps to be reduced. M-IEE, especially magnified narrow-band imaging and blue laser imaging-bright, has surpassed WLI in terms of sensitivity and specificity for the diagnosis of MA and IM in gastric mucosa, while linked color imaging accurately evaluates all segments of the stomach by searching for endoscopic findings according to the Kyoto classification of gastritis. Artificial intelligence may be able to further support an update of the Kyoto classification of gastritis that would allow us to improve the diagnosis of chronic gastritis, precancerous gastric conditions, lesions of the stomach, and early-stage gastric cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kishikawa H, Japan; Tsuji Y, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50:657-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1174] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 4. | Jin P, Ji X, Kang W, Li Y, Liu H, Ma F, Ma S, Hu H, Li W, Tian Y. Artificial intelligence in gastric cancer: a systematic review. J Cancer Res Clin Oncol. 2020;146:2339-2350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Makristathis A, Hirschl AM, Mégraud F, Bessède E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter. 2019;24 Suppl 1:e12641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Zhao Q, Chi T. Deep learning model can improve the diagnosis rate of endoscopic chronic atrophic gastritis: a prospective cohort study. BMC Gastroenterol. 2022;22:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | International Agency for Research on Cancer. Globocan 2020 – Stomach Cancer. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-factsheet.Pdf. |
| 8. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4877] [Article Influence: 696.7] [Reference Citation Analysis (1)] |
| 9. | Sakitani K, Nishizawa T, Arita M, Yoshida S, Kataoka Y, Ohki D, Yamashita H, Isomura Y, Toyoshima A, Watanabe H, Iizuka T, Saito Y, Fujisaki J, Yahagi N, Koike K, Toyoshima O. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter. 2018;23:e12503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 13. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 304] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 14. | Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM Jr, Correa P, Wilson KT, Piazuelo MB. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 15. | IARC monographs on the evaluation of the carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. Volume 61. Lyon: International Agency for Research on Cancer, 1994: 177-241. [PubMed] |
| 16. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 18. | Fukushima M, Fukui H, Watari J, Ito C, Hara K, Eda H, Tomita T, Oshima T, Miwa H. Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Shin SH, Jung DH, Kim JH, Chung HS, Park JC, Shin SK, Lee SK, Lee YC. Helicobacter pylori Eradication Prevents Metachronous Gastric Neoplasms after Endoscopic Resection of Gastric Dysplasia. PLoS One. 2015;10:e0143257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 724] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 21. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 22. | Zagari RM, Pozzato P, Martuzzi C, Fuccio L, Martinelli G, Roda E, Bazzoli F. 13C-urea breath test to assess Helicobacter pylori bacterial load. Helicobacter. 2005;10:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Haruma K, Kato M, Inoue K, Murakami K, Kamada T. Kyoto Classification of Gastritis. 1st ed. Tokyo Japan: Nihon Medical Center, 2017. |
| 24. | Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (3)] |
| 25. | Takao T, Ishikawa T, Ando T, Takao M, Matsumoto T, Isozaki Y, Okita M, Nagao Y, Oyamada H, Yokoyama K, Tatebe A, Uchiyama K, Handa O, Takagi T, Yagi N, Kokura S, Naito Y, Yoshikawa T. Multifaceted Assessment of Chronic Gastritis: A Study of Correlations between Serological, Endoscopic, and Histological Diagnostics. Gastroenterol Res Pract. 2011;2011:631461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 27. | Luzza F, Pensabene L, Imeneo M, Mancuso M, Contaldo A, Giancotti L, La Vecchia AM, Costa MC, Strisciuglio P, Docimo C, Pallone F, Guandalini S. Antral nodularity identifies children infected with Helicobacter pylori with higher grades of gastric inflammation. Gastrointest Endosc. 2001;53:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Shiotani A, Kamada T, Kumamoto M, Nakae Y, Nakamura Y, Kakudo K, Haruma K. Nodular gastritis in Japanese young adults: endoscopic and histological observations. J Gastroenterol. 2007;42:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Kamada T, Tanaka A, Yamanaka Y, Manabe N, Kusunoki H, Miyamoto M, Tanaka S, Hata J, Chayama K, Haruma K. Nodular gastritis with Helicobacter pylori infection is strongly associated with diffuse-type gastric cancer in young patients. Dig Endosc. 2007;19:180-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Nomura S, Terao S, Adachi K, Kato T, Ida K, Watanabe H, Shimbo T; Research Group for Establishment of Endoscopic Diagnosis of Chronic Gastritis. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig Endosc. 2013;25:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010;72:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017-2025.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Yamamichi N, Hata K, Seto Y, Koike K, Watanabe H, Suzuki H. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J Gastroenterol. 2018;24:4061-4068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Toyoshima O, Nishizawa T, Arita M, Kataoka Y, Sakitani K, Yoshida S, Yamashita H, Hata K, Watanabe H, Suzuki H. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol. 2018;24:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 36. | Nishizawa T, Sakitani K, Suzuki H, Yamakawa T, Takahashi Y, Yamamichi N, Watanabe H, Seto Y, Koike K, Toyoshima O. A combination of serum anti-Helicobacter pylori antibody titer and Kyoto classification score could provide a more accurate diagnosis of H pylori. United European Gastroenterol J. 2019;7:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Toyoshima O, Nishizawa T, Yoshida S, Matsuno T, Odawara N, Toyoshima A, Sakitani K, Watanabe H, Fujishiro M, Suzuki H. Consistency between the endoscopic Kyoto classification and pathological updated Sydney system for gastritis: A cross-sectional study. J Gastroenterol Hepatol. 2022;37:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (2)] |
| 40. | Kaji K, Hashiba A, Uotani C, Yamaguchi Y, Ueno T, Ohno K, Takabatake I, Wakabayashi T, Doyama H, Ninomiya I, Kiriyama M, Ohyama S, Yoneshima M, Koyama N, Takeda Y, Yasuda K. Grading of Atrophic Gastritis is Useful for Risk Stratification in Endoscopic Screening for Gastric Cancer. Am J Gastroenterol. 2019;114:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging vs white light vs mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Okubo M, Tahara T, Shibata T, Nakamura M, Kamiya Y, Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T, Hirata I. Usefulness of magnifying narrow-band imaging endoscopy in the Helicobacter pylori-related chronic gastritis. Digestion. 2011;83:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Dekker E, Houwen BBSL, Puig I, Bustamante-Balén M, Coron E, Dobru DE, Kuvaev R, Neumann H, Johnson G, Pimentel-Nunes P, Sanders DS, Dinis-Ribeiro M, Arvanitakis M, Ponchon T, East JE, Bisschops R. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020;52:899-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 45. | Inoue T, Uedo N, Ishihara R, Kawaguchi T, Kawada N, Chatani R, Kizu T, Tamai C, Takeuchi Y, Higashino K, Iishi H, Tatsuta M, Tomita Y, Tóth E. Autofluorescence imaging videoendoscopy in the diagnosis of chronic atrophic fundal gastritis. J Gastroenterol. 2010;45:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Sugimoto M, Kawai Y, Morino Y, Hamada M, Iwata E, Niikura R, Nagata N, Koyama Y, Fukuzawa M, Itoi T, Kawai T. Efficacy of high-vision transnasal endoscopy using texture and colour enhancement imaging and narrow-band imaging to evaluate gastritis: a randomized controlled trial. Ann Med. 2022;54:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Dohi O, Yagi N, Naito Y, Fukui A, Gen Y, Iwai N, Ueda T, Yoshida N, Kamada K, Uchiyama K, Takagi T, Konishi H, Yanagisawa A, Itoh Y. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: a randomized controlled study. Gastrointest Endosc. 2019;89:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Ono S, Dohi O, Yagi N, Sanomura Y, Tanaka S, Naito Y, Sakamoto N, Kato M. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion. 2020;101:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Matsumura S, Dohi O, Yamada N, Harusato A, Yasuda T, Yoshida T, Ishida T, Azuma Y, Kitae H, Doi T, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Takagi T, Ishikawa T, Konishi H, Morinaga Y, Kishimoto M, Yagi N, Naito Y, Itoh Y. Improved Visibility of Early Gastric Cancer after Successful Helicobacter pylori Eradication with Image-Enhanced Endoscopy: A Multi-Institutional Study Using Video Clips. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 50. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 812] [Article Influence: 135.3] [Reference Citation Analysis (3)] |
