Published online Dec 28, 2020. doi: 10.37126/aige.v1.i3.44
Peer-review started: November 1, 2020
First decision: November 25, 2020
Revised: November 29, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: December 28, 2020
Processing time: 57 Days and 7.4 Hours
Over the past few years, emerging new approaches in endoscopic imaging technologies facilitate a high-quality assessment of lesions found in the gastrointestinal (GI) tract. Endocytoscopy (EC), as a novel tool in endoscopy, aids the more accurate evaluation of superficial mucosal surface. This review article aims to represent the most relevant information related to the latest EC technology and its clinical application in the lower GI tract diagnostic. We discuss EC-computer-aided diagnosis capability to differentiate between non-neoplastic and neoplastic lesion that offers a closer look to in-vivo assessment and diagnosis of cancerous tissue. Nevertheless, artificial-assisted EC diagnostics could also be employed with benefits in patients with inflammatory bowel disease (IBD) by accurately highlighting the presence of mucosal injury. In our review we included those studies comprising data about colonoscopy with narrow banding imaging and computer-aided diagnosis, as well as EC. Last but not least, artificial-assisted EC facilitates in-vivo diagnosis of the lower GI tract and may, in the future, remodel the field of in-vivo endoscopic diagnosis of colorectal lesions, representing another step towards the so-called optical biopsy.
Core Tip: The possibility of obtaining "real-time histology" by endocytoscopy (EC) provides a time-saving and low-cost high-quality diagnosing process. It provides detailed detection and characterizations of gastrointestinal neoplasms, where EC defines the degree of neoplastic cellular transformation by visualizing variation in cell size, disorders of polarity, and nuclei deformity. Moreover, the EC system can evaluate the depth of cancer invasion and predict the therapeutic outcome. In line with this, one of the significant benefits from artificial intelligence (AI)-supported EC is avoiding unnecessary polypectomies and other pathological examinations and reducing redundant surgical procedures. Another major benefit of AI-assisted EC is to ensure enhanced delineation between benign and neoplastic colonic lesions. Furthermore, emerging EC-computer-aided diagnosis provides a novel endoscopic tool that contributes to the dramatic improvement of inflammatory bowel disease diagnosing and management.
- Citation: Peshevska-Sekulovska M, Velikova TV, Peruhova M. Artificial intelligence assisted endocytoscopy: A novel eye in endoscopy. Artif Intell Gastrointest Endosc 2020; 1(3): 44-52
- URL: https://www.wjgnet.com/2689-7164/full/v1/i3/44.htm
- DOI: https://dx.doi.org/10.37126/aige.v1.i3.44
In the last decade, the improvement in endoscopic detection of lower gastrointestinal (GI) lesions has dramatically improved. Advancement in endoscopic imaging technologies leads to a high-quality assessment of lesions found in the GI tract. One of the novel tools in endoscopy is endocytoscopy (EC), based on the principle of ultra-high magnification with intraprocedural stains[1]. This innovative endoscopic technique facilitates a more accurate evaluation of the superficial mucosal surface[2]. It allows real-time examination with the capability to distinguish normal from abnormal mucosa. EC allows evaluating the "in vivo" histological structure of colon epithelium by differentiation of colonic polyps and distinguishing invasive carcinoma from adenoma[3]. The aim of the “real-time” endoscopic diagnosis is time-saving and reduce medical patient costs. EC is a promising tool for the detection of GI abnormalities, which involves a contact light microscopy system with an ultra magnification capability (380-fold ultra-magnifying endoscopy), integrated into the distal tip of colonoscopy[4-7]. Using EC, we can perform "virtual histology" with high accuracy. It enables observation of cell stroma and nucleus, making it a perfect tool for diagnosing colorectal lesions[8].
In the last few years, with recent progress in artificial intelligence (AI), there is increasing interest in the application of computer-aided diagnosis (CAD) systems as a novel tool in improving the quality of EC[4]. The EC-CAD system’s diagnostic algorithm includes three major steps: Nuclear segmentation, mucosal feature extraction, and output of predicted pathological classification. The major benefits of using EC-CAD systems are “real-time” high diagnostic ability during colonoscopy by expert and trainee endoscopists[9]. Many recent studies showed a correspondence between EC-CAD and pathological findings of lower GI lesions, which allowed this diagnostic endoscopic method to serves as a form of on-site “optical biopsy”[5,8].
The main goal of this review article is to represent the most relevant information related to the latest EC technology and its clinical use in the diagnostic of the lower GI tract. We included those studies comprising data about colonoscopy with narrow banding imaging (NBI) and CAD, as well as EC.
There are many directions in which this new endoscopic tool finds implications. We discussed the current situation of EC-CAD in the diagnostic process. EC observation could show not only cellular atypia with lumen observation and nuclei of the mucosal surface layer. Thus, differentiation between non-neoplastic and neoplastic lesion offers a closer look at in-vivo assessment and cancerous tissue diagnosis. Another critical point discussed concerns EC-CAD diagnostics in patients with inflammatory bowel disease (IBD), by highlighting the importance of accurate evaluation of mucosal injury.
First-generation EC was introduced in the clinical practice in 2003 (XEC120U; Olympus Medical Systems Corp., Tokyo, Japan). Afterward, the improved version of EC with double integrated-type lens was launched in 2005 (GIF-Y0001; Olympus Medical Systems Corp., Tokyo, Japan). Four years later, in 2009, the third generation of EC appeared with a single integrated-type lens and smaller outer diameter (GIF-Y0002; Olympus Medical Systems Corp., Tokyo, Japan). The latest version of EC arouses on the horizon in 2015 with the ability to provide high-quality colonoscopy (GIF-H290ECI; Olympus Medical Systems Corp., Tokyo, Japan). This latest model of EC comprises Magnified-NBI and EC observation with 520 × magnification[10]. Except for integrated EC, in the clinical practice exist a novel model of probe-based EC with higher magnification (1390×), providing simultaneously biopsy obtaining from the regions of interest[11].
In 2019, a new real-time interpretation of EC images, based on AI software, was introduced by Olympus. This new development is called "Endobrain" (EndoBRAIN; Cybernet Systems, Tokyo, Japan) and finds an application into ordinary colonoscopy as a helping tool for real-time diagnosis, allowing directly therapeutic decision[12].
EC requires good mucosal preparation to provide detailed images of colonic lesions. After intense washing of the mucosa with water, the second step of preparation is the application of simethicone and N-acetylcysteine[13]. The type of dye solution for mucosal staining is another crucial factor for informative imaging acquisition. Based on the literature data, three types of staining with different concentrations exist methylene blue (MB), toluidine blue, and crystal violet (CV). In 2006, Kodashima et al[14] published a protocol using 0.25% toluidine blue in the stomach and colon, with 60-sec time-exposure. According to Ichimasa et al[15], a mixture of 1% MB and 0.05% CV for colonic EC is superior to other staining combinations.
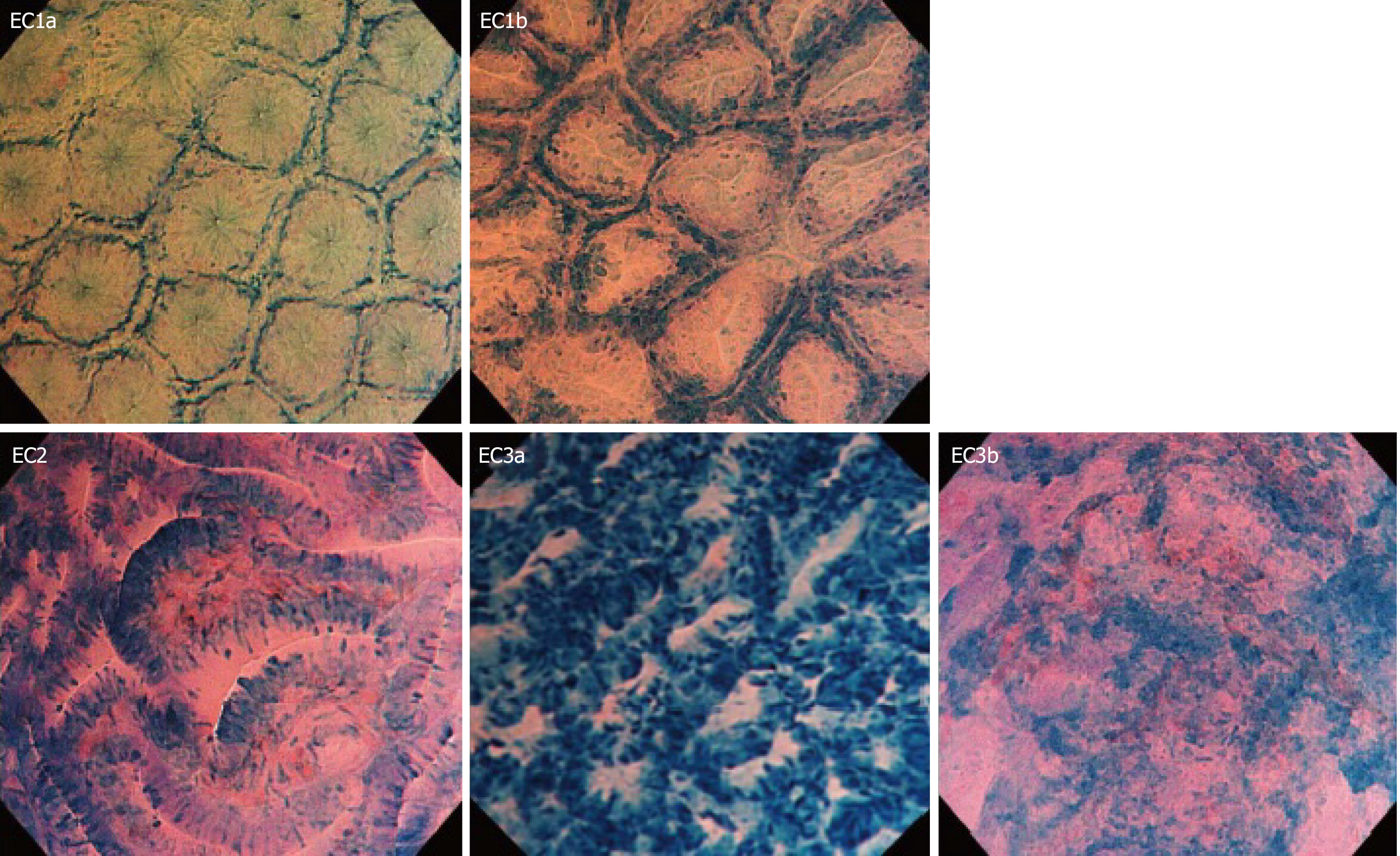
The possibility of obtaining "real-time histology" by EC provides time-saving and low-cost high-quality endoscopy. EC defines the degree of neoplastic cellular transformation by visualizing variation in cell size, disorders of polarity, and nuclei deformity[2]. Another significant EC system contribution is evaluating cancer invasion depth and predicting the therapeutic outcomes[16]. An interesting prospective study published by Kudo et al[5] in 2011 has demonstrated data about the feasibility of new EC classification in colorectal lesions. This classification was especially indicated to differentiate neoplastic from non-neoplastic colorectal lesions[5].
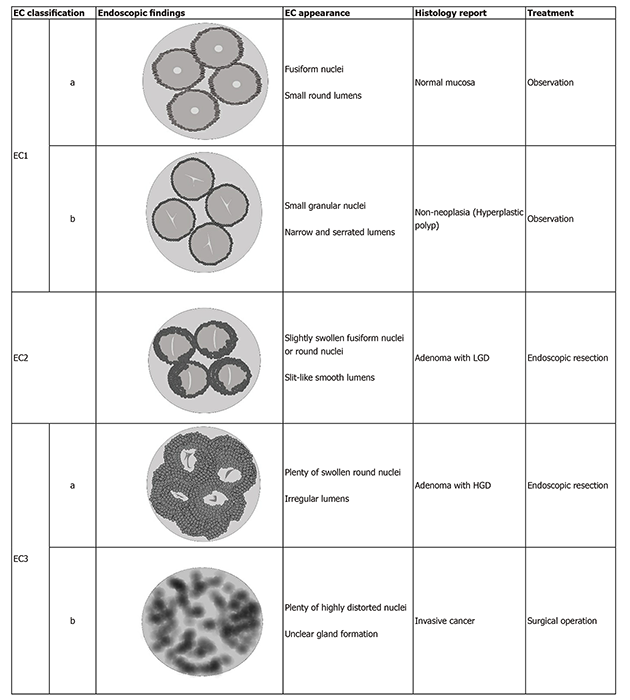
EC classification has five categories, which showed the glandular lumen changes and cellular nuclei of the target lesions. This evaluation system includes: EC1a, which indicates normal mucosa, EC1b show non-neoplasia (hyperplastic polyps), EC2 – adenoma with low-grade dysplasia; EC3a indicates adenoma with high-grade dysplasia (HGD), EC3b stands for invasive cancer (Figure 1)[5]. Histological findings have verified the abovementioned classification according to the Vienna classification (Figure 2).
Utsumi et al[17] conducted a study to differentiate neoplastic from non-neoplastic diminutive polyps (DP). They compared the results from EC in EC1b and EC2 DP with those obtained by histopathological results. The data showed that EC could be a potential tool for real-time histology in distinguishing benign from malignant colorectal lesions[17].
Over the past several years, a new understanding of colorectal carcinogenesis has emerged. In the past, lesions diagnosed as hyperplastic polyps (HPs) were thought to have no malignant potential. Nowadays, these allegations have changed. In this context, HPs may predispose to cancer because of their ability to transform into serrated lesions. These lesions could be found anywhere in the colon, but they are mostly placed in the distal colon (70%-80%). It was established that HPs, with right-side localization are more likely to have malignant potential.
Furthermore, although there are insufficient data on different microRNAs (miRNAs) expression profiles, they might play a role in serrated adenomas with different dysplasia grades. Compared to traditional colorectal carcinogenesis, miRNAs’ pivotal role and their related signaling mechanisms in the serrated pathway of carcinogenesis await to be elucidated[18]. In line with this, AI-assisted endoscopy could be an excellent complementary tool to provide the right and timely diagnosis.
According to the 5th edition of WHO classification of colorectal serrated lesions and polyps, they are classified into three histopathological subtypes: HPs, sessile serrated lesions (SSLs), and traditional serrated adenomas (TSAs)[19]. TSAs are extremely rare < 1% of all colorectal polyps, while HPs are the most common, comprising approximately 75% of all serrated polyps. SSLs (previously known as sessile serrated adenomas or sessile serrated polyps) cause nearly 25% of serrated polyps[20]. Thus, the management of serrated lesions depends on the accurate endoscopic diagnosis.
To provide a better understanding of serrated carcinogenesis and therapeutic strategies of these lesions, Kutsukawa et al[21] shed light on the accurate EC criteria for their proper diagnosis. In their study were included 785 SL, 712 were not observed with EC because of the smaller size (< 5 mm). The remaining 73 Lesions found out 12 mixed serrated polys, 3 of them with the carcinoma component, which led to their exclusion from the study. The remaining 58 polyps were divided into 27 HPs, 12 SSLs, and 19 TSAs. There were no polyps with HGD among the obtained specimens. The EC characteristic subdivided serrated polyps as follows: HP has star-like lumens and round nuclei; SSLs have oval lumens and round nuclei, and TSA has serrated or villous lumens fusiform nuclei. Their results pointed out that EC could be a feasible diagnostic tool in managing SL’s therapeutic options. They concluded that SSLs and TSAs should be removed entirely. Indeed, many studies should be conducted regarding future therapeutic strategies related to SL[21].
Takeda’s recent study evaluated the EC’s diagnostic and therapeutic potential in juvenile polyps (JP). In the study, 154 JP were included, assessed by magnifying chromoendoscopy, 20 were analyzed by EC. The EC findings indicated that JP was characterized by dilatated ductal openings surrounded by normal glandular cells, greater distances between basal gland layers, and interstitial infiltration by inflammatory cells. This study showed that EC might be an additional diagnostic method for detecting JPs[22].
These findings indicate a tetralogy of magnifying chromoendoscopic findings characteristic of JPs: Reddish surfaces, surface erosion, open pits, and low pit density. There is also a triad of EC findings characteristic of JPs, namely dilated ductal openings surrounded by normal glandular cells, greater distances between basal gland layers, and interstitial infiltration by inflammatory cells. The aforementioned magnifying chromoendoscopic and EC characteristics of JPs could be very useful in diagnosing JPs.
One of the GE field’s critical issues is the ability of endoscopist to detect appropriately and characterize the different types of colon polyps. The combination of EC-CAD ameliorates the competence of endoscopists. Hence, the learning curve could be dramatically improved using EC-CAD as a diagnostic tool in lower GI endoscopy.
In line with this, an interesting study by Mori et al[23] revealed that EC-CAD could be a handy endoscopic device for the detection of DP as well as small polyps. The study was an international web-based trial, including 139 DPs and 205 small polyps (147 neoplastic and 58 non-neoplastic). The results showed 89% accuracy for detecting DPs by EC-CAD compared to results obtained by experts. Additionally, they reported 89% sensitivity and 88% specificity for small polyps detection and differentiation[23].
For the first time in 2019, Kudo et al[24] performed an Endobrain analysis of images based on EC-NBI. Five academic centers in Japan participated in this study, where ten experts and 20 trainees made the endoscopic diagnosis. The endoscopists estimated images from 100 cases using white light endoscopy, EC with methylene blue, and EC- NBI images. Only EC images were assessed by the Endobrain system. The results showed 96.9% sensitivity and 100% specificity of Endobrain in distinguishing benign from malignant colorectal lesions compared with endoscopists and pathologists’ findings[24].
On the other hand, Hassan et al[25], in 2020, published a comprehensive meta-analysis that aimed to summarize all reported information related to CAD system performance in colorectal dysplasia. The authors intended to emphasize the paramount importance of the CAD system in colorectal neoplasia detection because of the high percentage of missed lesions at screening colonoscopy.
The meta-analysis included 4354 randomizes patients (2163 in the CAD-group and 2191 in the control group). Five of them were performed in China and one in Italy from five studies. Their results give insight into how CAD could significantly increase the detection of colon polyps (DP, small and large adenomas), despite their location and their superficial morphology (flat and polypoid). Furthermore, they assumed that CAD significantly improved the detection rate of SSLs during colonoscopy. More interestingly, Hassan et al[25] reported nearly 2-fold enhanced diagnostics of advanced neoplasia. In detail, they concluded that CAD could lead to an increase in adenoma detection rate per colonoscopy, resp. 44% and 70%. The author emphasized that additional studies testing CAD in Western populations should be conducted to properly assess CAD’s role in the polyp detection rate[25].
An interesting and significant publication by Mori et al[26] investigated the cost-effectiveness of AI in colonoscopy. The study investigated the performance of AI in the differentiation of colorectal polyps (neoplastic vs non-neoplastic). They included 207 patients with 250 rectosigmoid DP. The authors analyzed the colonoscopy’s cost between two groups of patients with rectosigmoid polyps (≤ 5 mm). The first group included patients who underwent colonoscopy with a "diagnose and leave" strategy based on AI prediction. The second diagnostic and therapeutic strategy was "resect-all-polyps”. Their results demonstrated that AI-assisted colonoscopy had 93.3% sensitivity, 95.2% specificity, and 95.2% negative predictive value in diagnostic colorectal neoplastic polyps. Moreover, they found out that the "diagnose and leave" strategy leads to a significant reduction in average colonoscopy costs. One of the study’s significant benefits was that colonoscopy supported by AI can save a large amount of money spent on excessive polypectomies and pathological examinations[26].
A massive breakthrough in technological developments in the last decade allowed performing in vivo real-time histology of the GI tract by simply pushing a button. Emerging EC-CAD provides enhanced delineation between benign and neoplastic colonic lesions. Furthermore, this novel diagnostic tool contributes to the detailed detection and characterizations of GI neoplasms.
With the emerging AI in endoscopy, therapeutic options for treating large colonic lesions become more accessible and accurate. AI technology provides "real-time" histology, thus determines whether a sizeable colonic lesion (> 2 cm) should be treated by endoscopic resection or surgery. AI endoscopy significantly shortens the process for making the final endoscopic and histological diagnosis of colonic lesions and avoids unnecessary tissue biopsy.
Lui et al[27]’s group has advocated a study that aimed to evaluate the application of AI-assisted image classifier to define the feasibility of curative endoscopic resection for large colonic lesions based on non-magnified endoscopic images. They trained the AI image classifier by 8000 endoscopic images of large colonic lesions. In comparison, the validation set comprises 567 endoscopic images from 76 patients. Histology findings of resected specimens have been used as a gold standard for validation in the study. Curative endoscopic resection was performed only in patients with well-differentiated adenocarcinoma, ≤ 1 mm submucosal invasion as well as without any lymphovascular invasion. The results obtained by the AI image classifier were compared with those taken by endoscopists (seniors and juniors). In patients with the lesions mentioned earlier, which are indicated for endoscopic curative resection, AI has excellent accuracy (85.5%). This study highlights the clinical implication of AI in predicting endoscopic curative resection of large colonic lesions (> 2 cm)[27].
To the best of our knowledge, Ichimasa et al were the first to publish an article about the role of AI in predicting lymph node metastasis (LNM) in patients with T1 colorectal cancers (CRC). Their study aimed to point out that AI provides valuable information about the necessity for additional surgery after endoscopic resection for pT1 CRCs. One of the major key-points in deciding on additional surgery in patients who underwent endoscopic resection of T1 CRC is the presence of LNM. To minimize the necessity for additional surgery, the authors have used an AI model for predicting the possibility of LMN metastasis in patients with T1 CRC. The predicting LNM data were compared with those of the Japanese, European and American guidelines. The study results showed a 100% sensitivity of prediction LNM and a significant reduction of the unnecessary surgical procedure after endoscopic resection of T1 CRC without missing LNM positivity[28].
With the implication of EC-CAD in clinical practice, the diagnostics of patients with inflammatory bowel disease (IBD) have dramatically improved. This novel endoscopic method allows for real-time histology diagnosis and predicts disease outcomes. Bessho et al[29] established an EC score system (ECSS) for assessment patients with IBD. ECSS assesses the shape, distance between crypts, and visibility of superficial microvessels. The system evaluated the severity of the disease according to the histological changes of the colonic mucosa. The authors also demonstrated a good correlation between this scoring system and Matt’s histological grading[29]. ECSS was up-graded by Ueda et al[30] in 2018 by adding additional indicators: The mucosa pits’ characteristics. Another benefit of this upgraded score system is the ability to predict disease relapse[30]. Using a probe-based EC with 1390× magnification, Neumann et al[31] shed light on EC’s role in identifying mucosa’s cellular structures in patients with IBD. This system allowed achieving a detailed analysis of ultrastructural patterns such as the nucleus - cytoplasm ratio and size and shape of the nucleus. The collected data provide the reliable distinction of different types of inflammatory cells in colonic mucosa[31]. In another study by Neumann et al[32], a concordance of 100% between standard histopathological grading and EC data was established. Another fascinating study by Nakazato et al[33], including 64 patients in clinical remission (Mayo 0 and Geboes score ≤ 2), revealed that ECSS has high accuracy for histological remission. In conclusion, they accept that ECSS could be a reliable assessment tool for histological healing evaluation[33].
A study by Maeda et al[34] reports about developing the EC-CAD system (520-fold ultra-magnifying endoscope), predicting persistent histological inflammation of colonic mucosa in patients with ulcerative colitis. The study’s goal was to evaluate the colonic mucosa with the EC-CAD system to predict the onset of clinical exacerbation based on persistent inflammation of the mucosa. In their study, 187 patients with ulcerative colitis were included. They performed white light endoscopy to define the Mayo endoscopic score of colonic mucosa. After identifying the most severe inflamed area, they used EC with NBI mode. Their analyses showed that EC-CAD identified persistent histologic inflammation with 74% sensitivity and 97% specificity. Maeda et al[34] showed that EC-CAD has an incremental benefit for future therapeutic strategy. However, the authors considered more studies to be conducted because of the insufficient number of learning images[34].
Although the gold standard of histological observations of GI lesions based on a light microscopic analysis of hematoxylin and eosin-stained thin-slice specimens, a definition of optical biopsy has recently been introduced. Moreover, real-time EC evaluation can spare the histopathological diagnosis and allows the detection of cell-level lesions and the assessment of cellular and structural atypia in vivo. Both methods showed a significant correlation. Emerging novel AI-assisted EC is radically shifting our approach to treating gastrointestinal lesions. Indeed, not every lesion detected through colonoscopy needs to be excreted or sent for histopathological assessment.
However, before AI-assisted EC becomes a universal method, significant hurdles such as acceptance by patients or performing by less qualified endoscopists and regulatory issues need to be carefully handled. The development of CAD and AI algorithms can promote, form, and improve decision-making in managing colorectal lesions. Overall, EC has shown an excellent diagnostic accuracy, offering to aid in the in-vivo diagnosis of lesions in the lower GI tract.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Lu B, Rácz I S-Editor: Wang JL L-Editor: A P-Editor: Wang LL
| 1. | Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R, Neurath MF. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther. 2011;33:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Takamaru H, Wu SYS, Saito Y. Endocytoscopy: technology and clinical application in the lower GI tract. Transl Gastroenterol Hepatol. 2020;5:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2005;2:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Inoue H, Kazawa T, Sato Y, Satodate H, Sasajima K, Kudo SE, Shiokawa A. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, "Endo-Cytoscopy system". Gastrointest Endosc Clin N Am. 2004;14:589-594, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, Yamamura F, Ohtsuka K, Inoue H, Hamatani S. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Mori Y, Kudo SE, Wakamura K, Misawa M, Ogawa Y, Kutsukawa M, Kudo T, Hayashi T, Miyachi H, Ishida F, Inoue H. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc. 2015;81:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Kumagai Y, Takubo K, Kawada K, Higashi M, Ishiguro T, Sobajima J, Fukuchi M, Ishibashi KI, Mochiki E, Aida J, Kawano T, Ishida H. A newly developed continuous zoom-focus endocytoscope. Endoscopy. 2017;49:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Rath T, Morgenstern N, Vitali F, Atreya R, Neurath MF. Advanced Endoscopic Imaging in Colonic Neoplasia. Visc Med. 2020;36:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 13. | Elvas L, Areia M, Brito D, Alves S, Saraiva S, Cadime AT. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: a double-blind randomized trial. Endoscopy. 2017;49:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi H, Yamamura F, Ohkoshi S, Hamatani S, Inoue H. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc. 2014;26:403-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Li X, Chen H, Gao Y, Chen X, Ge Z. Prediction of histology and invasive depth of colorectal neoplasia based on morphology of surface depression using magnifying chromocolonoscopy. Int J Colorectal Dis. 2010;25:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Utsumi T, Sano Y, Iwatate M, Sunakawa H, Teramoto A, Hirata D, Hattori S, Sano W, Hasuike N, Ichikawa K, Fujimori T. Prospective real-time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (≤ 5 mm). World J Gastrointest Oncol. 2018;10:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Peruhova M, Peshevska-Sekulovska M, Krastev B, Panayotova G, Georgieva V, Konakchieva R, Nikolaev G, Velikova TV. What could microRNA expression tell us more about colorectal serrated pathway carcinogenesis? World J Gastroenterol. 2020;26:6556-6571. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2406] [Article Influence: 481.2] [Reference Citation Analysis (3)] |
| 20. | Fan C, Younis A, Bookhout CE, Crockett SD. Management of Serrated Polyps of the Colon. Curr Treat Options Gastroenterol. 2018;16:182-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kutsukawa M, Kudo SE, Ikehara N, Ogawa Y, Wakamura K, Mori Y, Ichimasa K, Misawa M, Kudo T, Wada Y, Hayashi T, Miyachi H, Inoue H, Hamatani S. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc. 2014;79:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Takeda K, Kudo SE, Mori Y, Misawa M, Kudo T, Wakamura K, Hayashi T, Miyachi H, Ishida F, Inoue H. Magnifying chromoendoscopic and endocytoscopic findings of juvenile polyps in the colon and rectum. Oncol Lett. 2016;11:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Mori Y, Kudo SE, Chiu PW, Singh R, Misawa M, Wakamura K, Kudo T, Hayashi T, Katagiri A, Miyachi H, Ishida F, Maeda Y, Inoue H, Nimura Y, Oda M, Mori K. Impact of an automated system for endocytoscopic diagnosis of small colorectal lesions: an international web-based study. Endoscopy. 2016;48:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Kudo T, Suzuki K, Mori Y, Misawa M, Ichimasa K, Takeda K, Nakamura H, Maeda Y, Ogawa Y, Hayashi T, Wakamura K, Ishida F, Inoue H, Kudo SE. Endocytoscopy for the differential diagnosis of colorectal low-grade adenoma: a novel possibility for the "resect and discard" strategy. Gastrointest Endosc. 2020;91:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 307] [Article Influence: 76.8] [Reference Citation Analysis (1)] |
| 26. | Mori Y, Kudo SE, East JE, Rastogi A, Bretthauer M, Misawa M, Sekiguchi M, Matsuda T, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Kudo T, Mori K. Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: an add-on analysis of a clinical trial (with video). Gastrointest Endosc 2020; 92: 905-911. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Lui TKL, Wong KKY, Mak LLY, Ko MKL, Tsao SKK, Leung WK. Endoscopic prediction of deeply submucosal invasive carcinoma with use of artificial intelligence. Endosc Int Open. 2019;7:E514-E520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Ichimasa K, Kudo SE, Mori Y, Misawa M, Matsudaira S, Kouyama Y, Baba T, Hidaka E, Wakamura K, Hayashi T, Kudo T, Ishigaki T, Yagawa Y, Nakamura H, Takeda K, Haji A, Hamatani S, Mori K, Ishida F, Miyachi H. Correction: Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy. 2018;50:C2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Bessho R, Kanai T, Hosoe N, Kobayashi T, Takayama T, Inoue N, Mukai M, Ogata H, Hibi T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol. 2011;46:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Ueda N, Isomoto H, Ikebuchi Y, Kurumi H, Kawaguchi K, Yashima K, Ueki M, Matsushima K, Akashi T, Uehara R, Takeshima F, Hayashi T, Nakao K. Endocytoscopic classification can be predictive for relapse in ulcerative colitis. Medicine (Baltimore). 2018;97:e0107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Neumann H, Atreya R, Vieth M. Endocytoscopy enables accurate differentiation of mucosal inflammatory cells. Zeitschrift fur Gastroenterologie. 2010;48:4. |
| 32. | Neumann H, Vieth M, Neurath MF, Atreya R. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis. 2013;19:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Nakazato Y, Naganuma M, Sugimoto S, Bessho R, Arai M, Kiyohara H, Ono K, Nanki K, Mutaguchi M, Mizuno S, Kobayashi T, Hosoe N, Shimoda M, Abe T, Inoue N, Ogata H, Iwao Y, Kanai T. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy. 2017;49:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Maeda Y, Kudo SE, Mori Y, Misawa M, Ogata N, Sasanuma S, Wakamura K, Oda M, Mori K, Ohtsuka K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019;89:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |