Published online Apr 28, 2022. doi: 10.37126/aige.v3.i2.9
Peer-review started: January 16, 2022
First decision: March 8, 2022
Revised: March 22, 2022
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: April 28, 2022
Processing time: 101 Days and 15.9 Hours
In recent years there have been major developments in the field of artificial intelligence. The different areas of medicine have taken advantage of this tool to make various diagnostic and therapeutic methods more effective, safe, and user-friendly. In this way, artificial intelligence has been an increasingly present reality in medicine. In the field of Gastroenterology, the main application has been in the detection and characterization of colonic polyps, but an increasing number of studies have been published on the application of deep learning systems in other pathologies of the gastrointestinal tract. Evidence of the application of artificial intelligence in the assessment of biliary tract is still scarce. Some studies support the usefulness of these systems in the investigation and treatment of choledocholithiasis, demonstrating that they have the potential to be integrated into clinical practice and endoscopic procedures, such as endoscopic retrograde cholangiopancreatography. Its application in cholangioscopy for the investigation of undetermined biliary strictures also seems to be promising. Assessing the bile duct through endoscopic ultrasound can be challenging, especially for less experienced operators, thus becoming an area of potential interest for artificial intelligence. In this review, we summarize the state of the art of artificial intelligence in the endoscopic diagnosis and treatment of biliary diseases.
Core Tip: In recent times, artificial intelligence has played an increasing role in Gastroenterology. There have been numerous studies that show the potential of this technology in clinical practice. Despite this, evidence of the application of these systems in the investigation and treatment of biliary diseases is still scarce. The complexity and challenge that may underlie these processes make this symbiosis very promising. We reviewed the state of the art regarding the application of artificial intelligence in biliary pathology.
- Citation: Correia FP, Lourenço LC. Artificial intelligence in the endoscopic approach of biliary tract diseases: A current review. Artif Intell Gastrointest Endosc 2022; 3(2): 9-15
- URL: https://www.wjgnet.com/2689-7164/full/v3/i2/9.htm
- DOI: https://dx.doi.org/10.37126/aige.v3.i2.9
The concept of artificial intelligence (AI) began to be explored in 1950, by Alan Turing, when he proposed to think about the question: ‘Can machines think?’[1]. This concept was defined as the ability of a computer to achieve human performance in cognitive tasks[2]. AI systems have evolved over the years, with increasingly complex algorithms and increasingly similar performance to the human brain. From this evolution came machine learning and, later, deep learning, two subfields of artificial intelligence. Machine learning identifies and learns patterns and applies them to information in similar future scenarios. Deep learning, currently one of the most used systems, is based on an artificial neural network capable of learning and making decisions by itself, like a human brain[3].
In recent years, several AI systems have been developed for application in several areas of medicine. They are used in the most diverse functions, such as to help assess medical scans, pathology slides, skin lesions, retinal images, electrocardiograms, endoscopy, faces, and vital signs[4]. Gastroenterology, a versatile medical specialty with a wide area of knowledge and an important intervention component, has been one of the areas where AI has been applied more frequently. Some of the application in Gastroenterology are the investigation of dysplasia in areas of Barrett's esophagus, the diagnosis of gastroesophageal reflux disease, the differentiation of acute and chronic pancreatitis, the detection and classification of colorectal polyps, the characterization of colic inflammatory activity in patients with inflammatory bowel disease, among others[3,5].
Despite the many studies on the application of AI in Gastroenterology, the evidence of the use of this technology in the diagnosis and treatment of biliary tract diseases is still scarce. In this review, we conduct research, across multiple platforms and with no time limit, on the application of AI in the diagnosis and treatment of biliary pathology.
Gallstones are a very prevalent pathology in the Western population, often asymptomatic, however in some cases complicating with choledocholithiasis, cholangitis or acute pancreatitis. The diagnosis of choledocholithiasis is not always immediate and linear and may involve several diagnostic methods, from less invasive tests such as abdominal ultrasound, computed tomography (CT) and magnetic resonance cholangiopancreatography to more invasive methods such as endoscopic ultrasound (EUS). Until a few years ago, endoscopic retrograde choangiopancreatography (ERCP) was the first-line method in the diagnosis and treatment of common bile duct (CBD) stones. Since this is an invasive procedure with associated risks of complications (for example, post-ERCP pancreatitis, bleeding, and perforation), ERCP is no longer used for an exclusively diagnosis purposes, maintaining an important therapeutic role in patients with a high likelihood or confirmed choledocholithiasis[6].