| 51. | Horiuchi Y, Aoyama K, Tokai Y, Hirasawa T, Yoshimizu S, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig Dis Sci. 2020;65:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 52. | Ikenoyama Y, Hirasawa T, Ishioka M, Namikawa K, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, Takeuchi Y, Shichijo S, Katayama N, Fujisaki J, Tada T. Detecting early gastric cancer: Comparison between the diagnostic ability of convolutional neural networks and endoscopists. Dig Endosc. 2021;33:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Itoh T, Kawahira H, Nakashima H, Yata N. Deep learning analyzes Helicobacter pylori infection by upper gastrointestinal endoscopy images. Endosc Int Open. 2018;6:E139-E144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 54. | Miyaki R, Yoshida S, Tanaka S, Kominami Y, Sanomura Y, Matsuo T, Oka S, Raytchev B, Tamaki T, Koide T, Kaneda K, Yoshihara M, Chayama K. A computer system to be used with laser-based endoscopy for quantitative diagnosis of early gastric cancer. J Clin Gastroenterol. 2015;49:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Chen PC, Lu YR, Kang YN, Chang CC. The Accuracy of Artificial Intelligence in the Endoscopic Diagnosis of Early Gastric Cancer: Pooled Analysis Study. J Med Internet Res. 2022;24:e27694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 56. | Moribata K, Iguchi JK, Nakachi K, Maeda Y, Shingaki N, Niwa T, Deguchi H, Inoue I, Maekita T, Tamai H, Ichinose M. Endoscopic features associated with development of metachronous gastric cancer in patients who underwent endoscopic resection followed by Helicobacter pylori eradication. Dig Endosc. 2016;28:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Majima A, Dohi O, Takayama S, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest Endosc. 2019;90:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Miceli E, Vanoli A, Lenti MV, Klersy C, Di Stefano M, Luinetti O, Caccia Dominioni C, Pisati M, Staiani M, Gentile A, Capuano F, Arpa G, Paulli M, Corazza GR, Di Sabatino A. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther. 2019;50:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 59. | Kishino M, Nonaka K. Endoscopic Features of Autoimmune Gastritis: Focus on Typical Images and Early Images. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Toyoshima O, Nishizawa T, Yoshida S, Sakaguchi Y, Nakai Y, Watanabe H, Suzuki H, Tanikawa C, Matsuda K, Koike K. Endoscopy-based Kyoto classification score of gastritis related to pathological topography of neutrophil activity. World J Gastroenterol. 2020;26:5146-5155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (4)] |
| 61. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Kinoshita K, Torii A, Yamada A, Suzuki H, Koike K. Helicobacter pylori eradication improved the Kyoto classification score on endoscopy. JGH Open. 2020;4:909-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Panarese A, Galatola G, Armentano R, Pimentel-Nunes P, Ierardi E, Caruso ML, Pesce F, Lenti MV, Palmitessa V, Coletta S, Shahini E. Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions. World J Gastroenterol. 2020;26:3834-3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016;31:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 64. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 65. | Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, Genta RM, Graham DY, Hattori T, Malfertheiner P, Nakajima S, Sipponen P, Sung J, Weinstein W, Vieth M. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 66. | Shichijo S, Nomura S, Aoyama K, Nishikawa Y, Miura M, Shinagawa T, Takiyama H, Tanimoto T, Ishihara S, Matsuo K, Tada T. Application of Convolutional Neural Networks in the Diagnosis of Helicobacter pylori Infection Based on Endoscopic Images. EBioMedicine. 2017;25:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |