Published online Aug 28, 2021. doi: 10.37126/aige.v2.i4.136
Peer-review started: May 1, 2021
First decision: June 18, 2021
Revised: June 21, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: August 28, 2021
Processing time: 127 Days and 11.6 Hours
Capsule endoscopy (CE) is a recently developed diagnostic method for diseases of the small bowel that is non-invasive, safe, and highly tolerable. Its role in patients with inflammatory bowel disease has been widely validated in suspected and established Crohn’s disease (CD) due to its ability to assess superficial lesions not detected by cross-sectional imaging and proximal lesions of the small bowel not evaluable by ileocolonoscopy. Because CE is a highly sensitive but less specific technique, differential diagnoses that can simulate CD must be considered, and its interpretation should be supported by other clinical and laboratory indicators. The use of validated scoring systems to characterize and estimate lesion severity (Lewis score, Capsule Endoscopy Crohn’s Disease Activity Index), as well as the standardization of the language used to define the lesions (Delphi Consensus), have reduced the interobserver variability in CE reading observed in clinical practice, allowing for the optimization of diagnoses and clinical management strategies. The appearance of the panenteric CE, the incorporation of artificial intelligence, magnetically-guided capsules, and tissue biopsies are elements that contribute to CE being a promising, unique diagnostic tool in digestive tract diseases.
Core Tip: Capsule endoscopy (CE) is the non-invasive diagnostic method of choice for visualizing the small bowel. Its utility is widely validated in both suspected and established Crohn’s disease (CD) due to its high sensitivity for detecting early lesions and a high negative predictive value. CE enables estimating the activity and extent of disease, establishing prognosis, and evaluating the therapeutic response in patients with CD. New technologies, such as the panenteric CE and the recent incorporation of artificial intelligence to CE image analysis, render CE an attractive, unique diagnostic tool for diseases of the digestive tract in the future.
- Citation: Pérez de Arce E, Quera R, Núñez F P, Araya R. Role of capsule endoscopy in inflammatory bowel disease: Anything new? Artif Intell Gastrointest Endosc 2021; 2(4): 136-148
- URL: https://www.wjgnet.com/2689-7164/full/v2/i4/136.htm
- DOI: https://dx.doi.org/10.37126/aige.v2.i4.136
Capsule endoscopy (CE) is a non-invasive diagnostic method of increasing development in the study of small bowel diseases. Since its appearance in 2000[1], it has shown its greatest utility in studying obscure gastrointestinal bleeding, celiac disorder, polyposis syndromes, and Crohn’s disease (CD). The role of CE in inflammatory bowel disease (IBD), especially in CD, has been extensively investigated in the diagnosis of suspected CD and the management of established CD for the evaluation of disease severity, extent, and response to treatment[2]. The main advantage of CE over ileocolonoscopy is its ability to visualize the mucosa of the proximal small bowel, and compared to imaging studies, its ability to detect superficial mucosal ulcerations missed on magnetic resonance enterography (MRE)[3] and computed tomography enterography[4]. This point is fundamental, considering that studies report that the involvement of the small bowel affects up to 66% of patients diagnosed with CD[5], corresponding to up to 90% of lesions located in the terminal ileum accessible by ileocolonoscopy[6]. Before CE development, the proximal small bowel was examined using indirect imaging methods such as radiography, cross-sectional imaging, and enteroscopy. Detection of lesions in the proximal small bowel is critical due to the implications for managing patients with CD. Jejunal lesions visualized by CE have been found in up to 56% of patients with CD, and these are associated with more severe disease and more rapid progression[7]. Moreover, the role of CE in ulcerative colitis (UC) is not well established because the evidence remains limited. In recent years, remarkable advances in CE technology and design, and the recent use of artificial intelligence, have improved its diagnostic yield in CD.
This review aims to assess the role of CE in IBD and discusses advances in the field and their implications for clinical practice going forward.
Studies comparing the diagnostic yield of CE with other diagnostic techniques in patients with CD conclude that CE has a high sensitivity[4,8] and a high negative predictive value (NPV)[9]. However, the diagnostic accuracy of CE has not been determined due to the lack of a gold standard for the diagnosis of CD. A meta-analysis[8] found that CE had a better diagnostic yield than small bowel radiography [52% vs 16%; incremental yield (IY) 32%, P < 0.0001, 95% confidence interval (CI) = 16%-48%], computed tomography enterography (68 vs 21%; IY 47%, w = 47%, P < 0.00001, 95%CI = 31%-63%), and ileocolonoscopy (47 vs 25%; IY 22%, P = 0.009, 95%CI = 5%-39%) in unsuspected CD patients. Similarly, in patients with established CD, CE also outperformed these diagnostic tests[8]. Furthermore, CE was superior to MRE in detecting small bowel lesions in patients with CD, mainly superficial and proximal lesions[10]. A subsequent meta-analysis of 13 studies[3] compared the diagnostic performance of CE with MRE and small bowel contrast ultrasound imaging for the evaluation of small bowel CD. These authors found that the diagnostic yield of CE was similar to MRE [odds ratio (OR) 1.17; 95%CI: 0.83–1.67] and small bowel contrast ultrasound (OR 0.88; 95%CI: 0.51–1.53) when detecting lesions in the small bowel for both established and suspected CD. However, CE was superior to MRE in detecting proximal small bowel lesions (OR 2.79; 95%CI: 1.2–6.48).
In a recent study among the pediatric population, CE was as sensitive as MRE in identifying inflammatory activity in the terminal ileum and the proximal small bowel; however, the distribution of small bowel inflammation was more extensive when characterized by CE[11].
In summary, the diagnostic yield of CE is at least similar to MRE for established CD in the evaluation of the small bowel. However, the main advantage of CE is the detection of the most proximal and superficial lesions missed on MRE. In suspected CD, CE is more useful when the ileocolonoscopy results are negative.
At the moment, there are no established diagnostic criteria for the diagnosis of CD by CE. Currently, the Lewis score (LS)[12,13] and Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI)[14] are the two validated diagnostic indexes for the evaluation of CE images. Their results must be interpreted in the patient’s clinical setting because lesions are not pathognomonic for CD and can be found in other inflammatory conditions. The LS was the first and most widely used index for evaluating inflammatory changes in the mucosa of the small intestine, which is divided into three tertiles according to the transit time estimated using CE. Each characteristic CD finding (villous edema, ulceration, stenosis) is assigned a score for each tertile. The final result of the LS corresponds to the tertile with the highest score, in addition to the stenoses score. A score < 135 is considered normal or clinically insignificant inflammation; from 135 to 790 indicates mild inflammation; and > 790 moderate to severe inflammation[12]. The CECDAI evaluates the proximal and distal segments of the small bowel using an inflammation score (A; 0–5), an extent score (B; 0–3), and a stricture score (C; 0–3), which are combined using the formula A × B + C. The total score (from 0–26) results from adding both the proximal and distal segments. A higher CECDAI score reflects more severe mucosal inflammation[14]. Although there is a good correlation between the LS and CECDAI (Pearson’s = 0.81, P = 0.0001)[15], a recent study of 102 patients with CD found that CECDAI was superior to LS in reflecting active intestinal inflammation[16]. Recently, Eliakim et al[17] published the Eliakim score, a quantitative measure for PillCamTM Crohn’s with excellent reliability that significantly correlates with LS and fecal calprotectin (FC).
So far, manual video review is the method of choice for the detection of lesions in CE. However, a fast-reading method is offered by TOP100, a new software tool in RAPID Reader version 9.0[18]. TOP100 automatically selects the 100 best images from the video with relevant findings, allowing the LS to be calculated quickly. An initial study that compared both reading techniques found agreement in 89.6% of cases calculated by TOP100 as having LS > 135 and those calculated by manual review of the video. Despite these encouraging results, TOP100 should not replace the traditional reading method but rather constitutes a complementary tool for quick LS calculation[18].
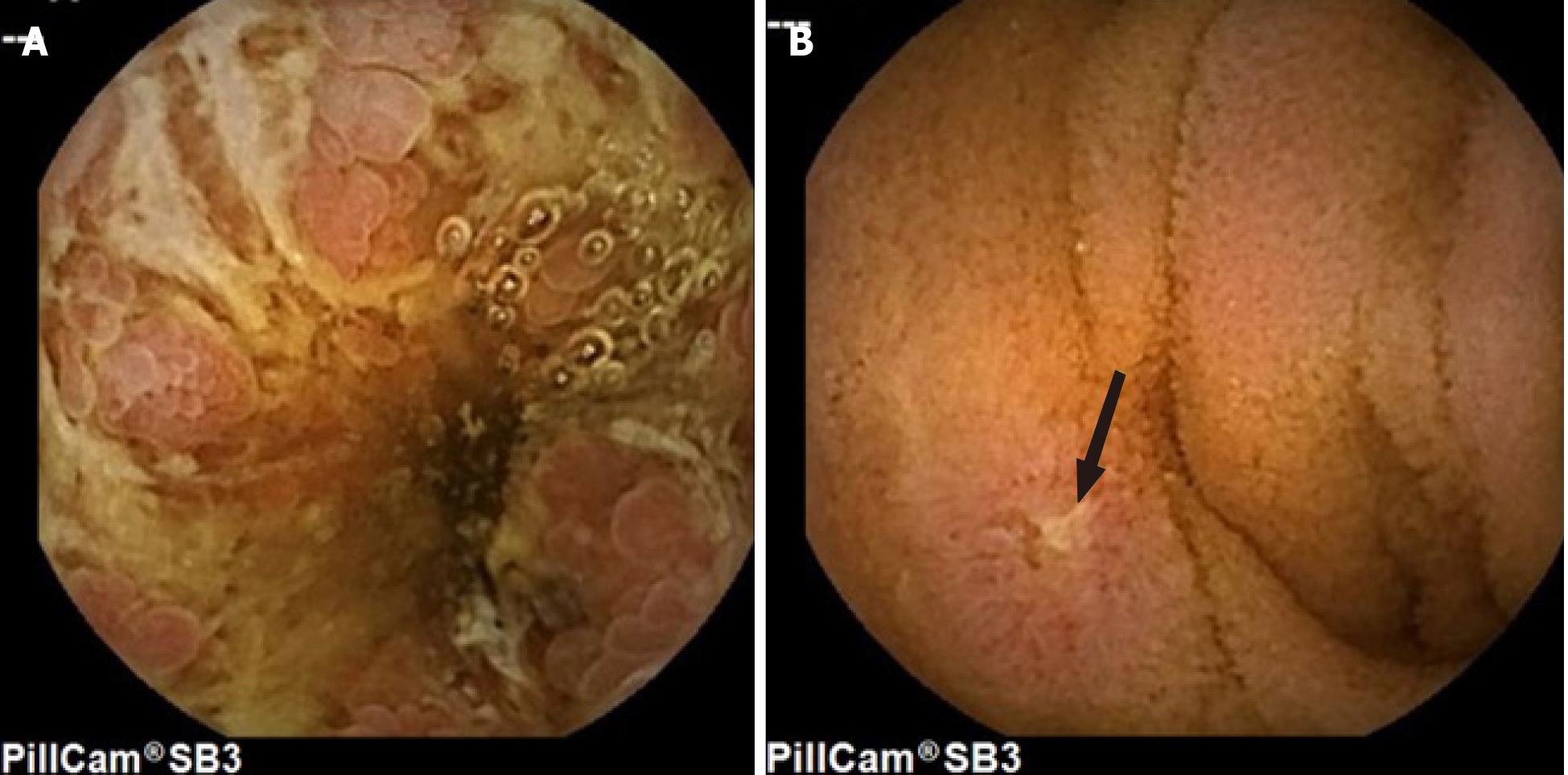
Although studies have shown the usefulness of CE in identifying small bowel lesions, one of the difficulties in IBD studies was the lack of nomenclature and descriptions of small bowel lesions. The high interobserver variability in the interpretation and evaluation of the severity of the lesions has both clinical and research implications. Published in 2005, the Capsule Endoscopy Structured Terminology (CEST)[19] is an international consensus on standardized terminology for the findings or lesions detected by CE and also contains guidelines for reporting these findings (structure and content). However, the description of ulcerative and inflammatory lesions in the CEST is ambiguous and limited and, as such, fails to inform clinicians as to which type of lesion is most suspicious for the diagnosis of CD. Therefore, the international Delphi consensus statement established seven definitions describing the ulcerative and inflammatory lesions seen in CD by CE: aphthoid erosion, deep ulceration, superficial ulceration, stenosis, edema, hyperemia, and denudation[20]. The use of a common language enables standardizing the results of clinical studies and improves patients’ health care (Figure 1).
The use of virtual chromoendoscopy, such as flexible spectral color enhancement (FICE), can also be applied in the revision of CE images to improve the visualization of any lesions. FICE enhances mucosal surface patterns using software to convert white light images to certain ranges of wavelengths (red, blue, green). A systematic review and meta-analysis of 13 studies found that the use of FICE failed to significantly improve the injury detection rate in CE[21].
The CE retention rate (not passed in more than two weeks post-ingestion or less if endoscopic or surgical intervention is required)[22] in the general population ranges from 1.0% to 2.5%[23]. Due to the potential occurrence of stenosis in patients with CD, the retention rate in patients with suspected CD is 2.35%, and with established CD is up to 4.63%[24]. The risk of EC retention can be estimated with a patency capsule (PillcamTM), a capsule with a lactose body, and a barium section for follow-up by fluoroscopy. The disintegration that induces deformation of the capsule or non-expulsion after 30 h suggests small bowel stenosis[23]. The NPV of the patency capsule to predict CE retention ranges from 98% to 100%[25,26]. Given the high risk of CE retention in patients with established CD, and due to the impossibility of distinguishing high from low-risk retention in the clinic, the use of a patency capsule is recommended before CE[24].
The main clinical scenarios for the application of EC for IBD are both suspected and established CD. CE studies in UC are limited.
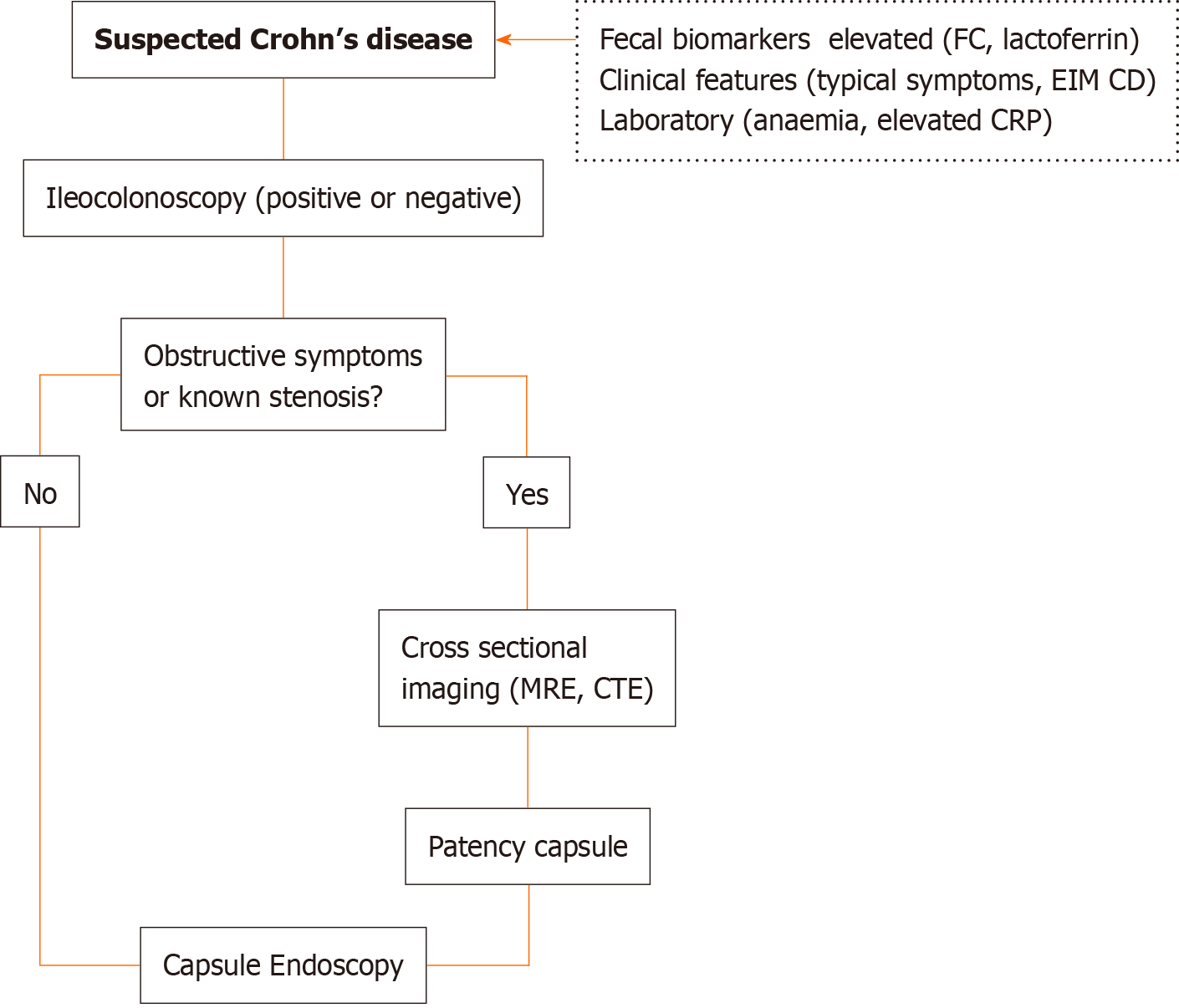
The European Society of Gastrointestinal Endoscopy Clinical Guideline[23] and the Clinical practice guidelines for the use of CE[27] recommend the use of CE in patients with suspected CD and negative ileocolonoscopy[23,27] and imaging results[27] as a diagnostic method for the evaluation of the small bowel, in the absence of obstructive symptoms or known stricture.
In a study with 95 patients, CE excluded the diagnosis of CD if the result was negative (NPV of 96%). Only 3% of the cases with negative CEs were diagnosed with CD after 15 mo of follow-up[9]. Moreover, minor lesions detected by CE may be present in more than 10% of healthy subjects[28]. Non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy is one of the main differential diagnoses of small bowel lesions. In this setting, lesions can appear as early as 2 wk from the onset of NSAID therapy[29,30]. Other differential diagnoses include radiation enteritis, ischemia, Bechet’s disease, lymphoma, and gastrointestinal infections[30]. Then, the interpretation of the findings from CE against suspected CD must be supported for other clinical elements due to the impossibility of obtaining tissue samples by CE.
The use of biomarkers as a screening method for intestinal inflammation, such as FC, could be useful in patients with suspected CD. FC is a cytosolic protein present in neutrophils that is released during inflammation; as such, its elevation in stool samples is a good indicator of intestinal inflammation[31]. Although it is highly sensitive, it is not specific since its levels can increase in IBD, colon cancer, ischemic colitis, and NSAID-induced enteropathy, among others[31]. Although FC has shown higher sensitivity and a stronger correlation with inflammatory activity in UC[32], in CD, the usefulness of FC is less established[33,34], particularly in the small bowel. However, recent studies have shown that FC could be a useful tool for selecting which patients should undergo CE for suspected CD when the ileocolonoscopy results are negative due to its ability to predict inflammatory activity in CE in patients with suspected CD[35-37]. Monteiro et al[35] found a moderate positive correlation (r = 0.56, P < 0.0019) between FC and the LS. FC > 100 µg/g were correlated with LS > 135 in 89% of patients, showing a sensitivity of 78.6%, specificity of 87.9%, positive predictive value of 89.2%, and NPV of 76.3%[35]. Similar findings for FC[27,36,38] and, to a lesser degree, for CRP[36,38] were described by other authors. In a subsequent meta-analysis of 463 patients from seven studies, FC had a significant diagnostic accuracy in detecting small bowel CD, and with FC values < 50 µg/g, the probability of a positive diagnosis was very low[39].
Considering the available evidence and due to CE’s ability to diagnose early disease, in patients with suspected CD (typical symptoms, elevated fecal and plasma biomarkers, anemia, or extraintestinal manifestations of CD), CE should be performed even if ileocolonoscopy results are positive due to the need to evaluate proximal lesions that could determine prognosis and treatment strategies (Figure 2).
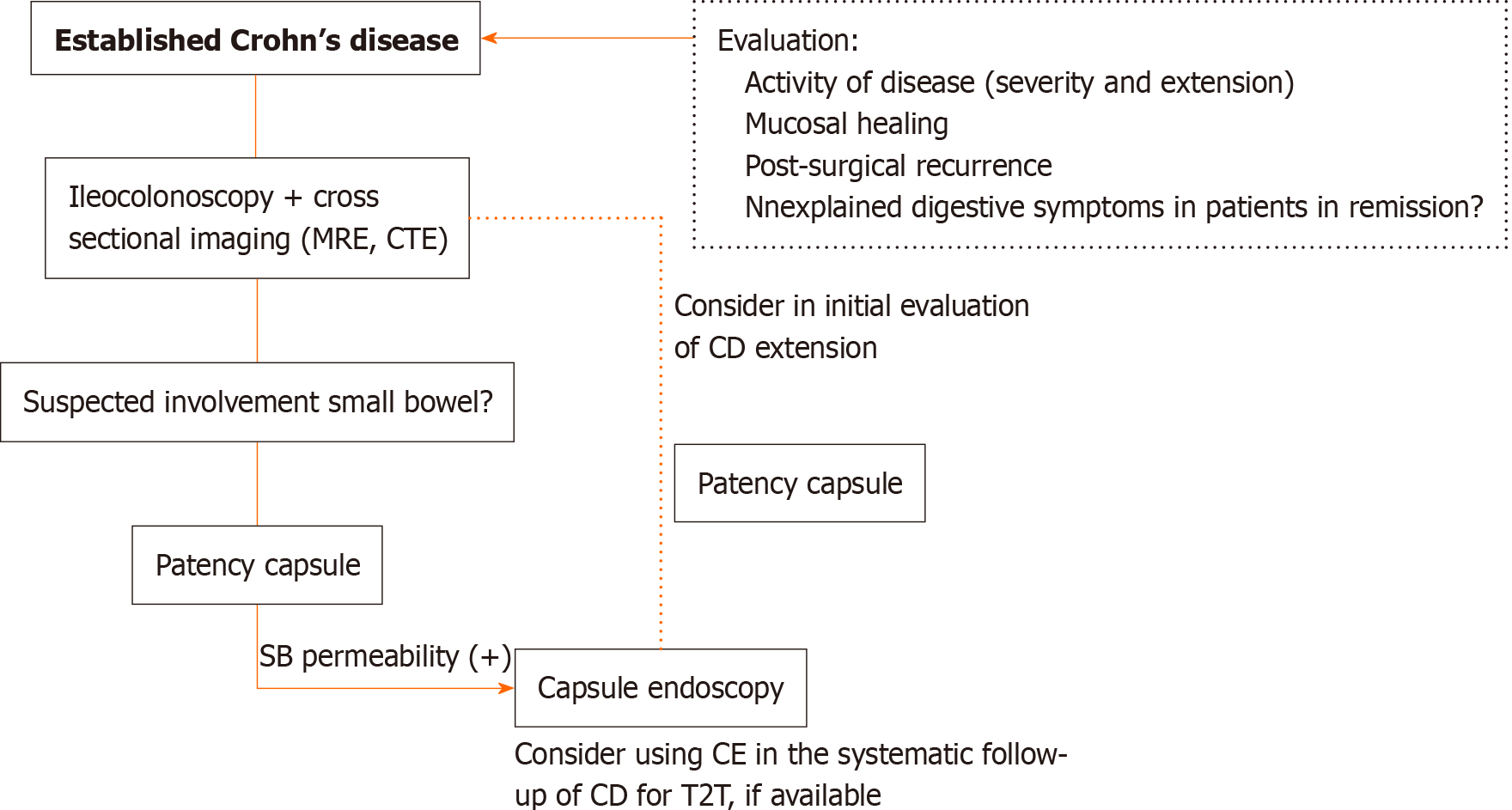
The American consensus guidelines for the use of CE recommends its use in patients with established CD when: (1) Clinical features unexplained by ileocolonoscopy or imaging studies are present; (2) The assessment of small bowel mucosal healing (not evaluable by ileocolonoscopy) is needed; and (3) Small bowel recurrence of CD after colectomy is suspected, undiagnosed by ileocolonoscopy or imaging studies[27]. Recently, the European Crohn’s and Colitis Organisation and the European Society of Gastrointestinal and Abdominal Radiology guidelines recommend CE along with intestinal ultrasound and MRE for initial evaluation and follow-up of established CD[40] (Figure 3).
The persistence of irritable bowel disease-like symptoms in patients with IBD in remission can occur in almost one-third of patients[41,42], being more frequent in patients with CD[42]. In a scenario where traditional diagnostics tests (ileocolonoscopy and cross-sectional imaging) are normal, CE could play a role in evaluating the small bowel to rule out disease activity as the symptom origin. Another clinical scenario is the study of persistent anemia in patients with CD in remission.
Studies have shown that the clinical response to treatment does not correlate with mucosal healing in patients with CD of the small bowel evaluated by CE[43]. Therefore, objective monitoring of disease activity in the small bowel is necessary. Hall et al[43] conducted the first prospective study in 43 patients with CD evaluated with CE at baseline and after 52 wk of treatment. The authors found that 90% of the patients had an active CD in their small bowel at baseline, yet only 65% at week 52 of treatment, with 42% of the patients achieving complete mucosal healing at week 52 (P < 0.0001, 95%CI: 0.62-0.22). Stenosis detected by CE was a poor prognostic factor for the response to treatment in this study[43]. In a subsequent prospective study in 43 patients with CD in clinical remission, fecal biomarkers (FC, lactoferrin, and S100A12) were good predictors of mucosal healing assessed by CE, proving useful in monitoring the CD progression[44]. Finally, a recent prospective observational cohort study assessed the ability of MRE, FC, and CE to predict flare-ups in patients with quiescent CD. CE predicted both short-term (3 mo) and long-term [24 mo, area under curve (AUC) 0.79, 95%CI: 0.66–0.88; P = 0.0001] flares, while FC only predicted short-term flares within 3 mo (AUC 0.81, 95%CI: 0.76-0.85), and MRE correlated with 2-year flare risk (AUC 0.71, 95%CI: 0.58-0.82; P = 0.024)[45].
In a recent study, Shiga et al[46] compared the postoperative follow-up for CE in patients with CD who underwent intestinal resection with the appearance of clinical symptoms for treatment adjustment. In the CE group, 87% residual or recurrent lesions were found at the 3rd postoperative month. Adjusted treatment based on EC findings revealed a strong protective effect (0.30, 0.10–0.75)[46]. This study did not compare the use of CE with ileocolonoscopy in the postoperative follow-up. However, it included 37% of small bowel resections not evaluable by ileocolonoscopy. Previous studies have shown post-surgical recurrence by CE that was not detected by ileocolonoscopy, which has allowed active treatment in this group of patients[47]. Although ileocolonoscopy continues to be the gold standard for the search for postoperative recurrence, CE is an excellent complementary tool, if available, that improves diagnostic performance in this clinical setting.
The “treat to target” strategy in CD[48] is based on the regular assessment of disease activity by using validated outcome measures and the subsequent adjustment of treatment of disease activity, following targets, where the main target is mucosal healing. A recent systematic review that included 47 studies highlighted CE as an objective method of evaluating CD activity that enables reclassifying patients with CD, monitoring the effect of medical treatment through the evaluation of mucosal healing, and detecting postoperative recurrence[49]. Owing to its diagnostic accuracy, CE could be incorporated into the “treat to target” management of patients with CD[49]. However, larger, randomized, controlled trials are necessary to confirm these findings.
CE allows the classification of patients with a diagnosis of IBD-undefined (IBD-U)[50-53], where the inflammatory involvement of the colon cannot differentiate between UC and CD. IBD-U occurs in up to 10% to 15% of patients[54], and at least 15% to 30% of patients will be reclassified as having CD during their disease[50,55]. Establishing this difference is important from a surgical point of view regarding the selection of the type of surgery and which complications to expect in patients with CD and, from the medical point of view, in the selection of the type of biological therapies.
The role of CE in the evaluation of the colonic mucosa in UC is unclear. Colon CE (CCE and later CCE-2 or second-generation) was developed in 2006 and was designed for non-invasive visualization of the colon[56]. A systematic review showed that the diagnostic accuracy of CCE in the colon is comparable with ileocolonoscopy in assessing the severity and extent of the disease[57]. However, some studies with a small number of patients have found a weak correlation between the findings from CCE and colonoscopy, which supports the latter for the evaluation of the mucosa in UC[58,59].
Regarding the evaluation of the small intestine in UC, a prospective observational study (capcolitis) on CE in 127 patients with known UC found that only 4% of the diagnoses changed to CD upon evaluating the small bowel with CE[60].
Panenteric CE (PCE) is a new type of CE similar to PillCamTM COLON 2 (CCE-2) and is currently known as PillCamTM Crohn’s System (Medtronic, Dublin, Ireland)[61]. PillCamTM Crohn’s System is designed for the evaluation of the mucosa of patients with CD. This capsule has a field of view that allows for a 344º view between both capsule heads to provide a pan-intestinal panoramic visualization. The rate frame of PillCamTM Crohn’s System ranges from 4–35 frames per second depending on the speed of the capsule into the gut and has an operating time of more than 12 h[61]. PCE was first described in a multicenter prospective study where it demonstrated a better diagnostic yield of PCE than ileocolonoscopy in 66 patients with active CD who underwent both modalities[62]. The authors found that the per-subject diagnostic yield rate for active CD lesions was 83.3% for PCE and 69.7% for ileocolonoscopy (yield difference 13.6%; 95%CI: 2.6%–24.7%), and the per-segment diagnostic yield rate was 40.6% for PCE and 32.7% for ileocolonoscopy (yield difference 7.9%; 95%CI: 3.3%–12.4%)[62].
In an observational cohort study performed on 93 patients (established CD: 71 and suspected CD: 22), the use of PCE allowed to change the treatment in 38.7% of patients[63]. Moreover, Montreal classification was up-staged in 33.8% of patients with established CD, and identifying proximal small bowel disease in 12.7% predicted treatment intensification[63]. A recent prospective, multicenter study in patients with established CD found that sensitivity of PCE was superior to MRE for proximal small bowel inflammation (97% vs 71%, P = 0.021) and similar to MRE and/or ileocolonoscopy in the terminal ileum and colon[64]. However, the overall sensitivity for active enteric inflammation for CE vs MRE and/or ileocolonoscopy was similar (94% vs 100%, P = 0.125), but the specificity was 74% vs 22%, respectively (P = 0.001)[64]. In the pediatric population, a prospective study in 48 children with CD found that PCE led to a change in therapy for 71% of patients at baseline and 23% at 24 wk. A “treat to target” strategy in these children led to increased mucosal healing and deep remission from 21% at baseline to 54% at week 24 and 58% at week 52[65]. A recent multicenter study[66] compared the 344° panoramic-view recorded by PillCamTM Crohn’s System (lesions detected by cameras A and B) with the standard 172°-view (lesions detected by one camera only) in 41 patients who underwent CE for suspected or established CD. The study found that the panoramic 344º-view increased small bowel CE accuracy vs the standard 172º-view, detecting a greater number of relevant lesions (56.1% vs 39.0%; P = 0.023), resulting in higher LS (222.8 vs 185.7; P = 0.031), and improved clinical management (48.8% vs 31.7%, P = 0.023)[66].
PCE, as the only study modality, could reduce costs associated with the evaluation of patients with CD, considering the need for MRE and ileocolonoscopy for the complete evaluation of the intestine in these patients. Furthermore, PCE is a safe method preferred by patients[64] that does not require sedation, representing advantages for the pediatric population[65].
Table 1 presents the main characteristics of the capsules used in IBD.
| SB CE | Colon CE | PillCam CrohnÒ | |
| (Pillcam SB3Ò) | |||
| Dimensions | 26 mm × 11 mm | 32 mm × 11 mm | 32.3 mm ± 0.5 mm × 11.6 mm |
| Weight | 3.0 g | 2.9 g | 2.9 g |
| Camera | One | 2-one at each end | 2-one at each end |
| Field of view | 156º ISO-8600-3 | 344º: 172° ISO-8600-3 per camera | 344º: 172° ISO-8600-3 per camera |
| Frame rate | 2-6 fps (2-6) | 4-35 fps (AFR) | 4-35 fps (AFR) |
| operating time | ≥ 8 or longer (max.15) | 10 h | Minimum of 10 hr |
| Operating temperature | 20-40 ºC | 20-40 ºC | 20-40 ºC |
In summary, based on the available literature, CE is essential in evaluating patients with CD. The finding of lesions in the small bowel detected by CE and not observed in conventional studies (cross-sectional imaging, ileo-colonoscopy) determines changes in the Montreal classification in patients with CD[10]. This leads to a modification of the therapeutic strategies, with the earlier introduction of immunomodulators and/or biological therapy, improving the prognosis of these patients[67].
In recent years, the development of artificial intelligence (AI) in medicine has made it possible to apply this technology to the automated identification of images on CE. AI, through deep learning artificial neural network (ANN) algorithms[68], facilitates image recognition according to which characteristics the algorithm chooses for itself based on what it considers best for that task, which requires much less time than conventional readings by endoscopists (5.9 min vs 96.6 min)[69]. Convolutional neural network (CNN), a type of ANN[68] applied to CE, has shown excellent performance for the detection of ulcers, polyps, celiac disease, and bleeding[69].
A recent study by Klang et al[70] evaluated the accuracy of CNN for the detection of ulcers in CD on CE for image sets from 49 patients. They reported an AUC of 0.99 for split images and accuracies ranging from 95.4% to 96.7%. The AUC for individual patients was 0.94 to 0.99[69]. Also, the use of CNN enabled characterizing the severity of ulcers on CE images in patients with CD with high accuracy in the detection of severe CD ulcerations and better differentiation between mild and severe ulceration (accuracy 0.91, 95%CI: 0.867-0.954) but a less accurate separation of moderate from severe: (Accuracy 0.78, 95%CI: 0.716–0.844) and mild vs moderate (accuracy 0.624, 95%CI: 0.547–0.701)[71]. Undoubtedly, this technology provides accurate and rapid detection of ulcers from CE images, thereby decreasing reading times. Moreover, deep neural networks are highly accurate in detecting stenosis in CE images (accuracy 93.5%) and differentiating between stenosis and healthy mucosa (AUC 0.989), stenosis, and all ulcers (AUC 0.942), and stenosis and different degrees of ulcer severity[72]. In another area, recent studies suggest that CNN would allow for the automatic evaluation of the degree of intestinal cleansing in CE studies, which could serve as a means of comparing different intestinal preparation methods and thus design recommendations[73].
Despite the encouraging results on the use of AI on CE in IBD, prospective studies are necessary to evaluate its usefulness in the diagnosis and follow-up in CD.
Because CE passage is passive and dependent on the peristalsis of the intestine, only 80 to 90% of patients have their entire intestine visualized. Thus, up to 30% of minor injuries may not be seen during the study[23]. One of the new challenges is the possibility of directing the navigation of the CE in the intestine. Magnetically-assisted CE (MACE) has been tested as a screening tool in gastric cancer[74], Barrett’s esophagus, and esophageal varix[75]. MACE has generated results comparable with esophagogastroduodenoscopy in detecting focal lesions[76] and the study of iron deficiency anemia[77]; however, it has not been evaluated in patients with IBD.
Other CE prototypes in development include biopsy[78] and drug delivery[79] capabilities, which could be clinically relevant for patients with IBD in the future.
The use of CE has played a fundamental role in evaluating the small bowel of patients with IBD, mainly in those with suspected CD and established CD. The development of new types of capsules, such as the panenteric capsule, and the integration of AI into CE image analysis, have improved the visualization and automated the identification of lesions in the digestive tract using a non-invasive, safe, highly tolerated method. Treatment optimization for patients with CD, thanks to CE findings, has improved the course of the disease. More studies are needed to support the use of CE in the evaluation of all patients with CD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cotter J S-Editor: Liu M L-Editor: A P-Editor: Wang LYT
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1386] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 2. | McCain JD, Pasha SF, Leighton JA. Role of Capsule Endoscopy in Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am. 2021;31:345-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Kopylov U, Yung DE, Engel T, Vijayan S, Har-Noy O, Katz L, Oliva S, Avni T, Battat R, Eliakim R, Ben-Horin S, Koulaouzidis A. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn's disease: Systematic review and meta-analysis. Dig Liver Dis. 2017;49:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-8; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (36)] |
| 5. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 6. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Ileoscopy reduces the need for small bowel imaging in suspected Crohn's disease. Dan Med J. 2012;59:A4491. [PubMed] |
| 7. | Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn's disease. Inflamm Bowel Dis. 2013;19:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol. 2006;101:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 368] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 9. | Hall B, Holleran G, Costigan D, McNamara D. Capsule endoscopy: High negative predictive value in the long term despite a low diagnostic yield in patients with suspected Crohn's disease. United European Gastroenterol J. 2013;1:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | González-Suárez B, Rodriguez S, Ricart E, Ordás I, Rimola J, Díaz-González Á, Romero C, de Miguel CR, Jáuregui A, Araujo IK, Ramirez A, Gallego M, Fernández-Esparrach G, Ginés Á, Sendino O, Llach J, Panés J. Comparison of Capsule Endoscopy and Magnetic Resonance Enterography for the Assessment of Small Bowel Lesions in Crohn's Disease. Inflamm Bowel Dis. 2018;24:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Prichard DO, Hamilton Z, Savage T, Smyth M, Penner C, Lakhani A, Carroll MW, Al Sarkhy A, Lemberg DA, Enns R, Jamieson D, Jacobson K. Capsule Endoscopy Complements Magnetic Resonance Enterography and Endoscopy in Evaluating Small Bowel Crohn's Disease. J Can Assoc Gastroenterol. 2020;3:279-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 13. | Cotter J, Dias de Castro F, Magalhães J, Moreira MJ, Rosa B. Validation of the Lewis score for the evaluation of small-bowel Crohn's disease activity. Endoscopy. 2015;47:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn's disease activity index (CECDAI). Dig Dis Sci. 2008;53:1933-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Yablecovitch D, Lahat A, Neuman S, Levhar N, Avidan B, Ben-Horin S, Eliakim R, Kopylov U. The Lewis score or the capsule endoscopy Crohn's disease activity index: which one is better for the assessment of small bowel inflammation in established Crohn's disease? Therap Adv Gastroenterol. 2018;11:1756283X17747780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Omori T, Kambayashi H, Murasugi S, Ito A, Yonezawa M, Nakamura S, Tokushige K. Comparison of Lewis Score and Capsule Endoscopy Crohn's Disease Activity Index in Patients with Crohn's Disease. Dig Dis Sci. 2020;65:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Eliakim R, Yablecovitch D, Lahat A, Ungar B, Shachar E, Carter D, Selinger L, Neuman S, Ben-Horin S, Kopylov U. A novel PillCam Crohn's capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn's disease. United European Gastroenterol J. 2020;8:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Freitas M, Arieira C, Carvalho PB, Rosa B, Moreira MJ, Cotter J. Simplify to improve in capsule endoscopy - TOP 100 is a swift and reliable evaluation tool for the small bowel inflammatory activity in Crohn's disease. Scand J Gastroenterol. 2020;55:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, Weinstein M, Shetzline M, Cave D, de Franchis R. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Leenhardt R, Buisson A, Bourreille A, Marteau P, Koulaouzidis A, Li C, Keuchel M, Rondonotti E, Toth E, Plevris JN, Eliakim R, Rosa B, Triantafyllou K, Elli L, Wurm Johansson G, Panter S, Ellul P, Pérez-Cuadrado Robles E, McNamara D, Beaumont H, Spada C, Cavallaro F, Cholet F, Fernandez-Urien Sainz I, Kopylov U, McAlindon ME, Németh A, Tontini GE, Yung DE, Niv Y, Rahmi G, Saurin JC, Dray X. Nomenclature and semantic descriptions of ulcerative and inflammatory lesions seen in Crohn's disease in small bowel capsule endoscopy: An international Delphi consensus statement. United European Gastroenterol J. 2020;8:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Yung DE, Boal Carvalho P, Giannakou A, Kopylov U, Rosa B, Rondonotti E, Toth E, Plevris JN, Koulaouzidis A. Clinical validity of flexible spectral imaging color enhancement (FICE) in small-bowel capsule endoscopy: a systematic review and meta-analysis. Endoscopy. 2017;49:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Cave D, Legnani P, de Franchis R, Lewis BS; ICCE. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P, Barbaro F, Cellier C, Charton JP, Delvaux M, Despott EJ, Domagk D, Klein A, McAlindon M, Rosa B, Rowse G, Sanders DS, Saurin JC, Sidhu R, Dumonceau JM, Hassan C, Gralnek IM. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 24. | Pasha SF, Pennazio M, Rondonotti E, Wolf D, Buras MR, Albert JG, Cohen SA, Cotter J, D'Haens G, Eliakim R, Rubin DT, Leighton JA. Capsule Retention in Crohn's Disease: A Meta-analysis. Inflamm Bowel Dis. 2020;26:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Cebrián García A, Elosua González A, Fernández-Urién Sainz I. Use of patency capsule in daily practice. Rev Esp Enferm Dig. 2019;111:491-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Albuquerque A, Cardoso H, Marques M, Rodrigues S, Vilas-Boas F, Lopes S, Dias CC, Macedo G. Predictive factors of small bowel patency in Crohn's disease patients. Rev Esp Enferm Dig. 2016;108:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Enns RA, Hookey L, Armstrong D, Bernstein CN, Heitman SJ, Teshima C, Leontiadis GI, Tse F, Sadowski D. Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterology. 2017;152:497-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 28. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG; Investigators. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 451] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 29. | Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, Bjarnason I. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Bar-Meir S. Review article: capsule endoscopy - are all small intestinal lesions Crohn's disease? Aliment Pharmacol Ther. 2006;24 Suppl 3:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Pérez de Arce E, Sedano R, Quera R. [Biomarkers in inflammatory bowel disease]. Rev Med Chil. 2020;148:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ, Feagan BG. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2015;110:802-19; quiz 820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 33. | Stawczyk-Eder K, Eder P, Lykowska-Szuber L, Krela-Kazmierczak I, Klimczak K, Szymczak A, Szachta P, Katulska K, Linke K. Is faecal calprotectin equally useful in all Crohn's disease locations? Arch Med Sci. 2015;11:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Sipponen T, Haapamäki J, Savilahti E, Alfthan H, Hämäläinen E, Rautiainen H, Koskenpato J, Nuutinen H, Färkkilä M. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn's disease detected by wireless capsule endoscopy. Scand J Gastroenterol. 2012;47:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Monteiro S, Barbosa M, Cúrdia Gonçalves T, Boal Carvalho P, Moreira MJ, Rosa B, Cotter J. Fecal Calprotectin as a Selection Tool for Small Bowel Capsule Endoscopy in Suspected Crohn's Disease. Inflamm Bowel Dis. 2018;24:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Egea Valenzuela J, Pereñíguez López A, Pérez Fernández V, Alberca de Las Parras F, Carballo Álvarez F. Fecal calprotectin and C-reactive protein are associated with positive findings in capsule endoscopy in suspected small bowel Crohn's disease. Rev Esp Enferm Dig. 2016;108:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Koulaouzidis A, Nemeth A, Johansson GW, Toth E. Dissecting Lewis score under the light of fecal calprotectin; an analysis of correlation of score components with calprotectin levels in capsule endoscopy. Ann Gastroenterol. 2015;28:259-264. [PubMed] |
| 38. | Höög CM, Bark LÅ, Broström O, Sjöqvist U. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn's disease. Scand J Gastroenterol. 2014;49:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Kopylov U, Yung DE, Engel T, Avni T, Battat R, Ben-Horin S, Plevris JN, Eliakim R, Koulaouzidis A. Fecal calprotectin for the prediction of small-bowel Crohn's disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1169] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 41. | Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 190] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 42. | Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 43. | Hall B, Holleran G, Chin JL, Smith S, Ryan B, Mahmud N, McNamara D. A prospective 52 week mucosal healing assessment of small bowel Crohn's disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (37)] |
| 44. | Aggarwal V, Day AS, Connor S, Leach ST, Brown G, Singh R, Friedman A, Zekry A, Craig PI. Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn's disease to assess remission and predict relapse. Gastrointest Endosc. 2017;86:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Ben-Horin S, Lahat A, Amitai MM, Klang E, Yablecovitch D, Neuman S, Levhar N, Selinger L, Rozendorn N, Turner D, Chowers Y, Odes S, Schwartz D, Yanai H, Dotan I, Braun T, Haberman Y, Kopylov U, Eliakim R; Israeli IBD Research Nucleus (IIRN). Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn's disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol. 2019;4:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Shiga H, Abe I, Kusaka J, Shimoyama Y, Moroi R, Kuroha M, Kakuta Y, Kinouchi Y, Masamune A. Capsule Endoscopy Is Useful for Postoperative Tight Control Management in Patients with Crohn's Disease. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Han ZM, Qiao WG, Ai XY, Li AM, Chen ZY, Feng XC, Zhang J, Wan TM, Xu ZM, Bai Y, Li MS, Liu SD, Zhi FC. Impact of capsule endoscopy on prevention of postoperative recurrence of Crohn's disease. Gastrointest Endosc. 2018;87:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, Hanauer SB, Sandborn WJ. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:1042-50.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 49. | Le Berre C, Trang-Poisson C, Bourreille A. Small bowel capsule endoscopy and treat-to-target in Crohn's disease: A systematic review. World J Gastroenterol. 2019;25:4534-4554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 50. | Mehdizadeh S, Chen G, Enayati PJ, Cheng DW, Han NJ, Shaye OA, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU). Endoscopy. 2008;40:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified). Inflamm Bowel Dis. 2007;13:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Cohen SA, Gralnek IM, Ephrath H, Saripkin L, Meyers W, Sherrod O, Napier A, Gobin T. Capsule endoscopy may reclassify pediatric inflammatory bowel disease: a historical analysis. J Pediatr Gastroenterol Nutr. 2008;47:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Monteiro S, Dias de Castro F, Boal Carvalho P, Rosa B, Moreira MJ, Pinho R, Saraiva MM, Cotter J. Essential role of small bowel capsule endoscopy in reclassification of colonic inflammatory bowel disease type unclassified. World J Gastrointest Endosc. 2017;9:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Eliakim R. The impact of wireless capsule endoscopy on gastrointestinal diseases. South Med J. 2007;100:235-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 57. | Shi HY, Chan FKL, Higashimori A, Kyaw M, Ching JYL, Chan HCH, Chan JCH, Chan AWH, Lam KLY, Tang RSY, Wu JCY, Sung JJY, Ng SC. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest Endosc. 2017;86:1139-1146.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Meister T, Heinzow HS, Domagk D, Dortgolz A, Lenze F, Ross M, Domschke W, Lügering A. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol. 2013;17:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Manes G, Ardizzone S, Cassinotti A. PillCam Colon and ulcerative colitis: what do physicians need to know? Endoscopy. 2013;45:325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Bokemeyer B, Luehr D, Helwig U, Maaser C, Jessen P, Schreiber S. Small bowel capsule endoscopy in ulcerative colitis: the capcolitis study: a prospective observational study. Eur J Gastroenterol Hepatol. 2019;31:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Eliakim R, Spada C, Lapidus A, Eyal I, Pecere S, Fernández-Urién I, Lahat A, Costamagna G, Schwartz A, Ron Y, Yanai H, Adler S. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease - feasibility study. Endosc Int Open. 2018;6:E1235-E1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Leighton JA, Helper DJ, Gralnek IM, Dotan I, Fernandez-Urien I, Lahat A, Malik P, Mullin GE, Rosa B. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn's disease: a feasibility study. Gastrointest Endosc. 2017;85:196-205.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Tai FWD, Ellul P, Elosua A, Fernandez-Urien I, Tontini GE, Elli L, Eliakim R, Kopylov U, Koo S, Parker C, Panter S, Sidhu R, McAlindon M. Panenteric capsule endoscopy identifies proximal small bowel disease guiding upstaging and treatment intensification in Crohn's disease: A European multicentre observational cohort study. United European Gastroenterol J. 2021;9:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 64. | Bruining DH, Oliva S, Fleisher MR, Fischer M, Fletcher JG; BLINK study group. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn's disease: a multicentre, prospective study. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 65. | Oliva S, Aloi M, Viola F, Mallardo S, Civitelli F, Maccioni F, Hassan C, Papoff P, Cucchiara S, Cohen SA. A Treat to Target Strategy Using Panenteric Capsule Endoscopy in Pediatric Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2019;17:2060-2067.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Tontini GE, Rizzello F, Cavallaro F, Bonitta G, Gelli D, Pastorelli L, Salice M, Vecchi M, Gionchetti P, Calabrese C. Usefulness of panoramic 344°-viewing in Crohn's disease capsule endoscopy: a proof of concept pilot study with the novel PillCam™ Crohn's system. BMC Gastroenterol. 2020;20:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn's disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 68. | Klang E. Deep learning and medical imaging. J Thorac Dis. 2018;10:1325-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 69. | Ding Z, Shi H, Zhang H, Meng L, Fan M, Han C, Zhang K, Ming F, Xie X, Liu H, Liu J, Lin R, Hou X. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology. 2019;157:1044-1054.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 70. | Klang E, Barash Y, Margalit RY, Soffer S, Shimon O, Albshesh A, Ben-Horin S, Amitai MM, Eliakim R, Kopylov U. Deep learning algorithms for automated detection of Crohn's disease ulcers by video capsule endoscopy. Gastrointest Endosc. 2020;91:606-613.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 71. | Barash Y, Azaria L, Soffer S, Margalit Yehuda R, Shlomi O, Ben-Horin S, Eliakim R, Klang E, Kopylov U. Ulcer severity grading in video capsule images of patients with Crohn's disease: an ordinal neural network solution. Gastrointest Endosc. 2021;93:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Klang E, Grinman A, Soffer S, Margalit Yehuda R, Barzilay O, Amitai MM, Konen E, Ben-Horin S, Eliakim R, Barash Y, Kopylov U. Automated Detection of Crohn's Disease Intestinal Strictures on Capsule Endoscopy Images Using Deep Neural Networks. J Crohns Colitis. 2021;15:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 73. | Noorda R, Nevárez A, Colomer A, Pons Beltrán V, Naranjo V. Automatic evaluation of degree of cleanliness in capsule endoscopy based on a novel CNN architecture. Sci Rep. 2020;10:17706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Zhao AJ, Qian YY, Sun H, Hou X, Pan J, Liu X, Zhou W, Chen YZ, Jiang X, Li ZS, Liao Z. Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc. 2018;88:466-474.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Beg S, Card T, Warburton S, Rahman I, Wilkes E, White J, Ragunath K. Diagnosis of Barrett's esophagus and esophageal varices using a magnetically assisted capsule endoscopy system. Gastrointest Endosc. 2020;91:773-781.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Liao Z, Hou X, Lin-Hu EQ, Sheng JQ, Ge ZZ, Jiang B, Hou XH, Liu JY, Li Z, Huang QY, Zhao XJ, Li N, Gao YJ, Zhang Y, Zhou JQ, Wang XY, Liu J, Xie XP, Yang CM, Liu HL, Sun XT, Zou WB, Li ZS. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol. 2016;14:1266-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 77. | Ching HL, Hale MF, Kurien M, Campbell JA, Chetcuti Zammit S, Healy A, Thurston V, Hebden JM, Sidhu R, McAlindon ME. Diagnostic yield of magnetically assisted capsule endoscopy versus gastroscopy in recurrent and refractory iron deficiency anemia. Endoscopy. 2019;51:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 78. | Son D, Gilbert H, Sitti M. Magnetically Actuated Soft Capsule Endoscope for Fine-Needle Biopsy. Soft Robot. 2020;7:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 79. | Stewart FR, Newton IP, Näthke I, Huang Z, Cox BF, Cochran S. Development of a therapeutic capsule endoscope for treatment in the gastrointestinal tract: bench testing to translational trial. 2017 IEEE International Ultrasonics Symposium (IUS); 2017 Sep 6-9; Washington, D.C., United States. Piscataway (NJ): IEEE, 2017: 1-4. [DOI] [Full Text] |