The importance of correctly selecting patients with an indication for ERCP has led to the development of several models to predict the presence of stones in the CBD. Currently, it is known that models based on Artificial Neural Networks (ANNs) are more suitable than logistic regression models (used in predictive models for dichotomous outcomes) in the evaluation of biological systems. As such, these models have also been proven to be the most effective at predicting the likelihood of CBD stones and thus discriminating patients who will benefit from ERCP[7]. In addition to ANN models based on clinical data, artificial intelligence systems are currently being developed to facilitate the detection of gallstones in imaging exams (CT and abdominal ultrasound)[8,9] and, in this way, contribute to a more careful selection of patients for ERCP.
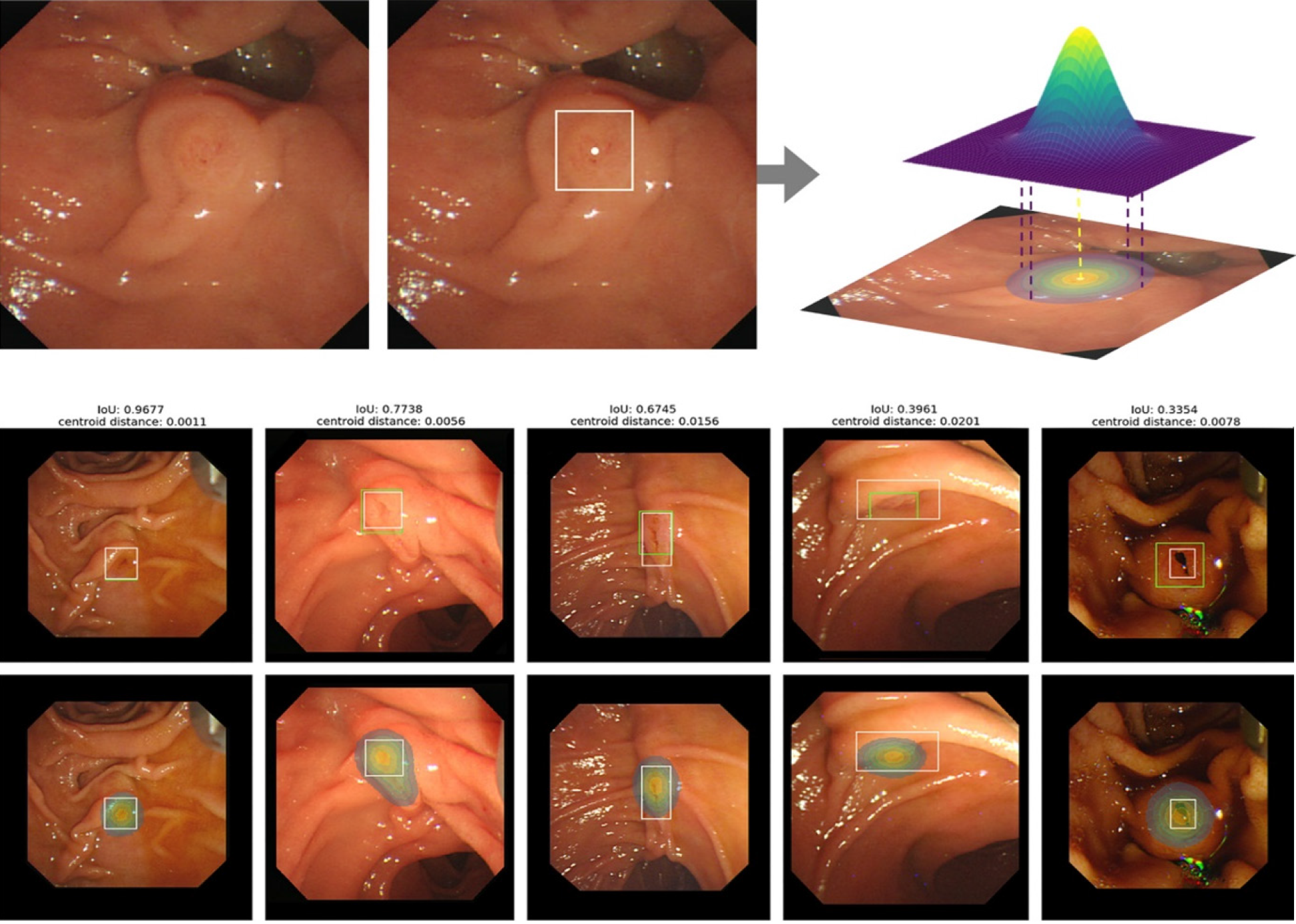
One of the most important steps for the success of ERCP is the cannulation of the major papilla. Several studies report failure in selective biliary cannulation in up to 20% of cases, even when performed by experienced endoscopists[10]. The European Society of Gastrointestinal Endoscopy has defined criteria for difficult biliary cannulation (at least one of the following): (1) More than five contacts with the papilla whilst attempting to cannulate; (2) time to cannulation greater than five minutes; or (3) more than one unintentional pancreatic cannulation. In these cases, longer manipulation of the papilla and multiple attempts at cannulation increase the risk of post-ERCP pancreatitis[11]. Recently, Kim et al[12] developed an artificial intelligence system that predict the location of the ampulla of Vater (AOV) and its difficulty to cannulate. In this model, the identification of the papilla is not based on a bounding box, but on a pixel-wise soft mask, which is a density map where each pixel has a probability of belonging to an AOV (Figure 1). In a fivefold cross-validation study, the model detected the ampulla with mean intersection-over-union 64.1%, precision 76.2%, recall 78.4%, and centroid distance 0.021. These results demonstrate a comparable performance with the human expert in recognizing the range of AOV and to pinpoint the location of AOV, although expert achieve a better deletion of unnecessary parts (precision 91.7% vs 78.9%).
Regarding the prediction of cannulation difficulty, the results were not as consistent: High performance for estimating easy cases for selective cannulation with the average precision and recall of 0.802 and 0.719, respectively, but low recall of 0.611 in the selection of difficult cases. The study showed, however, a good performance in predicting the need for additional cannulation techniques during the performance of ERCP.
After cannulation, there are several factors associated to more complex procedure and a lower probability of complete clearance of gallstones, including a more acute distal CBD angulation and a shorter length of the distal CBD arm[13]. With the aim to predict the technical difficulty of retrieving CBD stones and help the endoscopist to select the best therapeutic approach and accessories during the ERCP, Huang et al[14] developed a system based in deep convolutional neural networks, named intelligent difficulty scoring and assistance system (DSAS). This system was evaluated in a retrospective study where 1954 cholangiograms were used - 1381 images for training and 573 images for validation (internal and external). The system showed good accuracy, sensitivity, and specificity (91.45%, 94.57% and 81.13%, respectively) in detecting common bile duct stones, in addition to good results in image segmentation of the stone, common bile duct and duodenoscope - mean Intersection over Union was 68.35%, 86.42% and 95.85%, respectively. In the assessment of technical difficulty scoring of CBD stone extraction during ERCP, the DSAS was consistent with expert's endoscopists. This system provides a score value, with scores ≥ 2 being associated with greater difficulty in achieving complete CBD clearance (stone clearance rate - score < 2: 86%; score ≥ 2: 36%) and more frequently associated with the use of endoscopic papillary-balloon dilation.
Indeterminate biliary strictures still represent a diagnostic challenge nowadays. Despite the wide differential diagnosis, including benign and malignant causes, the main concern remains the exclusion of a potential malignant cause[15,16]. The methods initially used in the investigation of these strictures, which include imaging, laboratory evaluation and ERCP, although having a high specificity, they have a low sensitivity. Thus, it is difficult to definitively rule out a malignant pathology, which compromises the subsequent approach to the patient[16]. A meta-analysis confirmed the low sensitivity of both cytology (45%) and intraductal biopsies (48.1%) guided by ERCP, in the diagnosis of biliary strictures. Even combining both techniques, the sensitivity is suboptimal[17].
Cholangioscopy has emerged in recent years as a valuable tool in the characterization of these lesions, allowing direct visualization of the stricture and guided biopsies. A recent meta-analysis confirmed the high sensitivity (94%), specificity (95%) and accuracy (94%) of the cholangioscopy in the visual interpretation of biliary malignancies[18]. There are some features suggesting a malignant pathology, namely irregular and tortuous vessels, masses, papillary projections, or infiltrative lesions. Currently, there is no widely accepted system for the visual diagnosis of the stricture, which leads to some non-negligible degree of interobserver variability[19,20].
To overcome that problem, Saraiva et al[21] developed a convolutional neural network-based algorithm with the aim of automatically detecting and differentiating between benign and malignant strictures during cholangioscopy. To train and validate this system, they used 11855 images - 9695 for malignant strictures and 2160 for benign findings (benign biliary strictures or normal segments of the biliary tract). In a 5-fold cross validation study, the sensitivity, specificity, accuracy, and AUC in differentiating malignant from benign lesions was 94.7%, 92.1%, 94.9%, and 0.988 respectively, with a processing speed of 7 ms per frame. Due to its potential for use in real-time, this system may be useful in choosing the area to be biopsied, to obtain a better histological sample. Ghandour et al[22] also developed an artificial intelligence system that detects features suggestive of malignancy in cholangioscopy images with a sensitivity of 81%, specificity of 91%, positive predictive value of 93%, negative predictive value of 77% and AUC of 0.86. Ribeiro et al[23] created a system for automatic detection of papillary projections in cholangioscopic images, which, like the previous ones, showed very promising results. Although these studies show very promising results regarding the application of artificial intelligence in cholangioscopy, only isolated images were used, and they need to be validated using full videos in real time and in clinical practice.
The intimate location of the distal stomach, proximal duodenum and biliary tract makes EUS a great diagnostic method for biliary tract conditions. The relevance of EUS has increased in recent decades, including its application in investigation of hepatobiliary diseases. EUS has shown an excellent performance in the diagnosis of several biliary pathologies, namely choledocholithiasis, microlithiasis, biliary strictures, biliary obstruction, or cholangiocarcinoma[24,25]. EUS-guided interventions have also grown in the last years, being an option, in experienced centers, for drainage of biliary obstruction when ERCP fails, as well as in acute cholecystitis, biliary leaks and bilomas[26,27].
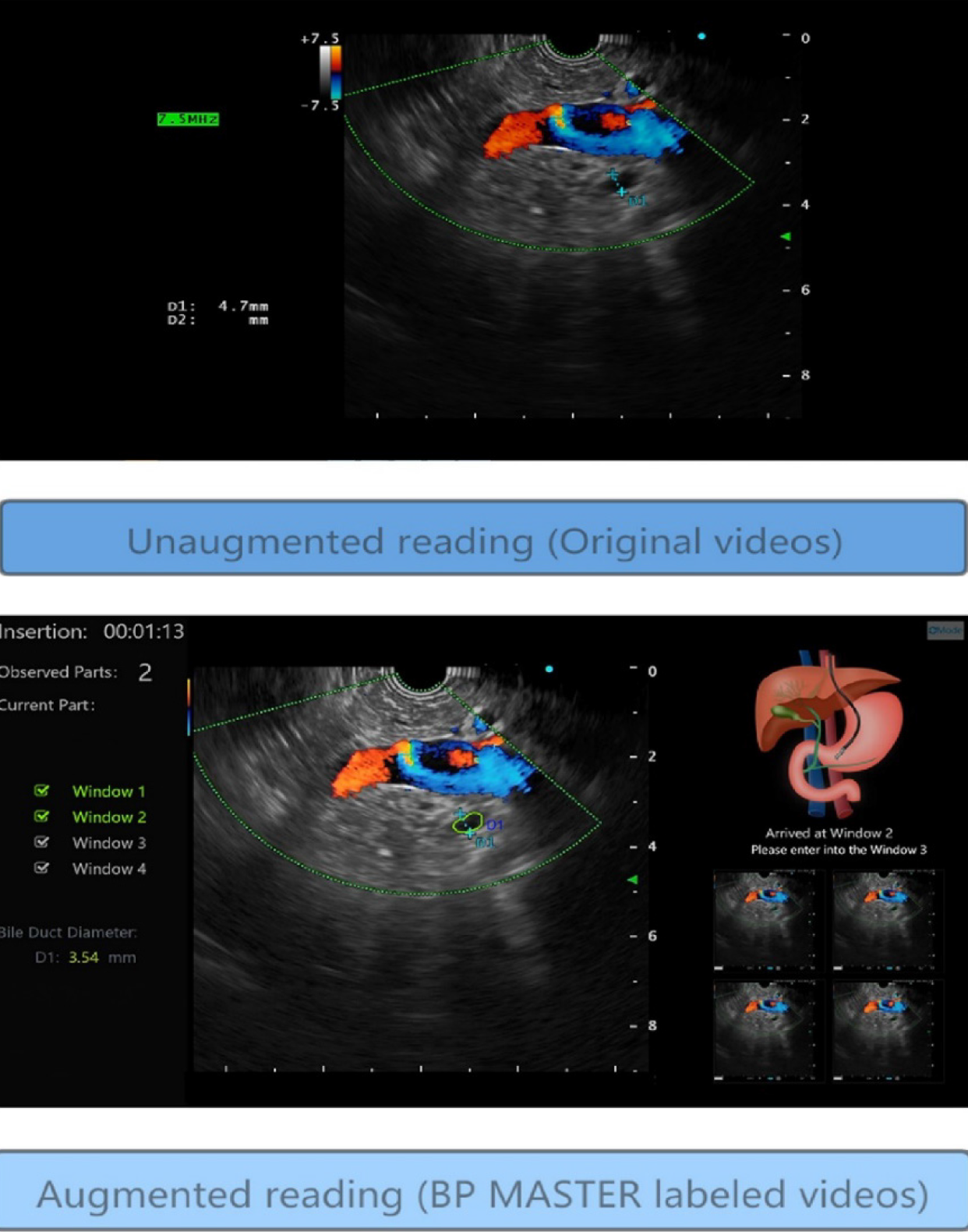
EUS is a challenging advanced endoscopic technique with a long learning curve[28]. As such, the development of systems that facilitate the interpretation of ultrasound endoscopy findings appears to be essential for the wide adoption of EUS. Yao et al[29] developed a deep learning-based system, BP MASTER, which, in real-time, recognizes the stations (the fundus of stomach; body of stomach and antrum; duodenal bulb; and descending duodenum) where the transducer is located and provide the corresponding operation instructions, delineates the bile duct, and gives an estimate of its diameter (Figure 2). To train the model, the authors used 10681 images in the bile duct station recognition and 2529 images in the bile duct annotation. For model validation, 2425 images and 515 video clips were used for internal validation and 799 images for external validation. This system showed an accuracy of 93.3% for station recognition in image validation set and 90.1% in video validation set and a Dice of 0.77 in the bile duct segmentation. The results obtained with this system were comparable to those of expert endoscopists. Furthermore, in a crossover study, this system showed an improvement in trainees' accuracy from 60.8% to 76.3%.
Another application of ultrasound endoscopy is the evaluation of polypoid lesions of the gallbladder. Recently, an artificial intelligence system applied to EUS was developed[30] that allows the distinction between gallstones and polypoid lesions with an accuracy of 95.7% and the differentiation of neoplastic and non-neoplastic polyps with an accuracy of 89.8%. At this last point, the accuracy of the EUS-AI was between mid-level and expert EUS endoscopists.
Despite being promising systems, with the potential to reduce endoscopic procedures with greater risks and even surgeries, further studies are needed to validate the results obtained.
The diagnostic and therapeutic complexity associated with bile tract diseases makes this an attractive area for the development of AI systems.
In choledocholithiasis, AI systems have proved to be useful both in diagnosis, allowing a more careful selection of patients with indication for ERCP; as well as treatment, assisting the endoscopist in the critical steps of the procedure (e.g., cannulation). The application of AI in cholangioscopy showed interest in the possibility of a more objective characterization of indeterminate biliary strictures and of directing biopsies to areas where the findings are more suspicious. Endoscopic ultrasound, an intervention area with a long learning curve, could benefit from the introduction of this technology, especially for less experienced endoscopists.
Despite this, there are still few studies focused on biliary condition, and most of them are retrospective, with small samples and high risk of bias. In the future, it is essential to continue to invest in the development of systems that optimize the diagnosis and facilitate the treatment of biliary pathologies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen BB, Taiwan; Cochior D, Romania; Huang Y, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 2. | Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (1)] |
| 4. | Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2826] [Article Influence: 471.0] [Reference Citation Analysis (0)] |
| 5. | Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smaïl-Tabbone M, Danese S, Peyrin-Biroulet L. Application of Artificial Intelligence to Gastroenterology and Hepatology. Gastroenterology. 2020;158:76-94.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (1)] |
| 6. | Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 7. | Jovanovic P, Salkic NN, Zerem E. Artificial neural network predicts the need for therapeutic ERCP in patients with suspected choledocholithiasis. Gastrointest Endosc. 2014;80:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Pang S, Ding T, Qiao S, Meng F, Wang S, Li P, Wang X. A novel YOLOv3-arch model for identifying cholelithiasis and classifying gallstones on CT images. PLoS One. 2019;14:e0217647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Yu CJ, Yeh HJ, Chang CC, Tang JH, Kao WY, Chen WC, Huang YJ, Li CH, Chang WH, Lin YT, Sufriyana H, Su EC. Lightweight deep neural networks for cholelithiasis and cholecystitis detection by point-of-care ultrasound. Comput Methods Programs Biomed. 2021;211:106382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guidewire-assisted cannulation of the common bile duct for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database Syst Rev. 2012;12:CD009662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 383] [Article Influence: 42.6] [Reference Citation Analysis (1)] |
| 12. | Kim T, Kim J, Choi HS, Kim ES, Keum B, Jeen YT, Lee HS, Chun HJ, Han SY, Kim DU, Kwon S, Choo J, Lee JM. Artificial intelligence-assisted analysis of endoscopic retrograde cholangiopancreatography image for identifying ampulla and difficulty of selective cannulation. Sci Rep. 2021;11:8381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Kim HJ, Choi HS, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi SH. Factors influencing the technical difficulty of endoscopic clearance of bile duct stones. Gastrointest Endosc. 2007;66:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Huang L, Lu X, Huang X, Zou X, Wu L, Zhou Z, Wu D, Tang D, Chen D, Wan X, Zhu Z, Deng T, Shen L, Liu J, Zhu Y, Gong D, Zhong Y, Liu F, Yu H. Intelligent difficulty scoring and assistance system for endoscopic extraction of common bile duct stones based on deep learning: multicenter study. Endoscopy. 2021;53:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015;3:22-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Bowlus CL, Olson KA, Gershwin ME. Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol. 2016;13:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 18. | de Oliveira PVAG, de Moura DTH, Ribeiro IB, Bazarbashi AN, Franzini TAP, Dos Santos MEL, Bernardo WM, de Moura EGH. Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis. Surg Endosc. 2020;34:3321-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Angsuwatcharakon P, Kulpatcharapong S, Moon JH, Ramchandani M, Lau J, Isayama H, Seo DW, Maydeo A, Wang HP, Nakai Y, Ratanachu-Ek T, Bapaye A, Hu B, Devereaux B, Ponnudurai R, Khor C, Kongkam P, Pausawasdi N, Ridtitid W, Piyachaturawat P, Khanh PC, Dy F, Rerknimitr R. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB (Oxford). 2022;24:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Stassen PMC, Goodchild G, de Jonge PJF, Erler NS, Anderloni A, Cennamo V, Church NI, Fernandez-Urien Sainz I, Huggett MT, James MW, Joshi D, Kylänpää L, Laleman W, Nayar MK, Oppong KW, Poley JW, Potts JR, Repici A, Udd M, Vila JJ, Wong T, Bruno MJ, Webster GJM; European Cholangioscopy Group. Diagnostic accuracy and interobserver agreement of digital single-operator cholangioscopy for indeterminate biliary strictures. Gastrointest Endosc. 2021;94:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Saraiva MM, Ribeiro T, Ferreira JPS, Boas FV, Afonso J, Santos AL, Parente MPL, Jorge RN, Pereira P, Macedo G. Artificial intelligence for automatic diagnosis of biliary stricture malignancy status in single-operator cholangioscopy: a pilot study. Gastrointest Endosc. 2022;95:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Ghandour B, Hsieh H, Akshintala V, Bejjani M, Szvarca D, Tejaswi S, DiMaio C, Trindade A, Manvar A, Itoi T, Slivka A, Goenka M, Moon J, Kushnir V, Sethi A, Bun Teoh A, Yan Tang R, Lau J, Rerknimitr R, Ang T, Maydeo A, Jonica E, Kaffes A, Saxena P, Nguyen N, Khokhar A, D'Souza L, Buscaglia J, Kaul V, Robles-Medranda C, Zulli C, Pons-Beltrán V, Bick B, Sherman S, Muniraj T, Jamidar P, Vedula S, Hager G, Khashab M. S1 Machine Learning for Classification of Indeterminate Biliary Strictures During Cholangioscopy. AJG. 2021;116:S1-S1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Ribeiro T, Saraiva MM, Afonso J, Ferreira JPS, Boas FV, Parente MPL, Jorge RN, Pereira P, Macedo G. Automatic Identification of Papillary Projections in Indeterminate Biliary Strictures Using Digital Single-Operator Cholangioscopy. Clin Transl Gastroenterol. 2021;12:e00418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Schwartz DA, Wiersema MJ. The role of endoscopic ultrasound in hepatobiliary disease. Curr Gastroenterol Rep. 2002;4:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Giljaca V, Gurusamy KS, Takwoingi Y, Higgie D, Poropat G, Štimac D, Davidson BR. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev. 2015;CD011549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Attasaranya S, Netinasunton N, Jongboonyanuparp T, Sottisuporn J, Witeerungrot T, Pirathvisuth T, Ovartlarnporn B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center's Experience in 31 Cases. Gastroenterol Res Pract. 2012;2012:680753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Canakis A, Baron TH. Relief of biliary obstruction: choosing between endoscopic ultrasound and endoscopic retrograde cholangiopancreatography. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 28. | Wani S, Keswani R, Hall M, Han S, Ali MA, Brauer B, Carlin L, Chak A, Collins D, Cote GA, Diehl DL, DiMaio CJ, Dries A, El-Hajj I, Ellert S, Fairley K, Faulx A, Fujii-Lau L, Gaddam S, Gan SI, Gaspar JP, Gautamy C, Gordon S, Harris C, Hyder S, Jones R, Kim S, Komanduri S, Law R, Lee L, Mounzer R, Mullady D, Muthusamy VR, Olyaee M, Pfau P, Saligram S, Piraka C, Rastogi A, Rosenkranz L, Rzouq F, Saxena A, Shah RJ, Simon VC, Small A, Sreenarasimhaiah J, Walker A, Wang AY, Watson RR, Wilson RH, Yachimski P, Yang D, Edmundowicz S, Early DS. A Prospective Multicenter Study Evaluating Learning Curves and Competence in Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography Among Advanced Endoscopy Trainees: The Rapid Assessment of Trainee Endoscopy Skills Study. Clin Gastroenterol Hepatol. 2017;15:1758-1767.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Yao L, Zhang J, Liu J, Zhu L, Ding X, Chen D, Wu H, Lu Z, Zhou W, Zhang L, Xu B, Hu S, Zheng B, Yang Y, Yu H. A deep learning-based system for bile duct annotation and station recognition in linear endoscopic ultrasound. EBioMedicine. 2021;65:103238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Jang SI, Kim YJ, Kim EJ, Kang H, Shon SJ, Seol YJ, Lee DK, Kim KG, Cho JH. Diagnostic performance of endoscopic ultrasound-artificial intelligence using deep learning analysis of gallbladder polypoid lesions. J Gastroenterol Hepatol. 2021;36:3548-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |