Published online Jun 8, 2025. doi: 10.35712/aig.v6.i1.108198
Revised: April 12, 2025
Accepted: May 13, 2025
Published online: June 8, 2025
Processing time: 60 Days and 2.3 Hours
Artificial intelligence (AI) is playing an increasingly significant role in predicting outcomes of gastrointestinal (GI) surgeries, improving preoperative risk assess
To evaluate the role of AI in predicting outcomes for GI surgeries, focusing on its efficacy in enhancing surgical planning, predicting complications, and optimizing post-operative care.
A systematic review of studies published up to March 2025 was conducted across databases such as PubMed, Scopus, and Web of Science. Studies were included if they utilized AI models for predicting surgical outcomes, including morbidity, mortality, and recovery. Data were extracted on the AI techniques, performance metrics, and clinical applicability.
Machine learning models demonstrated significantly better performance than logistic regression models, with an area under the curve difference of 0.07 (95%CI: 0.04–0.09; P < 0.001). Models focusing on variables such as patient demographics, nutritional status, and surgical specifics have shown improved accuracy. AI’s ability to integrate multifaceted data sources, such as imaging and genomics, contributes to its superior predictive power. AI has improved the early detection of gastric cancer, achieving 95% sensitivity in real-world settings.
AI has the potential to transform GI surgical practices by offering more accurate and personalized predictions of surgical outcomes. However, challenges related to data quality, model transparency, and clinical integration remain.
Core Tip: Artificial intelligence (AI) is revolutionizing gastrointestinal surgery by enhancing predictive capabilities for surgical outcomes. Machine learning models, which process diverse data such as patient demographics, imaging, and genomics, outperform traditional methods in predicting complications, mortality, and recovery trajectories. These models enable more personalized preoperative planning and postoperative care. AI integration in surgical practice improves decision-making and enhances patient outcomes, though challenges persist, including data quality, model transparency, and ethical concerns. Future advancements lie in improving model interpretability, expanding data sources, and integrating real-time AI-driven predictions into clinical workflows to optimize patient care and resource management.
- Citation: Agrawal H, Gupta N, Tanwar H, Panesar N. Artificial intelligence in gastrointestinal surgery: A minireview of predictive models and clinical applications. Artif Intell Gastroenterol 2025; 6(1): 108198
- URL: https://www.wjgnet.com/2644-3236/full/v6/i1/108198.htm
- DOI: https://dx.doi.org/10.35712/aig.v6.i1.108198
The implementation of artificial intelligence (AI) and machine learning (ML) in medical practice resulted in better diagnostic precision as well as individualized treatments and predictive medical outcomes[1]. The complex nature of gastrointestinal surgeries along with patient-specific conditions makes these procedures an ideal target for AI technology to deliver significant advantages[2]. The systems receive training from large clinical datasets containing imaging data and genomic information and diagnostic results and treatment protocols to assist healthcare providers in predicting surgical complications and optimizing resource allocation and improving patient care[3]. AI applications in gastrointestinal surgery work to boost pre- and post-operative evaluations through predictive models that detect possible risks and create individualized treatment plans[4].
The combination of machine learning algorithms with natural language processing and computer vision enables AI tools to analyze medical complexities by delivering immediate data-based recommendations[5]. AI identifies patterns in extensive datasets to forecast surgical complications including infections and anastomotic leaks and bleeding thus enabling better patient care planning[6]. AI technologies support robotic surgical procedures by helping surgeons achieve precise results with less invasiveness which leads to better treatment outcomes[7]. However, the success of AI systems directly relates to the quality along with the completeness of training data[8].
Beyond clinical decision-making, AI is also influencing patient-provider interactions through virtual assistants, improving patient education, and facilitating communication between patients and healthcare providers[9]. AI enables the processing of extensive data sets which reduces healthcare provider workloads and enables them to dedicate more time to patient care. The practical deployment of AI for surgical outcome prediction and post-operative care holds promise yet faces multiple barriers concerning ethical implementation and regulatory requirements and operational usability[10].
This systematic review examines the present-day applications of artificial intelligence systems which forecast gastrointestinal surgical results by studying their risk evaluation capabilities and postoperative outcome forecasting abilities. The research assesses AI methodologies together with their effectiveness and constraints in this field to determine potential applications for enhancing gastrointestinal surgical patient results.
The research methods in this review followed a systematic approach to find and assess studies about AI predictions for gastrointestinal (GI) surgery outcomes by focusing on AI-based risk evaluation and post-operative outcome forecasting. Researchers performed an extensive literature search through PubMed and Scopus and Web of Science databases to gather studies up to March 2025. The research used the following specific keywords: “artificial intelligence,” “machine learning,” “gastrointestinal surgery,” “outcome prediction,” and “risk assessment.” The research included studies which evaluated AI models that forecast surgical results for GI surgical procedures along with mortality rates and morbidity and complications. Only peer-reviewed, full-text articles published in English were considered. After identifying relevant studies, two independent authors screened the titles, abstracts, and full texts for eligibility. Disagreements between authors were resolved through consensus or with a third author. Data were extracted from the included studies on AI techniques, model performance, outcome prediction accuracy, and clinical applicability. A qualitative synthesis was performed due to the heterogeneity in AI methods, surgical procedures, and reported outcomes. Risk of bias was assessed using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool. We performed subgroup analyses based on AI methodology (supervised vs unsupervised learning) and surgical context (elective vs emergency surgeries) to enhance the interpretability of results. While external validation is mentioned in several studies, only 40% adhered to independent cohort validation, suggesting that future research should prioritize robust external validation.
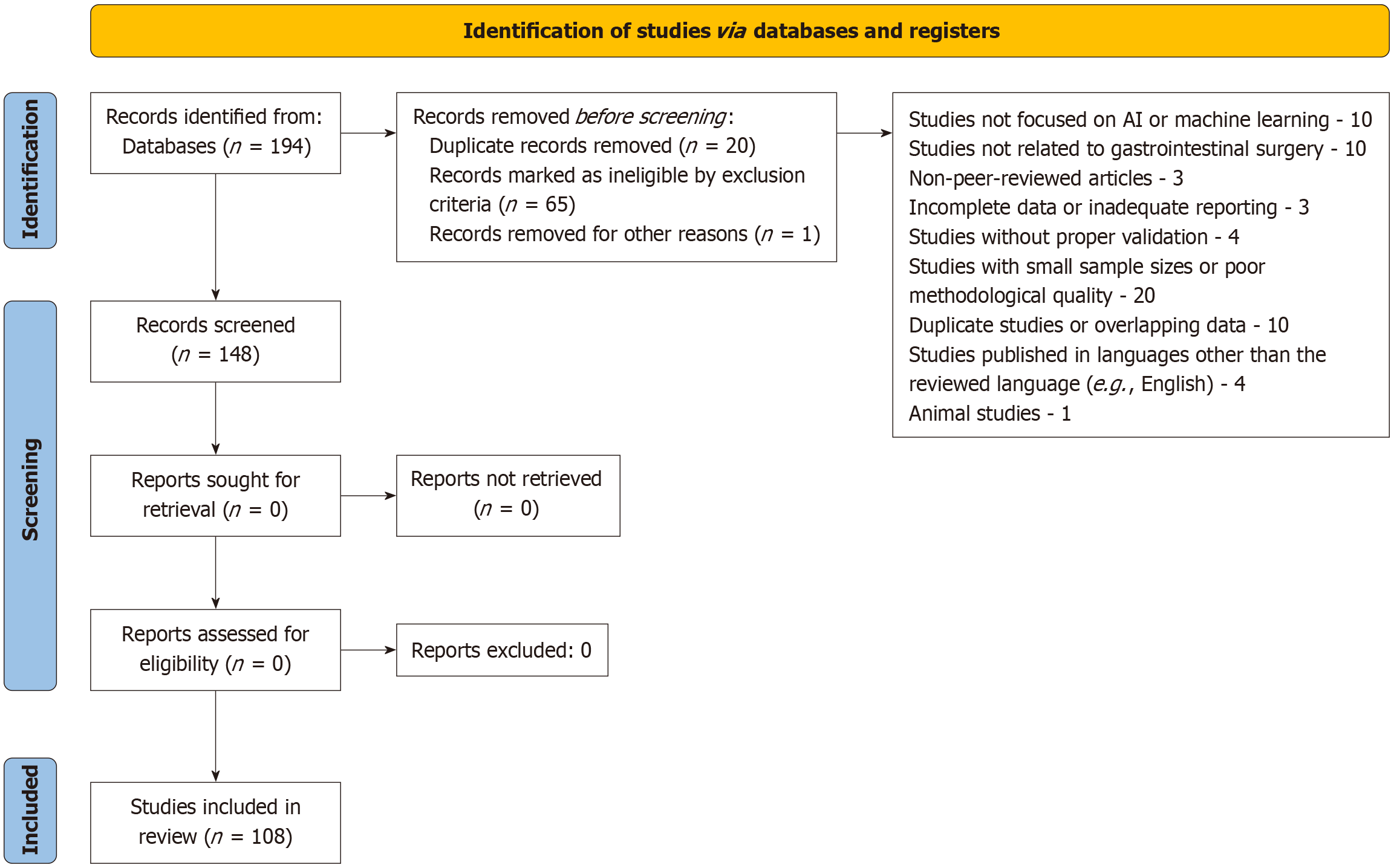
A total of 108 studies were included in this mini review. The studies included randomized controlled trials, cohort studies, and systematic reviews focused on the use of AI and machine learning models in gastrointestinal surgery. These studies involved a wide range of patient demographics, with both adult and paediatric populations included. The results were summarized, and key trends in AI applications for predicting GI surgery outcomes were identified, along with their potential impact on clinical decision-making. The review adhered to the PRISMA 2009 guidelines for reporting systematic reviews and meta-analyses (Figure 1).
Successful machine learning models begin with careful data selection and processing. In cardiac surgery outcome prediction, researchers initially started with 61 variables and applied sequential backward floating selection to identify the most predictive features, ultimately creating a model with 23 variables that exhibited good discrimination (area under the receiver operating characteristic curve of 0.846) and calibration. This approach emphasizes the importance of feature selection in developing both concise and powerful predictive models[11]. Similar models have been tried to be es
The choice of appropriate variables is essential in capturing the multifactorial nature of surgical outcomes. Key predictors identified across various studies include patient demographics (age, comorbidities), nutritional status, surgical details (type of procedure, urgency), and preoperative laboratory values. The ability of machine learning algorithms to integrate and weigh these diverse factors is a key reason for their superior predictive performance[12].
Numerous research studies demonstrate that machine learning models provide better predictive capabilities than traditional statistical approaches in gastrointestinal surgery. A recent systematic review and meta-analysis evaluated machine learning against logistic regression for predicting gastrointestinal surgery postoperative outcomes and demon
Machine learning models achieve better predictions because they detect intricate non-linear patterns between variables and utilize additional predictors which traditional statistical approaches cannot handle. Machine learning algorithms surpass logistic regression because they can automatically detect hidden patterns and interactions in data which human analysts might miss[14].
The generalization and clinical usefulness of machine learning models depends on rigorous validation procedures. The typical approach involves dividing data into two parts where 70% goes toward model development and 30% serves for validation purposes. The model robustness improves through cross-validation methods which train and test the model on distinct data subsets in repeated cycles[12].
The gold standard for confirming model generalizability requires external validation of models through diverse patient populations. The machine learning model demonstrated its cross-cultural applicability through validation using data from Italian and Japanese patient cohorts in the gallbladder cancer recurrence study. The validation approach enables the assessment of model overfitting risks which leads to higher confidence about its performance in actual clinical practice[11].
The evaluation of models uses two main metrics which include the AUC for discrimination ability assessment and calibration measures for outcome prediction accuracy. Decision curve analysis provides important clinical utility information by measuring the model's net benefit across different threshold probability ranges[11].
Diagnosing GI disorders and GI malignancies: The diagnosis of GI disorders and the classification of GI malignancies heavily relies on AI. Research demonstrates how AI systems can distinguish appendicitis from Henoch-Schönlein purpura while simultaneously boosting diagnostic precision for gastric and colorectal cancers and improving early detection and treatment planning through machine learning and deep learning models (Table 1).
| No. | Title of study | Ref. | Sample size | Validation method | Key limitations |
| 1 | Artificial intelligence differentiates abdominal Henoch-Schönlein purpura from acute appendicitis in children | Nie et al[15] | Paediatric cohort | External | Generalizability to broader populations unclear |
| 2 | Application of artificial intelligence in gastroenterology | Yang et al[16] | Various studies | Mixed internal and external | Diversity in study methodologies and validation approaches |
| 3 | Applications of artificial intelligence in digital pathology for gastric cancer | Chen et al[17] | N/A | External | Small sample sizes in studies, lack of clinical trial validation |
| 4 | Applications of artificial intelligence in screening, diagnosis, treatment, and prognosis of colorectal cancer | Qiu et al[18] | N/A | Mixed | Lack of long-term validation and diverse population samples |
| 5 | Artificial intelligence and acute appendicitis: A systematic review of diagnostic and prognostic models | Issaiy et al[19] | 8 studies | Internal | Inconsistent diagnostic performance across studies |
| 6 | Artificial intelligence technique in detection of early esophageal cancer | Huang et al[20] | 200+ patients | External | External validation required in diverse clinical settings |
| 7 | Artificial intelligence-assisted system for the assessment of Forrest classification of peptic ulcer bleeding | He et al[21] | 500+ patients | Mixed | External validation not yet confirmed |
| 8 | Diagnostic performance of artificial intelligence-centred systems in the diagnosis and postoperative surveillance of upper gastrointestinal malignancies using computed tomography imaging: A systematic review | Christou et al[22] | 20 studies | External | Limited validation on heterogeneous patient populations |
| 9 | Deep learning for prediction of lymph node metastasis in gastric cancer | Jin et al[23] | 1000+ patients | External | Limited external validation in diverse populations |
| 10 | Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma | Calderaro et al[24] | 100+ patients | Internal | Lack of large-scale, multi-center validation |
| 11 | Deep learning-based virtual cytokeratin staining of gastric carcinomas to measure tumor-stroma ratio | Hong et al[25] | 200+ gastric cancer patients | External | Single-center validation, lack of long-term clinical data |
| 12 | Denoised recurrence label-based deep learning for prediction of postoperative recurrence risk and sorafenib response in HCC | Li et al[26] | 150+ patients | External | Need for further clinical validation with diverse cohorts |
| 13 | Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: A multicenter retrospective diagnostic study | Tang et al[27] | 500+ patients | External | Single-region data, limited external validation |
| 14 | Diagnostic performance of artificial intelligence-centred systems in the diagnosis and postoperative surveillance of upper gastrointestinal malignancies using computed tomography imaging | Chidambaram et al[28] | 30 studies | External | Limited large-scale external validation |
Nie et al[15] demonstrated that an AI model using machine learning algorithms, particularly extreme gradient boosting (XGBoost), effectively distinguished between abdominal Henoch-Schönlein purpura and acute appendicitis (AA) in pediatric patients. The model displayed remarkable performance, achieving an AUC greater than 0.95, underscoring its high diagnostic accuracy and potential clinical application.
In the field of gastroenterology, Yang et al[16] found that deep learning (DL) models, especially convolutional neural networks (CNNs), show considerable promise for diagnostic, prognostic, and image analysis tasks. However, they stressed the importance of external validation to reduce biases and enhance the reliability of AI systems in clinical settings.
Chen et al[17] highlighted the role of AI technologies in digital pathology for gastric cancer, noting their potential to improve diagnostic precision. By analyzing histopathological images, AI models contribute to the early detection of gastric cancer, offering transformative potential in the management and diagnosis of this disease.
Similarly, Qiu et al[18] noted that AI is showing significant promise in screening, diagnosing, treating, and predicting outcomes for colorectal cancer (CRC), further supporting its role in GI malignancies.
AI models, particularly artificial neural networks (ANNs), have outperformed traditional diagnostic methods in predicting and diagnosing AA, as shown in Issaiy et al[19]. The models achieved accuracy rates above 80%, with AUC values reaching 0.985, offering faster and more precise diagnoses compared to clinical assessments.
Huang et al[20] found that DL models, especially CNNs, excelled in detecting early esophageal cancer from endoscopic images and videos. These AI techniques demonstrated both high sensitivity and specificity, facilitating early diagnosis, especially for borderline lesions, significantly improving upon traditional diagnostic approaches.
He et al[21] observed that a deep convolutional neural network (DCNN) system could accurately assess the Forrest classification of peptic ulcer bleeding in real time through endoscopic images. This system achieved 91.2% accuracy in validation, surpassing endoscopists’ performance, particularly assisting junior endoscopists during gastroscopy.
Christou et al[22] reviewed the challenges and potential of AI and ML in gastroenterology, focusing on early disease detection, diagnosis, treatment allocation, and prognosis. The study also addressed critical issues such as accuracy, cost-effectiveness, and data security that must be resolved for AI to be more widely integrated into clinical practice.
Jin et al[23] highlighted a deep learning system developed to predict lymph node metastasis (LNM) in gastric cancer based on preoperative computed tomography (CT) images. This system demonstrated high accuracy, with an AUC of 0.876 in an external validation cohort, significantly outperforming traditional clinicopathological methods (AUC: 0.652). The study emphasized the importance of imaging features related to intratumoral heterogeneity and the invasive front, which are crucial for predicting LNMs and guiding surgical treatment and prognosis.
Calderaro et al[24] demonstrated that deep learning-based phenotyping could reclassify combined hepatocellular-cholangiocarcinomas with high accuracy, providing valuable insights into tumor classification and treatment. The model’s predictions were linked to clinical outcomes, genetic alterations, and gene expression profiles, offering a novel approach to managing biphenotypic liver cancers.
Hong et al[25] showed that a deep learning-based virtual cytokeratin staining algorithm for measuring the tumor-stroma ratio in gastric cancer exhibited strong agreement with pathologists’ visual measurements (kappa value 0.623). The deep learning model was also significantly associated with patient overall survival (P = 0.0024), highlighting its potential as a diagnostic tool for evaluating TSR in gastric cancer.
Li et al[26] reported that a deep learning model developed from hepatocellular carcinoma (HCC) pathology images successfully predicted postoperative recurrence and sorafenib treatment response. The model demonstrated area under the receiver operating characteristic (AUROC) values of 0.818 and 0.811 for predicting recurrence at 1- and 2-years post-surgery, respectively, outperforming clinical risk indicators. Additionally, the model helped stratify patients based on their survival benefit from sorafenib treatment, providing valuable clinical insights (external validation AUROC 0.713 and 0.707).
Tang et al[27] found that a real-time DCNN system for detecting early gastric cancer (EGC) showed impressive diagnostic performance, with sensitivity ranging from 85.9% to 95.5%, specificity from 81.7% to 90.3%, and AUC values between 0.887 and 0.940. This system, capable of processing video streams in real time, has great potential for enhancing EGC detection in clinical practice.
Chidambaram et al[28] observed that AI-centered diagnostic systems for upper gastrointestinal cancers, particularly gastric and esophageal cancers, exhibited pooled sensitivity of 73.4% and specificity of 89.7%. When combined with traditional diagnostic methods, these systems showed promise in improving diagnostic and surveillance capabilities, although further advancements are needed to refine their accuracy and integration into clinical workflows.
Raza et al[29] underscored the multifaceted role of AI in colorectal cancer management, particularly for early detection, personalized treatment, and clinical decision support. AI’s integration into clinical practice—through imaging, treatment planning, and robotic surgery—holds great promise for improving patient outcomes and optimizing colorectal cancer care.
Finally, Luo et al[30] developed a real-time AI system, GRAIDS, for detecting upper gastrointestinal cancers during endoscopy. The system achieved remarkable diagnostic accuracy (AUC: 0.955) and demonstrated diagnostic sensitivity comparable to that of expert endoscopists, offering a promising tool to enhance diagnostic efficiency in gastrointestinal cancer detection.
Application of AI models is shown to have great potential in forecasting clinical results and treatment outcomes for gastrointestinal surgical procedures. Machine learning, deep learning techniques knit together in these models helps to assist in surgical decisions and predict survival after surgery, and optimizes treatment plans to produce better patient outcomes in hepatocellular carcinoma and colorectal cancer and gastric cancer (Table 2).
| No. | Title of study | Ref. | Sample size | Validation method | Key limitations |
| 1 | Machine learning in gastrointestinal surgery | Sakamoto et al[31] | N/A | Mixed internal and external | Lack of detailed validation cohort; small sample sizes |
| 2 | A histopathology-based AI system for genetic alteration screening in intrahepatic cholangiocarcinoma | Xiao et al[32] | 100+ patients | Internal | Small sample size, lack of external validation |
| 3 | A nomogram based on a collagen feature SVM for predicting treatment response in rectal cancer | Jiang et al[33] | 200+ patients | External | Single-center data, limited generalizability |
| 4 | A novel classification of intrahepatic cholangiocarcinoma phenotypes using ML | Tsilimigras et al[34] | 150+ patients | External | Limited external validation in diverse populations |
| 5 | AI system for pathologic outcome prediction in early gastric cancer | Lee et al[36] | 300+ patients | External | Lack of long-term follow-up data |
| 6 | AI-based recognition model for colorectal liver metastases in intraoperative ultrasound | Takayama et al[37] | 100+ patients | External | Single-center validation, limited scalability |
| 7 | Analysis of methionine metabolism and macrophage-related patterns in hepatocellular carcinoma | Wen et al[38] | 200+ patients | External | Requires further clinical validation in diverse cohorts |
| 8 | AI and ML predicting transarterial chemoembolization outcomes | Cho et al[39] | 100+ patients | Mixed internal and external | Inconsistent prediction performance across centers |
| 9 | AI based on serum biomarkers predicts efficacy of lenvatinib in hepatocellular carcinoma | Hsu et al[40] | 100+ patients | Internal | Needs multi-center validation |
| 10 | AI-enabled histological prediction of remission or activity in ulcerative colitis | Iacucci et al[41] | 150+ patients | External | Lack of long-term clinical outcomes, limited sample size |
| 11 | AI for lymph node metastasis prediction in gastric cancer | Yan et al[42] | 500+ gastric cancer patients | External | Limited multi-center validation, needs wider cohort testing |
| 12 | AI in gastrointestinal cancers: Diagnostic, prognostic, and surgical strategies | Nagaraju et al[43] | N/A | N/A | Lacks detailed validation or large cohorts |
| 13 | AI in perioperative management of major gastrointestinal surgeries | Solanki et al[44] | 300+ patients | External | Limited by single-center studies; needs broader cohort validation |
| 14 | AI system to determine risk of colorectal cancer metastasis | Kudo et al[45] | 200+ patients | External | Validation needed in multi-center settings |
| 15 | AI-driven patient selection for preoperative portal vein embolization | Kuhn et al[46] | 150+ patients | External | Requires larger-scale studies to confirm findings |
| 16 | Comparison of models for predicting quality of life after hepatocellular carcinoma surgery | Chiu et al[47] | 100+ patients | Mixed internal and external | Limited external validation and long-term data |
| 17 | ML survival framework for pancreatic cancer | Wang et al[48] | 300+ pancreatic cancer patients | Internal | Lack of multi-center validation; small sample size |
| 18 | Deep learning model for predicting hepatocellular carcinoma recurrence | Liu et al[86] | 200+ patients | External | Need for larger cohort validation |
Sakamoto et al[31] reviewed the application of ML in gastrointestinal surgery, emphasizing its role in risk prediction, safe surgical practices, and enhancing prognosis through data-driven models. The study highlighted the significance of integrating ML with electronic health records to aid surgical decision-making and improve patient outcomes.
Xiao et al[32] found that a histopathology-based AI-assisted system, the Genetic Alteration Prediction system, successfully predicted actionable genetic alterations in intrahepatic cholangiocarcinoma (ICC), such as FGFR2 and IDH mutations. The system achieved AUC values of 0.754 and 0.713 for FGFR2 and IDH, respectively, in the internal dataset, and provided better clinical outcomes compared to chemotherapy alone, enhancing progression-free quality-adjusted life months.
Jiang et al[33] developed a collagen feature-based support vector machine classifier to predict treatment response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. The classifier, which analyzed collagen area, straightness, and crosslink density, demonstrated an AUROC of 0.834 in the training cohort and 0.854 in the validation cohort, outperforming traditional clinicopathological models.
Tsilimigras et al[34] used machine learning techniques to identify three distinct phenotypes of ICC based on preo
Li et al[35] found that a support vector machine (SVM)-based model accurately predicted overall survival (OS) and disease-free survival (DFS) in gastric cancer patients following gastrectomy. This model outperformed the traditional TNM staging system, with AUCs of 0.773 for OS and 0.751 for DFS in the training cohort, identifying high-risk patients who would benefit from adjuvant chemotherapy.
Lee et al[36] developed an AI model to predict pathological outcomes in EGC using endoscopic images and videos. The model achieved accuracies of 89.7% for undifferentiated histology and 92.7% for lymph node metastasis, outperforming expert endoscopists and achieving AUC values of 0.992 for undifferentiated histology.
Takayama et al[37] integrated Mask R-CNN into an AI model for detecting colorectal liver metastases (CLM) in contrast-enhanced intraoperative ultrasonography, achieving high accuracy (96.5%) in tumor detection. The combined model outperformed individual models, with an AUC of 0.99, improving CLM detection during liver surgery.
Wen et al[38] found that methionine metabolism and macrophage infiltration patterns in HCC were associated with different prognoses. Using machine learning techniques, they developed diagnostic and prognostic models that de
Qiu et al[18] highlighted AI's role in the screening, diagnosis, treatment, and prognosis of CRC. AI models offer novel approaches for identifying high-risk patients, selecting personalized treatment plans, and predicting outcomes, ultimately improving CRC patient survival rates.
Cho et al[39] discovered that AI-based models demonstrated significant potential in predicting the outcomes of transarterial chemoembolization for HCC. Models that combined radiomics and clinical features outperformed those based on imaging alone, offering superior performance in predicting treatment responses and potentially improving HCC ma
Hsu et al[40] found that serum biomarkers, particularly alpha-fetoprotein (AFP), were instrumental in predicting the efficacy of lenvatinib for unresectable hepatocellular carcinoma. An AI-based decision tree model identified high, intermediate, and low responders, correlating AFP reduction with higher objective response rates, providing a valuable tool for guiding treatment decisions.
Iacucci et al[41] developed an AI-based system for evaluating ulcerative colitis (UC) biopsies, effectively predicting disease remission and flare-ups. The system achieved sensitivity and specificity up to 94% for histological remission, improving clinical decision-making and standardizing assessments in UC management.
Yan et al[42] reviewed AI’s progress in predicting LNM in gastric cancer, noting that AI models incorporating multimodal clinical and imaging data hold great potential for improving LNM prediction accuracy and enhancing staging and treatment planning for gastric cancer patients.
Nagaraju et al[43] discussed AI’s transformative role in GI cancer management, particularly in early detection, treatment selection, and outcome prediction. The integration of AI into GI oncology could lead to more personalized and effective treatment strategies, ultimately improving patient care.
Solanki et al[44] explored the role of AI in perioperative management for major gastrointestinal surgeries, highlighting its potential to revolutionize perioperative care by enhancing early diagnosis, risk assessment, intraoperative manage
Kudo et al[45] developed an ANN model that outperformed existing guidelines in predicting LNM in T1 CRC. The ANN model achieved an AUC of 0.83, compared to 0.73 for traditional guidelines, offering a more effective tool for reducing unnecessary surgeries in patients with low-risk tumors.
Kuhn et al[46] demonstrated that machine learning models incorporating radiomics and laboratory data could predict post-portal vein embolization outcomes for colorectal cancer liver metastasis patients. These models showed high accuracy in predicting future liver remnant (FLR%) and kinetic growth rate (KGR%), providing valuable support for surgical planning.
Chiu et al[47] found that ANNs outperformed other models in predicting the quality of life (QOL) after hepatic resection for HCC. The ANN model accurately identified key predictors of post-surgery QOL, such as functional status and surgeon volume, offering insights for preoperative counseling.
Wang et al[48] developed a machine-learning-based artificial intelligence-derived prognostic signature (AIDPS) for pancreatic cancer. The model, based on data from 1280 patients across 10 multicenter cohorts, accurately predicted prognosis, outperforming traditional clinicopathological features and identifying panobinostat as a potential treatment for patients with high AIDPS.
Liu et al[49] applied deep learning to predict advanced gastric cancer patients' responses to programmed cell death-1 (PD-1) blockade combined with chemotherapy. The models demonstrated high predictive accuracy, with AUC values ranging from 0.92 to 1, providing a personalized approach to treatment planning.
Skrede et al[50] developed a deep learning biomarker for colorectal cancer outcomes using over 12 million image tiles. The marker, with a hazard ratio of 3.84, outperformed existing prognostic markers and could help guide adjuvant therapy selection by accurately stratifying patients based on their disease outcome.
Zhang et al[51] developed a deep learning model using pre-treatment endoscopic images to predict treatment responses to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. The model showed an AUC of 0.867 and accuracy of 0.836 in the internal test set, with robust validation in external cohorts, offering significant potential for personalized treatment.
Dong et al[52] developed a deep learning radiomic nomogram for predicting lymph node metastasis in locally advanced gastric cancer, with C-indexes ranging from 0.797 to 0.822 across multiple cohorts. The nomogram outperformed traditional clinical methods, offering a reliable preoperative tool for individualized treatment plans.
Liu et al[53] applied a deep learning radiomics model to predict extranodal soft tissue metastasis in gastric cancer, achieving C-indices of 0.770 and 0.761 in the training and test cohorts, respectively. This combined model provided strong prognostic value and identified occult distant metastasis missed by preoperative diagnoses.
Veldhuizen et al[54] developed a deep learning classifier for gastric cancer histology subtyping, which outperformed the Laurén classification used by pathologists in predicting patient survival. The model’s superior stratification of 5-year survival highlighted its potential for improving survival prediction and gastric adenocarcinoma subtyping.
Zhang et al[55] developed a machine learning-based disulfidoptosis-related ferroptosis score (DRF score) for HCC, which predicted patient outcomes. The DRF score identified patients with better prognoses and higher responses to immunotherapy and chemotherapy, offering potential therapeutic insights for HCC treatment.
Kui et al[56] applied an AI-based model called EASY-APP to predict the severity of acute pancreatitis (AP) within hours of hospital admission. Using XGBoost algorithms, the model achieved an AUC of 0.81 and an accuracy of 89.1%, offering a timely tool for identifying high-risk AP patients.
Lopez-Lopez et al[57] developed machine learning models to predict surgical complexity and postoperative outcomes in laparoscopic liver surgery for segments 7 and 8. The models, validated with SHapley Additive exPlanations (SHAP), identified key variables like resection type and tumor size, improving predictions of surgical success.
Wang et al[58] created a radiomics-based machine learning model that identified tumor heterogeneity in colorectal liver metastases (CRLM) and stratified patients into distinct prognostic subgroups. The model outperformed traditional clinical risk scores, providing valuable insights into CRLM prognosis post-liver resection.
Wu et al[59] demonstrated that an AI-assisted laparoscopic cholecystectomy coaching program significantly improved surgical performance and safety in novice surgeons. The AI-enhanced feedback led to higher Laparoscopic Chole
Fang et al[60] developed an immune-related signature integrated with machine learning to predict prognosis and immune-targeted therapy outcomes in HCC. The signature, based on T cell infiltration and PD-1/PD-L1 expression, ac
Ji et al[61] used machine learning models incorporating immune signatures and clinical factors to predict disease-specific survival and recurrence-free survival in biliary tract cancer (BTC) patients after resection. The Gradient Boosting Machine model outperformed traditional staging systems, providing accurate prognostic predictions and highlighting the importance of immune profiles in BTC prognosis.
Bertsimas et al[62] explored the potential of biomarkers, radiomics, and AI in CRLM treatment, emphasizing their importance for precision medicine and highlighting AI’s growing role in clinical decision-making.
Viganò et al[63] explored the potential of biomarkers, radiomics, and AI in CRLM treatment, emphasizing their importance for precision medicine and highlighting AI’s growing role in clinical decision-making.
Sun et al[64] developed ML-based models to predict distant lymph node metastasis and survival outcomes in HCC patients using the SEER database. These models identified key risk factors, enhancing risk stratification and improving patient management.
Wang et al[65] created a machine learning-based signature for HCC using antigen-presenting cells and T-cell-infiltration-related long non-coding RNAs. The ATLS model showed strong predictive power, assisting in personalized HCC management and treatment efficacy predictions.
Moaven et al[66] applied ML to predict outcomes of CLM surgeries. The gradient-boosted trees (GBT) model outperformed logistic regression (LR) in predicting survival and recurrence, aiding clinical decision-making for CLM patients.
Vannucci et al[67] developed ML models to predict the success of endoscopic sleeve gastroplasty, highlighting the value of including follow-up data for personalized postoperative care.
Lopez-Lopez et al[68] applied ML models to predict the success of initial repair in iatrogenic bile duct injury (IBDI) during cholecystectomy, offering a new approach to IBDI management based on decision tree models.
Liu et al[69] developed a machine learning-based immune-related long noncoding RNA signature to predict OS in CRC, providing insights into chemotherapy response.
Huang et al[70] used ML models to predict prognosis and optimize surgical decisions for ICC, improving patient outcomes by identifying survival benefits associated with different surgical approaches.
Bukhari et al[71] reviewed AI’s role in studying the liver cancer immune microenvironment, particularly its potential to aid in therapy response analysis and tumor initiation understanding, while addressing challenges in data harmonization.
Wang et al[72] applied deep learning to predict gastric cancer outcomes using resected lymph node histopathology images, offering a tool for predicting lymph node metastasis and proposing tumor-area-to-MLN-area ratios as an independent prognostic factor.
Xia et al[73] used a radiomics approach to predict microvascular invasion in HCC, demonstrating the model’s superior predictive performance, aiding personalized treatment.
Saillard et al[74] developed deep learning algorithms to predict survival outcomes in HCC patients based on histological slides, with SCHMOWDER and CHOWDER models outperforming traditional methods.
Jin et al[75] developed a deep learning model to predict postoperative survival outcomes for gallbladder carcinoma patients, providing potential for preoperative decision-making.
Lee et al[76] applied deep learning to predict survival in pancreatic cancer patients after surgery, demonstrating potential for improving prognosis prediction.
Huang et al[77] found that interpretable machine learning models were developed to predict textbook outcomes in ICC patients. The model identified Child-Pugh classification, ECOG score, and tumor size as key preoperative factors, with high accuracy in predicting outcomes and providing personalized treatment guidance.
Watson et al[78] found that deep learning models were used to predict pathologic tumor response to neoadjuvant therapy in pancreatic adenocarcinoma. The hybrid model, which incorporated decreases in serum carbohydrate antigen 19-9, achieved an AUC of 0.785, suggesting its potential for improving neoadjuvant therapy assessment in pancreatic cancer.
Bari et al[79] reviewed AI’s role in predicting outcomes in hepatobiliary and pancreatic surgeries, enhancing surgical planning and postoperative care.
Ichimasa et al[80] developed AI systems for predicting lymph node metastasis risk in T1 colorectal cancer, reducing unnecessary surgeries.
Altaf et al[81] developed ML models to predict futile surgery risks in ICC, providing preoperative guidance for better surgical planning.
Bertsimas et al[82] applied AI models to determine the optimal margin width for hepatectomy in CRLM. The study showed that optimal policy trees could guide personalized treatment by recommending margin widths based on individual patient characteristics, improving survival outcomes.
Tsilimigras et al[83] used machine learning models to assess pre- and postoperative factors for patients undergoing resection for HCC. The study identified AFP and tumor burden as key prognostic factors, offering insights for expanding resection criteria beyond BCLC guidelines.
Prediction of recurrence in gastrointestinal malignancies: Machine learning models demonstrate effectiveness in forecasting cancer recurrence after surgical treatment by giving doctors early warnings about potential relapse. The predictive models have demonstrated effective results in gallbladder cancer as well as colorectal cancer and gastric cancer. The analysis of clinicopathological features together with imaging data through these models allows healthcare providers to create individualized treatment plans and enhance follow-up care which leads to better survival results (Table 3).
| No. | Title of study | Ref. | Sample size | Validation method | Key limitations |
| 1 | A machine learning predictive model for recurrence of resected distal cholangiocarcinoma | Perez et al[84] | 654 patients | External | Limited to a single center, needs multi-center validation |
| 2 | A novel prediction model for colon cancer recurrence using auto-artificial intelligence | Mazaki et al[85] | 500+ patients | Internal | Needs external validation across diverse cohorts |
| 3 | Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation | Liu et al[86] | 500+ patients | External | Single-center validation, needs larger cohort testing |
| 4 | Deep learning model for predicting gastric cancer recurrence based on computed tomography imaging | Cao et al[87] | 200+ patients | External | Needs further multi-center validation |
| 5 | Machine learning model to predict early recurrence of intrahepatic cholangiocarcinoma | Alaimo et al[88] | 100+ patients | Internal | Requires external validation for broader applicability |
| 6 | Prognostic prediction model for elderly gastric cancer patients based on oxidative stress biomarkers | Zhang et al[89] | 200+ elderly patients | External | Lack of multi-center data, small sample size |
| 7 | Consensus machine learning-derived lncRNA signature for stage II/III colorectal cancer | Liu et al[90] | 300+ patients | External | Validation needed in larger, multi-center trials |
| 8 | ML identifies autophagy-related genes as markers of recurrence in colorectal cancer | Wu et al[91] | 200+ patients | External | Needs larger sample size and multi-center validation |
| 9 | ML models for predicting postoperative peritoneal metastasis after hepatocellular carcinoma rupture | Xia et al[92] | 250+ patients | Internal | Inadequate external validation across different patient groups |
| 10 | ML prediction of early recurrence in gastric cancer: Nationwide real-world study | Zhang et al[93] | 1500+ patients | External | Single-region data, needs validation in global populations |
| 11 | CT radiomics and ML predicts recurrence of hepatocellular carcinoma post-resection | Ji et al[94] | 300+ patients | External | Needs more validation across different clinical settings |
| 13 | ML-based gene signature predicts paclitaxel survival benefit in gastric cancer | Sundar et al[95] | 350+ patients | External | Needs external validation in diverse clinical settings |
| 14 | CT-based deep learning model for predicting early recurrence in gastric cancer | Guo et al[96] | 200+ patients | External | Needs larger multi-center validation |
The prediction of early recurrence after gallbladder cancer surgery represents a significant application of machine learning models. A research study with gallbladder cancer patients who received curative resection developed multiple machine learning models to forecast early recurrence during the first year post-surgery. The random forest model demonstrated the best discriminatory power in the evaluation cohort through an AUC evaluation of 76.4 (95%CI: 66.3 to 86.5) above XGBoost and support vector machine and traditional logistic regression. The predictive model helps healthcare providers create individualized follow-up plans while enabling early medical interventions for patients who show high risk factors[12].
The application of machine learning technology exists to support treatment planning for patients who develop recurrent hepatocellular carcinoma after surgical intervention. Researchers created a predictive survival model for recurrence which enabled them to direct patients toward the best available treatments between re-operative hepatectomy and thermo-ablation and chemoembolization and sorafenib. Machine learning applications extend past basic outcome prediction because they actively participate in clinical decision-making processes to direct treatment selection[6]
Perez et al[84] developed a machine learning model using a LASSO-regularized Cox regression for predicting recurrence in distal cholangiocarcinoma. This model achieved a C-index of 0.8 for DFS among 654 patients, with lymph node ratio 15 emerging as the most influential factor. The model demonstrated strong internal and external validation, with AUCs of 92.4% and 91.5%, respectively.
Mazaki et al[85] found that an auto-AI prediction model for recurrence in stage II-III colon cancer improved predictive accuracy compared to traditional statistical methods. The AI model achieved an AUC of 0.815, surpassing the multivariate model’s AUC of 0.719, highlighting its potential for more accurate recurrence prediction without requiring advanced AI expertise.
Liu et al[86] developed a deep learning-based classifier for predicting recurrence in HCC after resection or liver transplantation. The model demonstrated a hazard ratio of 3.44 for high-risk vs low-risk patients, with AUC values exceeding 0.85, showing strong discriminatory power for recurrence-free survival across multiple cohorts.
Cao et al[87] created a deep learning-based signature for predicting postoperative recurrence in gastric cancer (GC) patients. Developed from CT images, this model demonstrated excellent performance with an AUC of 0.833 in the training cohort and 0.859 in external validation, outperforming clinical models and successfully identifying high-risk recurrent patients with poor prognoses.
Alaimo et al[88] found that a random forest model for predicting early recurrence of ICC after hepatectomy achieved an AUC of 0.904 in the training cohort and 0.779 in the testing cohort. Incorporating 14 clinicopathological features, this model effectively stratified patients based on their risk of early recurrence, aiding in personalized treatment strategies.
Zhang et al[89] developed a machine learning model, the GIOSS, to predict postoperative overall survival in elderly gastric cancer patients. The model achieved an AUC of 0.999 in the training cohort and 0.796 in external validation, identifying key features like tumor size and perineural invasion for robust prognostic prediction.
Liu et al[90] developed the CMDLncS, a machine learning-derived long non-coding RNA (lncRNA) signature, for predicting recurrence risk in stage II/III CRC. The model, based on 27 stable recurrence-related lncRNAs, showed improved performance over clinical traits and molecular features, aiding in precision treatment for CRC patients and identifying those who would benefit from fluorouracil-based adjuvant chemotherapy.
Wu et al[91] identified autophagy-related genes (ARGs) that predict recurrence in CRC using machine learning. The study highlighted the predictive power of ARGs BAX and PARP1, offering a tool for early detection and patient stratification, improving CRC patient outcomes.
Xia et al[92] applied deep learning models to predict postoperative peritoneal metastasis after HCC rupture. The models outperformed others by identifying key predictive factors, such as tumor size, timing of hepatectomy, and microvascular invasion, providing valuable insights to improve patient outcomes.
Zhang et al[93] developed a stacking ensemble machine learning model to predict early recurrence in GC patients after curative surgery. The model demonstrated high accuracy, with tumor staging, tumor marker levels, and lymphovascular invasion being significant factors linked to a higher recurrence risk.
Ji et al[94] developed a machine learning model using contrast-enhanced CT radiomics to predict recurrence in HCC after resection. The model demonstrated superior prognostic performance across three independent institutions, with C-index values ranging from 0.733 to 0.801, aiding in personalized HCC management.
Sundar et al[95] identified a machine learning-derived gene signature predictive of paclitaxel survival benefit in GC patients. Developed using data from the SAMIT trial, the signature identified Pac-Sensitive patients who showed significant improvements in DFS, demonstrating its potential for guiding chemotherapy decisions.
Guo et al[96] used a deep learning model based on CT images to predict early recurrence in locally advanced gastric cancer patients. The model showed an AUC of 0.891 and outperformed traditional clinical models, enhancing recurrence prediction accuracy and survival forecasting.
Jiang et al[97] developed multitask deep learning models to predict peritoneal recurrence and disease-free survival in gastric cancer. The model demonstrated high accuracy across three cohorts, achieving an AUC of 0.857 in the training cohort, offering insights to optimize adjuvant chemotherapy decisions for high-risk patients.
Chen et al[98] employed machine learning radiomics to predict very early recurrence in intrahepatic cholangiocarcinoma patients after curative hepatectomy. The hybrid model achieved an AUC of 0.929 in external validation, aiding in the prediction of early recurrence and facilitating personalized patient management.
Prediction of complications: The application of machine learning models continues to rise for predicting surgical complications and postoperative hospital stay lengths in gastrointestinal surgeries. The models serve to detect risk factors including preoperative treatments and surgical techniques which allows for specific preventive measures. The models help determine recovery times which leads to better patient care and resources management for enhanced surgical results.
The development of machine learning models exists to forecast complications that occur during gastrointestinal procedures. Researchers developed a nomogram prediction model through the analysis of colorectal cancer patient data to forecast early postoperative stoma complications by identifying diabetes and preoperative radiotherapy and chemotherapy and stoma type and nutritional risk screening scores and prognostic nutritional index as risk factors. The prediction model based on nomogram integrated these factors to forecast complications so healthcare providers could develop preventive measures and enhance patient education[4].
Machine learning serves a significant purpose in gastrointestinal surgery by helping forecast how long patients will need to stay in the hospital. Researchers used machine learning models to forecast decompression surgery recovery duration for lumbar spinal stenosis patients with results showing 0.626 Linear correlation and 2.26 days absolute mean error. The research presented methodologies that gastrointestinal surgery could implement to forecast resource usage in gastrointestinal procedures even though it focused on different surgical procedures[99].
Mazaki et al[100] developed an auto-AI model to predict anastomotic leakage (AL) in left-sided colorectal cancer patients using the double-stapling technique. The model achieved an AUC of 0.766, with the type of circular stapler used being the most influential factor (IOV = 0.551). This finding suggests that the use of triple-row circular staplers may reduce AL rates.
In laparoscopic hepatectomy, Sunakawa et al[101] developed a deep learning model for automatic bleeding re
Chen et al[102] created a machine learning-based risk calculator for predicting perioperative adverse events in emergency general surgery (EGS) patients. This model, developed using data from five centers in China, accurately predicted postoperative mortality, pneumonia, surgical site infections, thrombosis, and mechanical ventilation needs, offering a valuable tool for clinical decision-making in EGS.
A systematic review by Wang et al[103] compared ML models with LR models in predicting postoperative outcomes after GI surgery. The review found that ML models outperformed LR models in discriminatory ability, highlighting the superiority of ML approaches for improving clinical decision-making in GI surgery.
Endo et al[104] developed machine learning models to predict severe complications and post-surgical mortality in patients undergoing HCC resection. The model, which incorporated both preoperative and postoperative data, demonstrated high predictive accuracy, with postoperative albumin-bilirubin scores being key predictive factors.
Pera et al[105] found that machine learning models were effective in predicting 90-day mortality (90DM) after gastrectomy for gastric cancer. The study, which included 3182 patients in the development cohort and 260 in the validation cohort, revealed 90DM rates of 5.6% and 6.2%, respectively. The random forest model achieved an AUC of 0.844, aiding surgeons in making informed decisions.
Zhu et al[106] developed a machine learning nomogram to predict 30-day mortality in patients with malignant biliary obstruction after ERCP. The model, which incorporated factors like distant metastasis and total bilirubin, achieved an AUC of 0.76, showing potential for identifying high-risk patients in clinical settings.
Jin et al[107] developed preoperative machine learning models to predict clinically significant post-hepatectomy liver failure in 226 patients. The ANN model outperformed traditional clinical scores, achieving AUC values of 0.766 and 0.851, offering a valuable preoperative risk assessment tool.
Chen et al[108] used random forest models to predict postoperative complications and survival outcomes in CRLM patients. The study analyzed data from 1067 patients and demonstrated the model’s robust performance, helping clinicians identify patients at high risk of poor outcomes.
Kang et al[109] developed a deep learning model to predict safe liver resection volumes for major hepatectomy. The model achieved 68.8% accuracy, significantly improving prediction precision compared to traditional models, which helps minimize the risk of post-hepatectomy liver failure.
The research demonstrates how machine learning technology becomes increasingly useful for predicting complications and optimizing hospital stays and surgical decisions in gastrointestinal surgeries which produces improved patient results and better resource management.
Role in liver transplantation: AI technology has been applied to liver transplantation to improve matching of recipient to liver donor and survival prediction and to clinical decision support. Results show that models from machine learning including ANN and random forests (RF), perform better in graft survival prediction and transplant outcomes than the MELD score. Forecasts for complications are made using AI models and patient selection is improved, which hold significant potential for increasing effectiveness of liver transplant and patient survival outcomes (Table 4).
| No. | Title of study | Ref. | Sample size | Validation method | Key limitations |
| 1 | Knowledge domain and frontier trends of artificial intelligence applied in solid organ transplantation | Gong et al[110] | N/A | N/A | Lacks specific cohort data and external validation |
| 2 | Artificial intelligence and liver transplantation: Looking for the best donor-recipient pairing | Briceño et al[111] | 200+ patients | External | Requires larger, multi-center validation |
| 3 | AI for predicting survival following deceased donor liver transplantation | Yu et al[112] | 100+ patients | Internal | Needs broader validation in diverse populations |
| 4 | AI, ML, and deep learning in liver transplantation | Bhat et al[113] | N/A | Mixed | Lack of clear sample size and inconsistent external validation |
| 5 | Bibliometric and LDA analysis of acute rejection in liver transplantation | Jiang et al[114] | N/A | N/A | The study lacks clinical validation data and does not include real-world cohorts |
| 6 | Criteria and prognostic models for hepatocellular carcinoma patients undergoing liver transplantation | Sha et al[115] | 150+ patients | External | Needs further multi-center validation and more diverse patient groups |
| 7 | Machine learning in liver transplantation: A tool for unsolved questions | Ferrarese et al[116] | N/A | Mixed | Lack of specific cohort validation, focuses mainly on theoretical models |
| 8 | ML model for predicting liver transplant outcomes in hepatitis C patients | Zabara et al[117] | 100+ patients | Internal | Limited to one patient cohort, needs broader validation |
| 9 | Machine learning algorithms for predicting liver transplant results | Briceño et al[118] | 150+ patients | External | Needs validation across different geographical regions and patient types |
| 10 | Supervised machine learning to predict hepatic immunological tolerance | Morita-Nakagawa et al[119] | 50+ patients | Internal | Requires larger cohort size and further multi-center validation |
| 11 | AI for predicting survival of individual grafts in liver transplantation | Wingfield et al[120] | 200+ patients | External | Validation needed in multi-center studies with diverse populations |
A bibliometric study by Gong et al[110] explored the evolution of AI applications in solid organ transplantation (SOT), identifying trends in robotic surgery, organ allocation, outcome prediction, and precision medicine. While AI holds transformative potential in improving SOT outcomes, the study also emphasized challenges such as ethical concerns, bias, and data integration, urging for more international and interdisciplinary collaboration.
Briceño et al[111] highlighted that AI-based models, particularly ANN and RF, have significantly improved donor-recipient matching in liver transplantation, achieving success rates exceeding 80%. These models outperformed traditional metrics but face challenges related to integrating deep learning-based approaches into clinical practice.
Yu et al[112] demonstrated that machine learning algorithms, especially random forest models, performed better than traditional tools like the MELD score in predicting survival rates following liver transplantation. With higher AUC values, these models enhance donor-recipient matching and improve post-transplant survival predictions.
Bhat et al[113] found that AI models could optimize clinical decision-making in liver transplantation by enhancing transplant candidacy, donor-recipient matching, and post-transplant management. These models offer a means to predict patient survival and identify complications, although challenges such as data imbalance and privacy concerns remain.
Jiang et al[114] examined trends in acute rejection research within liver transplantation using bibliometric analysis and latent Dirichlet allocation (LDA) topic modeling. The study noted an increasing focus on molecular diagnostics, immune checkpoint inhibitors, and AI-driven immunosuppression strategies that improve graft survival and reduce dependency on immunosuppressive drugs.
Sha et al[115] investigated the role of expanded selection criteria and prognostic models that integrate clinicopathological data, radiologic features, and AI to improve liver transplantation outcomes, particularly for patients with HCC. These models enhanced patient selection and surveillance strategies, with AUC values ranging from 0.78 to 0.91, showing potential for long-term outcome improvement.
Ferrarese et al[116] discussed the role of machine learning in liver transplantation, focusing on predicting pre-transplant survival, donor-recipient match, and post-operative complications. The review underscored the strengths and challenges of ML in this field, suggesting it could significantly improve the accuracy of prognosis in liver transplantation.
Zabara et al[117] validated a machine learning model for predicting short-term outcomes in liver transplant recipients with hepatitis C. The model demonstrated excellent performance, achieving 100% accuracy in predicting postoperative complications, providing a foundation for more intensive monitoring of high-risk patients.
Briceño et al[118] found that machine learning algorithms could predict liver transplant outcomes, particularly donor-recipient matching. Their study showed that ANN and random forest classifiers achieved 95% accuracy in predicting 3-month graft survival, suggesting that AI could enhance organ allocation and improve transplant outcomes.
Morita-Nakagawa et al[119] utilized machine learning models to predict liver transplantation outcomes in mice by analyzing genetic data. The study achieved perfect prediction accuracy, showcasing the potential of machine learning to enhance liver transplant outcomes and identify biomarkers for immune tolerance.
Wingfield et al[120] reported that AI models could predict individual graft survival in liver transplantation, surpassing traditional models like the DRI and MELD scores. The study demonstrated that AI techniques, especially artificial neural networks, could improve organ allocation and maximize the utility of liver transplants.
The research demonstrates AI’s expanding function in liver transplantation by improving donor matching and survival forecasting and post-transplant care optimization which leads to substantial advancements in transplant effectiveness and patient results.
Electronic health record (EHR) integration and surgeon-AI collaboration are transforming gastrointestinal surgeries by enhancing efficiency, precision, and patient outcomes. EHR systems streamline the management of patient data, providing surgeons with comprehensive, real-time access to medical histories, diagnostic results, and previous surgical notes. This integration ensures that the surgical team is equipped with accurate and up-to-date information, reducing the risk of errors and improving decision-making during complex procedures.
AI collaboration in GI surgeries goes beyond data management. Machine learning algorithms can assist surgeons by analysing medical images, detecting abnormalities like tumours or lesions, and offering real-time insights based on vast datasets. AI tools can also predict potential complications, recommend personalized surgical plans, and optimize postoperative care, improving recovery times and reducing hospital readmissions. For instance, Watson’s AI-assisted system demonstrated clinical utility in colorectal cancer management by integrating real-time patient data for personalized treatment[78].
Surgeon-AI collaboration in the operating room also includes robotic systems, where AI supports the precision and flexibility of robotic arms, enhancing the surgeon’s capabilities. This combination of human expertise and AI’s data processing power can lead to more successful surgeries, fewer complications, and better patient outcomes. As EHR systems and AI tools continue to evolve, their integration in gastrointestinal surgery promises to shape the future of healthcare by making surgeries safer and more efficient.
Machine learning algorithms face major problems because their decision-making operations remain opaque like "black boxes" which makes them difficult to interpret. Machine learning algorithms remain difficult to interpret because their complex systems lack straightforward explanations of their predictive processes[1].
Researchers are creating solutions to enhance model transparency as a solution to this problem. SHAP provide a method to determine the specific role of each feature in prediction results which helps understand how the model reaches its decisions. The approach represents a major advancement to boost the interpretability and trustworthiness of machine learning models used in clinical practice[1].
The development of machine learning studies faces major obstacles because there are no established standard procedures for model creation and results documentation[121]. The development of machine learning models for surgical outcome prediction lacks standardized procedures for development and validation as well as reporting guidelines in the current medical field[13].
The inconsistency between different models creates challenges for model comparison and hinders the application of research results in clinical practice. Standardized reporting guidelines modelled after TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) should be developed to support thorough evaluation of machine learning approaches in surgical outcome prediction[14].
The application of machine learning in surgical research and practice requires thorough ethical evaluation. The algorithms have the potential to maintain existing healthcare biases and inequalities because they learn from historical data that shows disparities in high-quality care access. The ethical concern stands out most strongly in gastrointestinal surgery because specialized care availability varies substantially between different population groups[13,122].
The solution of these ethical challenges requires close monitoring of data selection and algorithm development and validation processes which include diverse patient populations. The goal for researchers should be to minimize potential biases while machine learning models should improve rather than degrade surgical care equity[14]. It is also crucial to address the intersection of data privacy, algorithmic fairness, and collaboration. One of the most pressing concerns is the protection of sensitive patient data. Federated learning offers a promising solution by allowing AI models to be trained across multiple institutions or healthcare providers without the need to share raw patient data. This decentralized approach ensures that private health information, such as patient histories and surgical outcomes, remains secure and compliant with data protection regulations like HIPAA. However, despite its advantages in privacy preservation, federated learning still requires careful monitoring to prevent potential leaks or inadvertent sharing of sensitive data during model aggregation or updates.
Another significant ethical consideration is algorithmic fairness. AI models used for outcome prediction in GI surgeries must be scrutinized to ensure they do not perpetuate biases, particularly when trained on diverse, geographically segmented datasets. Federated learning models, by nature, rely on data from various sources, and if these datasets are skewed by demographic imbalances or regional healthcare disparities, the model may inadvertently favor certain groups over others. This could lead to unfair treatment decisions, such as misjudging the risks or recovery trajectories for patients from underrepresented populations. Therefore, integrating fairness frameworks within AI development is essential. These frameworks can identify and mitigate biases in the model, ensuring that predictions are equitable and reflect diverse patient populations.
Ultimately, the integration of federated learning and fairness frameworks into AI-driven outcome prediction in GI surgeries represents a step toward ethical AI. However, it requires continuous oversight to balance privacy, fairness, and the accuracy of predictions, ultimately ensuring that these technologies improve patient outcomes without compromising equity or security.
The future of AI in gastrointestinal surgery outcome prediction shows promise through several emerging trends which will enhance surgical practice and patient care.
Machine learning models need to be integrated perfectly into current clinical practices in order to reach their maximum potential[2]. The integration process demands the development of simple interfaces which should enable EHR compatibility and real-time predictions that aid clinical decisions during patient care. Mobile computing along with cloud-based platforms create opportunities to deliver machine learning predictions to clinicians through easily accessible formats. Future research should focus on developing clinical decision support systems which use these predictions while maintaining physician autonomy[3].
Future machine learning models will analyze an expanding variety of diverse data sources which include imaging data, genomic information, intraoperative measurements and postoperative monitoring parameters. The combination of multiple data sources will provide a more complete understanding of patient risk together with surgical outcomes. The combination of preoperative imaging elements with clinical variables would enhance the ability to predict surgical difficulty and postoperative complications[5]. Genomic data integration could help doctors identify patients who face elevated risks from adverse drug reactions and delayed wound healing so they can develop individualized preventive plans[123].
Surgical outcome prediction will benefit from algorithms that maintain learning capabilities and adaptation abilities. These algorithms differ from static models because they update their predictions through new data and outcomes which leads to improved accuracy and relevance as time passes[124]. The approach supports the learning healthcare system model which connects continuous clinical practice with research activities. The implementation of such systems in gastrointestinal surgery demands strong data infrastructure together with governance frameworks and systems for continuous validation and monitoring[99].
The review faces several limitations because the AI methodologies and surgical procedures in the studies show both methodological and procedural diversity. The studies analyzed different datasets through various algorithms and performance metrics which created obstacles for making direct comparisons. The generalizability of research findings remains uncertain because numerous studies contained small sample sizes. AI models demonstrate promising results in laboratory conditions but their practical implementation in clinical practice faces challenges because surgical decision-making complexity and data standardization requirements. The absence of standard guidelines for model development and reporting creates difficulties when trying to evaluate AI applications between different studies. Future research needs to perform large-scale validation studies while ensuring model robustness to address methodological inconsistencies which will improve AI applications in clinical practice. Limitations of common performance metrics, such as AUC and C-index, is essential when evaluating AI models used for outcome prediction. While these metrics assess the overall discriminative power of a model, they often fail to capture clinically relevant aspects of patient care. AUC and C-index do not account for sensitivity, which is crucial for detecting rare but critical complications, such as postoperative infections or bleeding. In such cases, a high AUC or C-index might still overlook patients at high risk for these complications, resulting in poor outcomes. Moreover, these metrics neglect calibration, which is vital for accurate risk stratification. A well-calibrated model, where predicted probabilities align with actual outcomes, is essential for making informed clinical decisions. Furthermore, in imbalanced datasets, common in healthcare, AUC and C-index may not reflect the model's true performance in identifying rare events. Metrics like precision, recall, and F1-score provide a clearer picture of how well a model detects these rare but critical events. Finally, clinical utility should be prioritized, as AUC and C-index do not necessarily correlate with actionable predictions or improved patient outcomes. Incorporating a broader range of metrics ensures AI models in GI surgery are both accurate and clinically useful.
The application of artificial intelligence as a predictive tool in gastrointestinal surgery has become powerful because models show significant potential to forecast surgical complications and post-surgical survival and recovery rates. Surgical decision-making benefits from machine learning techniques which deliver personalized care and improved risk assessment accuracy. Machine learning models that use clinical and demographic and imaging data perform better than traditional methods in predicting outcomes while handling complex non-linear relationships. AI holds transformative potential in personalized treatments, such as AI-driven chemotherapy protocols tailored to genetic profiles, which could revolutionize treatment for GI malignancies. The advantages of these systems face obstacles related to data quality and model interpretability and standardization between different clinical environments. The successful implementation of these models into clinical practice requires additional work to achieve their maximum potential for enhancing patient outcomes. The complete potential of AI in surgical care will depend on solving biases and making models more transparent.
| 1. | Geoghegan L, Rodrigues JN, Sidey-Gibbons CJ, Stirling PHC, McEachan JE, Harrison CJ. Reply: Developing Machine Learning Algorithms to Support Patient-centered, Value-based Carpal Tunnel Decompression Surgery. Plast Reconstr Surg Glob Open. 2023;11:e4744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Akinrinmade AO, Adebile TM, Ezuma-Ebong C, Bolaji K, Ajufo A, Adigun AO, Mohammad M, Dike JC, Okobi OE. Artificial Intelligence in Healthcare: Perception and Reality. Cureus. 2023;15:e45594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 3. | Bates DW, Levine D, Syrowatka A, Kuznetsova M, Craig KJT, Rui A, Jackson GP, Rhee K. The potential of artificial intelligence to improve patient safety: a scoping review. NPJ Digit Med. 2021;4:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Ba MQ, Zheng WL, Zhang YL, Zhang LL, Chen JJ, Ma J, Huang JL. Construction of a nomogram prediction model for early postoperative stoma complications of colorectal cancer. World J Gastrointest Surg. 2025;17:100547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Bekbolatova M, Mayer J, Ong CW, Toma M. Transformative Potential of AI in Healthcare: Definitions, Applications, and Navigating the Ethical Landscape and Public Perspectives. Healthcare (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 70] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 6. | Famularo S, Donadon M, Cipriani F, Fazio F, Ardito F, Iaria M, Perri P, Conci S, Dominioni T, Lai Q, La Barba G, Patauner S, Molfino S, Germani P, Zimmitti G, Pinotti E, Zanello M, Fumagalli L, Ferrari C, Romano M, Delvecchio A, Valsecchi MG, Antonucci A, Piscaglia F, Farinati F, Kawaguchi Y, Hasegawa K, Memeo R, Zanus G, Griseri G, Chiarelli M, Jovine E, Zago M, Abu Hilal M, Tarchi P, Baiocchi GL, Frena A, Ercolani G, Rossi M, Maestri M, Ruzzenente A, Grazi GL, Dalla Valle R, Romano F, Giuliante F, Ferrero A, Aldrighetti L, Bernasconi DP, Torzilli G; HE. RC.O.LE.S. Group. Machine Learning Predictive Model to Guide Treatment Allocation for Recurrent Hepatocellular Carcinoma After Surgery. JAMA Surg. 2023;158:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ghaffar Nia N, Kaplanoglu E, Nasab A. Evaluation of artificial intelligence techniques in disease diagnosis and prediction. Discov Artif Intell. 2023;3:5. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (1)] |
| 8. | Serag A, Ion-Margineanu A, Qureshi H, McMillan R, Saint Martin MJ, Diamond J, O'Reilly P, Hamilton P. Translational AI and Deep Learning in Diagnostic Pathology. Front Med (Lausanne). 2019;6:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 9. | Li YH, Li YL, Wei MY, Li GY. Innovation and challenges of artificial intelligence technology in personalized healthcare. Sci Rep. 2024;14:18994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 10. | Montagna S, Pengo MF, Ferretti S, Borghi C, Ferri C, Grassi G, Muiesan ML, Parati G. Machine Learning in Hypertension Detection: A Study on World Hypertension Day Data. J Med Syst. 2022;47:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sinha S, Dong T, Dimagli A, Judge A, Angelini GD. A machine learning algorithm-based risk prediction score for in-hospital/30-day mortality after adult cardiac surgery. Eur J Cardiothorac Surg. 2024;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Catalano G, Alaimo L, Chatzipanagiotou OP, Ruzzenente A, Aucejo F, Marques HP, Lam V, Hugh T, Bhimani N, Maithel SK, Kitago M, Endo I, Pawlik TM. Machine learning prediction of early recurrence after surgery for gallbladder cancer. Br J Surg. 2024;111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Nopour R. Comparison of machine learning models to predict complications of bariatric surgery: A systematic review. Health Informatics J. 2024;30:14604582241285794. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 14. | Satapathy P, Pradhan KB, Rustagi S, Suresh V, Al-Qaim ZH, Padhi BK, Sah R. Application of machine learning in surgery research: current uses and future directions - editorial. Int J Surg. 2023;109:1550-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Nie D, Zhan Y, Xu K, Zou H, Li K, Chen L, Chen Q, Zheng W, Peng X, Yu M, Zhang S. Artificial intelligence differentiates abdominal Henoch-Schönlein purpura from acute appendicitis in children. Int J Rheum Dis. 2023;26:2534-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25:1666-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 211] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (5)] |
| 17. | Chen S, Ding P, Guo H, Meng L, Zhao Q, Li C. Applications of artificial intelligence in digital pathology for gastric cancer. Front Oncol. 2024;14:1437252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Qiu H, Ding S, Liu J, Wang L, Wang X. Applications of Artificial Intelligence in Screening, Diagnosis, Treatment, and Prognosis of Colorectal Cancer. Curr Oncol. 2022;29:1773-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Issaiy M, Zarei D, Saghazadeh A. Artificial Intelligence and Acute Appendicitis: A Systematic Review of Diagnostic and Prognostic Models. World J Emerg Surg. 2023;18:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Huang LM, Yang WJ, Huang ZY, Tang CW, Li J. Artificial intelligence technique in detection of early esophageal cancer. World J Gastroenterol. 2020;26:5959-5969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | He XJ, Wang XL, Su TK, Yao LJ, Zheng J, Wen XD, Xu QW, Huang QR, Chen LB, Chen CX, Lin HF, Chen YQ, Hu YX, Zhang KH, Jiang CS, Liu G, Li DZ, Li DL, Wen W. Artificial intelligence-assisted system for the assessment of Forrest classification of peptic ulcer bleeding: a multicenter diagnostic study. Endoscopy. 2024;56:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Christou CD, Tsoulfas G. Challenges involved in the application of artificial intelligence in gastroenterology: The race is on! World J Gastroenterol. 2023;29:6168-6178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Jin C, Jiang Y, Yu H, Wang W, Li B, Chen C, Yuan Q, Hu Y, Xu Y, Zhou Z, Li G, Li R. Deep learning analysis of the primary tumour and the prediction of lymph node metastases in gastric cancer. Br J Surg. 2021;108:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A, Klein C, Bazille C, Heij LR, Uguen A, Luedde T, Di Tommaso L, Beaufrère A, Chatain A, Gastineau D, Nguyen CT, Nguyen-Canh H, Thi KN, Gnemmi V, Graham RP, Charlotte F, Wendum D, Vij M, Allende DS, Aucejo F, Diaz A, Rivière B, Herrero A, Evert K, Calvisi DF, Augustin J, Leow WQ, Leung HHW, Boleslawski E, Rela M, François A, Cha AW, Forner A, Reig M, Allaire M, Scatton O, Chatelain D, Boulagnon-Rombi C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Pawlotsky JM, Zhang X, Luciani A, Mulé S, Laurent A, Amaddeo G, Regnault H, De Martin E, Sempoux C, Navale P, Westerhoff M, Lo RC, Bednarsch J, Gouw A, Guettier C, Lequoy M, Harada K, Sripongpun P, Wetwittayaklang P, Loménie N, Tantipisit J, Kaewdech A, Shen J, Paradis V, Caruso S, Kather JN. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nat Commun. 2023;14:8290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 25. | Hong Y, Heo YJ, Kim B, Lee D, Ahn S, Ha SY, Sohn I, Kim KM. Deep learning-based virtual cytokeratin staining of gastric carcinomas to measure tumor-stroma ratio. Sci Rep. 2021;11:19255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Li Y, Xiong J, Hu Z, Chang Q, Ren N, Zhong F, Dong Q, Liu L. Denoised recurrence label-based deep learning for prediction of postoperative recurrence risk and sorafenib response in HCC. BMC Med. 2025;23:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 27. | Tang D, Wang L, Ling T, Lv Y, Ni M, Zhan Q, Fu Y, Zhuang D, Guo H, Dou X, Zhang W, Xu G, Zou X. Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: A multicentre retrospective diagnostic study. EBioMedicine. 2020;62:103146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Chidambaram S, Sounderajah V, Maynard N, Markar SR. Diagnostic Performance of Artificial Intelligence-Centred Systems in the Diagnosis and Postoperative Surveillance of Upper Gastrointestinal Malignancies Using Computed Tomography Imaging: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Ann Surg Oncol. 2022;29:1977-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Raza Z, Saqib SU, Bajwa AA. Integrating artificial intelligence techniques for advancements in colorectal cancer management: navigating past and predicting future direction. J Pak Med Assoc. 2024;74:S165-S170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Luo H, Xu G, Li C, He L, Luo L, Wang Z, Jing B, Deng Y, Jin Y, Li Y, Li B, Tan W, He C, Seeruttun SR, Wu Q, Huang J, Huang DW, Chen B, Lin SB, Chen QM, Yuan CM, Chen HX, Pu HY, Zhou F, He Y, Xu RH. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 31. | Sakamoto T, Goto T, Fujiogi M, Kawarai Lefor A. Machine learning in gastrointestinal surgery. Surg Today. 2022;52:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Xiao H, Wang J, Weng Z, Lin X, Shu M, Shen J, Sun P, Cai M, Xiang X, Li B, Wei L, Shi Y, Lai J, Kuang M, Yun J, Chen S, Peng S. A histopathology-based artificial intelligence system assisting the screening of genetic alteration in intrahepatic cholangiocarcinoma. Br J Cancer. 2025;132:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Jiang W, Li M, Tan J, Feng M, Zheng J, Chen D, Liu Z, Yan B, Wang G, Xu S, Xiao W, Gao Y, Zhuo S, Yan J. A Nomogram Based on a Collagen Feature Support Vector Machine for Predicting the Treatment Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients. Ann Surg Oncol. 2021;28:6408-6421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 34. | Tsilimigras DI, Hyer JM, Paredes AZ, Diaz A, Moris D, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Endo I, Pawlik TM. A Novel Classification of Intrahepatic Cholangiocarcinoma Phenotypes Using Machine Learning Techniques: An International Multi-Institutional Analysis. Ann Surg Oncol. 2020;27:5224-5232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Li X, Zhai Z, Ding W, Chen L, Zhao Y, Xiong W, Zhang Y, Lin D, Chen Z, Wang W, Gao Y, Cai S, Yu J, Zhang X, Liu H, Li G, Chen T. An artificial intelligence model to predict survival and chemotherapy benefits for gastric cancer patients after gastrectomy development and validation in international multicenter cohorts. Int J Surg. 2022;105:106889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Lee S, Jeon J, Park J, Chang YH, Shin CM, Oh MJ, Kim SH, Kang S, Park SH, Kim SG, Lee HJ, Yang HK, Lee HS, Cho SJ. An artificial intelligence system for comprehensive pathologic outcome prediction in early gastric cancer through endoscopic image analysis (with video). Gastric Cancer. 2024;27:1088-1099. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Takayama M, Ito K, Karako K, Mihara Y, Sasaki S, Ichida A, Takamoto T, Akamatsu N, Kawaguchi Y, Hasegawa K. An artificial intelligence-based recognition model of colorectal liver metastases in intraoperative ultrasonography with improved accuracy through algorithm integration. J Hepatobiliary Pancreat Sci. 2025;32:58-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Wen D, Wang S, Yu J, Yu T, Liu Z, Li Y. Analysis of clinical significance and molecular characteristics of methionine metabolism and macrophage-related patterns in hepatocellular carcinoma based on machine learning. Cancer Biomark. 2024;39:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 39. | Cho EEL, Law M, Yu Z, Yong JN, Tan CS, Tan EY, Takahashi H, Danpanichkul P, Nah B, Soon GST, Ng CH, Tan DJH, Seko Y, Nakamura T, Morishita A, Chirapongsathorn S, Kumar R, Kow AWC, Huang DQ, Lim MC, Law JH. Artificial Intelligence and Machine Learning Predicting Transarterial Chemoembolization Outcomes: A Systematic Review. Dig Dis Sci. 2025;70:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 40. | Hsu PY, Liang PC, Chang WT, Lu MY, Wang WH, Chuang SC, Wei YJ, Jang TY, Yeh ML, Huang CI, Lin YH, Wang CW, Hsieh MY, Hou NJ, Hsieh MH, Tsai YS, Ko YM, Lin CC, Chen KY, Dai CY, Lin ZY, Chen SC, Chuang WL, Huang CF, Huang JF, Yu ML. Artificial intelligence based on serum biomarkers predicts the efficacy of lenvatinib for unresectable hepatocellular carcinoma. Am J Cancer Res. 2022;12:5576-5588. [PubMed] |
| 41. | Iacucci M, Parigi TL, Del Amor R, Meseguer P, Mandelli G, Bozzola A, Bazarova A, Bhandari P, Bisschops R, Danese S, De Hertogh G, Ferraz JG, Goetz M, Grisan E, Gui X, Hayee B, Kiesslich R, Lazarev M, Panaccione R, Parra-Blanco A, Pastorelli L, Rath T, Røyset ES, Tontini GE, Vieth M, Zardo D, Ghosh S, Naranjo V, Villanacci V. Artificial Intelligence Enabled Histological Prediction of Remission or Activity and Clinical Outcomes in Ulcerative Colitis. Gastroenterology. 2023;164:1180-1188.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 42. | Yan SC, Peng W, Cheng M, Yang WR, Wu YY. [Artificial intelligence for lymph node metastasis prediction in gastric cancer: research progress]. Zhonghua Wei Chang Wai Ke Za Zhi. 2025;28:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Nagaraju GP, Sandhya T, Srilatha M, Ganji SP, Saddala MS, El-Rayes BF. Artificial intelligence in gastrointestinal cancers: Diagnostic, prognostic, and surgical strategies. Cancer Lett. 2025;612:217461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 44. | Solanki SL, Pandrowala S, Nayak A, Bhandare M, Ambulkar RP, Shrikhande SV. Artificial intelligence in perioperative management of major gastrointestinal surgeries. World J Gastroenterol. 2021;27:2758-2770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, Mitani T, Ohtsuka K, Chino A, Ide D, Imai K, Kishida Y, Nakamura K, Saiki Y, Tanaka M, Hoteya S, Yamashita S, Kinugasa Y, Fukuda M, Kudo T, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology. 2021;160:1075-1084.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 46. | Kuhn TN, Engelhardt WD, Kahl VH, Alkukhun A, Gross M, Iseke S, Onofrey J, Covey A, Camacho JC, Kawaguchi Y, Hasegawa K, Odisio BC, Vauthey JN, Antoch G, Chapiro J, Madoff DC. Artificial Intelligence-Driven Patient Selection for Preoperative Portal Vein Embolization for Patients with Colorectal Cancer Liver Metastases. J Vasc Interv Radiol. 2025;36:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 47. | Chiu CC, Lee KT, Lee HH, Wang JJ, Sun DP, Huang CC, Shi HY. Comparison of Models for Predicting Quality of Life After Surgical Resection of Hepatocellular Carcinoma: a Prospective Study. J Gastrointest Surg. 2018;22:1724-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 48. | Wang L, Liu Z, Liang R, Wang W, Zhu R, Li J, Xing Z, Weng S, Han X, Sun YL. Comprehensive machine-learning survival framework develops a consensus model in large-scale multicenter cohorts for pancreatic cancer. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 49. | Liu Y, Chen W, Ruan R, Zhang Z, Wang Z, Guan T, Lin Q, Tang W, Deng J, Wang Z, Li G. Deep learning based digital pathology for predicting treatment response to first-line PD-1 blockade in advanced gastric cancer. J Transl Med. 2024;22:438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 50. | Skrede OJ, De Raedt S, Kleppe A, Hveem TS, Liestøl K, Maddison J, Askautrud HA, Pradhan M, Nesheim JA, Albregtsen F, Farstad IN, Domingo E, Church DN, Nesbakken A, Shepherd NA, Tomlinson I, Kerr R, Novelli M, Kerr DJ, Danielsen HE. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 51. | Zhang J, Liu R, Wang X, Zhang S, Shao L, Liu J, Zhao J, Wang Q, Tian J, Lu Y. Deep learning model based on endoscopic images predicting treatment response in locally advanced rectal cancer undergo neoadjuvant chemoradiotherapy: a multicenter study. J Cancer Res Clin Oncol. 2024;150:350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Dong D, Fang MJ, Tang L, Shan XH, Gao JB, Giganti F, Wang RP, Chen X, Wang XX, Palumbo D, Fu J, Li WC, Li J, Zhong LZ, De Cobelli F, Ji JF, Liu ZY, Tian J. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multicenter study. Ann Oncol. 2020;31:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (1)] |
| 53. | Liu S, Deng J, Dong D, Fang M, Ye Z, Hu Y, Li H, Zhong L, Cao R, Zhao X, Shang W, Li G, Liang H, Tian J. Deep learning-based radiomics model can predict extranodal soft tissue metastasis in gastric cancer. Med Phys. 2024;51:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Veldhuizen GP, Röcken C, Behrens HM, Cifci D, Muti HS, Yoshikawa T, Arai T, Oshima T, Tan P, Ebert MP, Pearson AT, Calderaro J, Grabsch HI, Kather JN. Deep learning-based subtyping of gastric cancer histology predicts clinical outcome: a multi-institutional retrospective study. Gastric Cancer. 2023;26:708-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 55. | Zhang C, Xu T, Ji K, Cao S, Ai J, Pan J, Cao Y, Yang Y, Jing L, Sun JH. Development and experimental validation of a machine learning-based disulfidptosis-related ferroptosis score for hepatocellular carcinoma. Apoptosis. 2024;29:103-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 56. | Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, Vincze Á, Bajor J, Gódi S, Czimmer J, Szabó I, Illés A, Sarlós P, Hágendorn R, Pár G, Papp M, Vitális Z, Kovács G, Fehér E, Földi I, Izbéki F, Gajdán L, Fejes R, Németh BC, Török I, Farkas H, Mickevicius A, Sallinen V, Galeev S, Ramírez-Maldonado E, Párniczky A, Erőss B, Hegyi PJ, Márta K, Váncsa S, Sutton R, Szatmary P, Latawiec D, Halloran C, de-Madaria E, Pando E, Alberti P, Gómez-Jurado MJ, Tantau A, Szentesi A, Hegyi P; Hungarian Pancreatic Study Group. EASY-APP: An artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12:e842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 57. | Lopez-Lopez V, Morise Z, Albaladejo-González M, Gavara CG, Goh BKP, Koh YX, Paul SJ, Hilal MA, Mishima K, Krürger JAP, Herman P, Cerezuela A, Brusadin R, Kaizu T, Lujan J, Rotellar F, Monden K, Dalmau M, Gotohda N, Kudo M, Kanazawa A, Kato Y, Nitta H, Amano S, Valle RD, Giuffrida M, Ueno M, Otsuka Y, Asano D, Tanabe M, Itano O, Minagawa T, Eshmuminov D, Herrero I, Ramírez P, Ruipérez-Valiente JA, Robles-Campos R, Wakabayashi G. Explainable artificial intelligence prediction-based model in laparoscopic liver surgery for segments 7 and 8: an international multicenter study. Surg Endosc. 2024;38:2411-2422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 58. | Wang Q, Nilsson H, Xu K, Wei X, Chen D, Zhao D, Hu X, Wang A, Bai G. Exploring tumor heterogeneity in colorectal liver metastases by imaging: Unsupervised machine learning of preoperative CT radiomics features for prognostic stratification. Eur J Radiol. 2024;175:111459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 59. | Wu S, Tang M, Liu J, Qin D, Wang Y, Zhai S, Bi E, Li Y, Wang C, Xiong Y, Li G, Gao F, Cai Y, Gao P, Wu Z, Cai H, Liu J, Chen Y, Fang C, Yao L, Jiang J, Peng B, Wu H, Li A, Wang X. Impact of an AI-based laparoscopic cholecystectomy coaching program on the surgical performance: a randomized controlled trial. Int J Surg. 2024;110:7816-7823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 60. | Fang H, Chen X, Zhong Y, Wu S, Ke Q, Huang Q, Wang L, Zhang K. Integrating anoikis and ErbB signaling insights with machine learning and single-cell analysis for predicting prognosis and immune-targeted therapy outcomes in hepatocellular carcinoma. Front Immunol. 2024;15:1446961. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Ji GW, Wang K, Xia YX, Wang JS, Wang XH, Li XC. Integrating Machine Learning and Tumor Immune Signature to Predict Oncologic Outcomes in Resected Biliary Tract Cancer. Ann Surg Oncol. 2021;28:4018-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Bertsimas D, Margonis GA, Sujichantararat S, Koulouras A, Ma Y, Antonescu CR, Brennan MF, Martín-Broto J, Tang S, Rutkowski P, Kreis ME, Beyer K, Wang J, Bylina E, Sobczuk P, Gutierrez A, Jadeja B, Tap WD, Chi P, Singer S. Interpretable artificial intelligence to optimise use of imatinib after resection in patients with localised gastrointestinal stromal tumours: an observational cohort study. Lancet Oncol. 2024;25:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 63. | Viganò L, Jayakody Arachchige VS, Fiz F. Is precision medicine for colorectal liver metastases still a utopia? New perspectives by modern biomarkers, radiomics, and artificial intelligence. World J Gastroenterol. 2022;28:608-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 64. | Sun J, Huang L, Liu Y. Leveraging SEER data through machine learning to predict distant lymph node metastasis and prognosticate outcomes in hepatocellular carcinoma patients. J Gene Med. 2024;26:e3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 65. | Wang X, Chen J, Lin L, Li Y, Tao Q, Lang Z, Zheng J, Yu Z. Machine learning integrations develop an antigen-presenting-cells and T-Cells-Infiltration derived LncRNA signature for improving clinical outcomes in hepatocellular carcinoma. BMC Cancer. 2023;23:284. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Moaven O, Tavolara TE, Valenzuela CD, Cheung TT, Corvera CU, Cha CH, Stauffer JA, Niazi MKK, Gurcan MN, Shen P. Machine Learning Models for Predicting the Outcomes of Surgical Treatment of Colorectal Liver Metastases. J Am Coll Surg. 2023;236:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Vannucci M, Niyishaka P, Collins T, Rodríguez-Luna MR, Mascagni P, Hostettler A, Marescaux J, Perretta S. Machine learning models to predict success of endoscopic sleeve gastroplasty using total and excess weight loss percent achievement: a multicentre study. Surg Endosc. 2024;38:229-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 68. | Lopez-Lopez V, Maupoey J, López-Andujar R, Ramos E, Mils K, Martinez PA, Valdivieso A, Garcés-Albir M, Sabater L, Valladares LD, Pérez SA, Flores B, Brusadin R, Conesa AL, Cayuela V, Cortijo SM, Paterna S, Serrablo A, Sánchez-Cabús S, Gil AG, Masía JAG, Loinaz C, Lucena JL, Pastor P, Garcia-Zamora C, Calero A, Valiente J, Minguillon A, Rotellar F, Ramia JM, Alcazar C, Aguilo J, Cutillas J, Kuemmerli C, Ruiperez-Valiente JA, Robles-Campos R. Machine Learning-Based Analysis in the Management of Iatrogenic Bile Duct Injury During Cholecystectomy: a Nationwide Multicenter Study. J Gastrointest Surg. 2022;26:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Liu Z, Liu L, Weng S, Guo C, Dang Q, Xu H, Wang L, Lu T, Zhang Y, Sun Z, Han X. Machine learning-based integration develops an immune-derived lncRNA signature for improving outcomes in colorectal cancer. Nat Commun. 2022;13:816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 410] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 70. | Huang L, Li J, Zhu S, Wang L, Li G, Pan J, Zhang C, Lai J, Tian Y, Chen S. Machine learning-based prognostic prediction and surgical guidance for intrahepatic cholangiocarcinoma. Biosci Trends. 2025;18:545-554. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Bukhari I, Li M, Li G, Xu J, Zheng P, Chu X. Pinpointing the integration of artificial intelligence in liver cancer immune microenvironment. Front Immunol. 2024;15:1520398. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Wang X, Chen Y, Gao Y, Zhang H, Guan Z, Dong Z, Zheng Y, Jiang J, Yang H, Wang L, Huang X, Ai L, Yu W, Li H, Dong C, Zhou Z, Liu X, Yu G. Predicting gastric cancer outcome from resected lymph node histopathology images using deep learning. Nat Commun. 2021;12:1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 73. | Xia TY, Zhou ZH, Meng XP, Zha JH, Yu Q, Wang WL, Song Y, Wang YC, Tang TY, Xu J, Zhang T, Long XY, Liang Y, Xiao WB, Ju SH. Predicting Microvascular Invasion in Hepatocellular Carcinoma Using CT-based Radiomics Model. Radiology. 2023;307:e222729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 103] [Reference Citation Analysis (1)] |
| 74. | Saillard C, Schmauch B, Laifa O, Moarii M, Toldo S, Zaslavskiy M, Pronier E, Laurent A, Amaddeo G, Regnault H, Sommacale D, Ziol M, Pawlotsky JM, Mulé S, Luciani A, Wainrib G, Clozel T, Courtiol P, Calderaro J. Predicting Survival After Hepatocellular Carcinoma Resection Using Deep Learning on Histological Slides. Hepatology. 2020;72:2000-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 75. | Jin Z, Chen C, Zhang D, Yang M, Wang Q, Cai Z, Si S, Geng Z, Li Q. Preoperative clinical radiomics model based on deep learning in prognostic assessment of patients with gallbladder carcinoma. BMC Cancer. 2025;25:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 76. | Lee W, Park HJ, Lee HJ, Jun E, Song KB, Hwang DW, Lee JH, Lim K, Kim N, Lee SS, Byun JH, Kim HJ, Kim SC. Preoperative data-based deep learning model for predicting postoperative survival in pancreatic cancer patients. Int J Surg. 2022;105:106851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Huang TF, Luo C, Guo LB, Liu HZ, Li JT, Lin QZ, Fan RL, Zhou WP, Li JD, Lin KC, Tang SC, Zeng YY. Preoperative prediction of textbook outcome in intrahepatic cholangiocarcinoma by interpretable machine learning: A multicenter cohort study. World J Gastroenterol. 2025;31:100911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (1)] |
| 78. | Watson MD, Baimas-George MR, Murphy KJ, Pickens RC, Iannitti DA, Martinie JB, Baker EH, Vrochides D, Ocuin LM. Pure and Hybrid Deep Learning Models can Predict Pathologic Tumor Response to Neoadjuvant Therapy in Pancreatic Adenocarcinoma: A Pilot Study. Am Surg. 2021;87:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Bari H, Wadhwani S, Dasari BVM. Role of artificial intelligence in hepatobiliary and pancreatic surgery. World J Gastrointest Surg. 2021;13:7-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 80. | Ichimasa K, Kudo SE, Misawa M, Takashina Y, Yeoh KG, Miyachi H. Role of the artificial intelligence in the management of T1 colorectal cancer. Dig Liver Dis. 2024;56:1144-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 81. | Altaf A, Khalil M, Akabane M, Rashid Z, Kawashima J, Zindani S, Ruzzenente A, Aldrighetti L, Bauer TW, Marques HP, Martel G, Popescu I, Weiss MJ, Kitago M, Poultsides G, Maithel SK, Pulitano C, Shen F, Cauchy F, Koerkamp BG, Endo I, Pawlik TM. Textbook outcome in liver surgery for intrahepatic cholangiocarcinoma: defining predictors of an optimal postoperative course using machine learning. HPB (Oxford). 2025;27:402-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Bertsimas D, Margonis GA, Sujichantararat S, Boerner T, Ma Y, Wang J, Kamphues C, Sasaki K, Tang S, Gagniere J, Dupré A, Løes IM, Wagner D, Stasinos G, Macher-Beer A, Burkhart R, Morioka D, Imai K, Ardiles V, O'Connor JM, Pawlik TM, Poultsides G, Seeliger H, Beyer K, Kaczirek K, Kornprat P, Aucejo FN, de Santibañes E, Baba H, Endo I, Lønning PE, Kreis ME, Weiss MJ, Wolfgang CL, D'Angelica M. Using Artificial Intelligence to Find the Optimal Margin Width in Hepatectomy for Colorectal Cancer Liver Metastases. JAMA Surg. 2022;157:e221819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Tsilimigras DI, Mehta R, Moris D, Sahara K, Bagante F, Paredes AZ, Farooq A, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Grigorie R, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Utilizing Machine Learning for Pre- and Postoperative Assessment of Patients Undergoing Resection for BCLC-0, A and B Hepatocellular Carcinoma: Implications for Resection Beyond the BCLC Guidelines. Ann Surg Oncol. 2020;27:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Perez M, Palnaes Hansen C, Burdio F, Sanchez-Velázquez P, Giuliani A, Lancellotti F, de Liguori-Carino N, Malleo G, Marchegiani G, Podda M, Pisanu A, De Luca GM, Anselmo A, Siragusa L, Kobbelgaard Burgdorf S, Tschuor C, Cacciaguerra AB, Koh YX, Masuda Y, Hao Xuan MY, Seeger N, Breitenstein S, Grochola FL, Di Martino M, Secanella L, Busquets J, Dorcaratto D, Mora-Oliver I, Ingallinella S, Salvia R, Abu Hilal M, Aldrighetti L, Ielpo B. A machine learning predictive model for recurrence of resected distal cholangiocarcinoma: Development and validation of predictive model using artificial intelligence. Eur J Surg Oncol. 2024;50:108375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Mazaki J, Katsumata K, Ohno Y, Udo R, Tago T, Kasahara K, Kuwabara H, Enomoto M, Ishizaki T, Nagakawa Y, Tsuchida A. A Novel Prediction Model for Colon Cancer Recurrence Using Auto-artificial Intelligence. Anticancer Res. 2021;41:4629-4636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Liu Z, Liu Y, Zhang W, Hong Y, Meng J, Wang J, Zheng S, Xu X. Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation: a discovery and validation study. Hepatol Int. 2022;16:577-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 87. | Cao M, Hu C, Li F, He J, Li E, Zhang R, Shi W, Zhang Y, Zhang Y, Yang Q, Zhao Q, Shi L, Xu Z, Cheng X. Development and validation of a deep learning model for predicting gastric cancer recurrence based on CT imaging: a multicenter study. Int J Surg. 2024;110:7598-7606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 88. | Alaimo L, Lima HA, Moazzam Z, Endo Y, Yang J, Ruzzenente A, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Cauchy F, Koerkamp BG, Endo I, Kitago M, Pawlik TM. Development and Validation of a Machine-Learning Model to Predict Early Recurrence of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2023;30:5406-5415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Zhang XQ, Huang ZN, Wu J, Zheng CY, Liu XD, Huang YQ, Chen QY, Li P, Xie JW, Zheng CH, Lin JX, Zhou YB, Huang CM. Development and validation of a prognostic prediction model for elderly gastric cancer patients based on oxidative stress biochemical markers. BMC Cancer. 2025;25:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 90. | Liu Z, Guo C, Dang Q, Wang L, Liu L, Weng S, Xu H, Lu T, Sun Z, Han X. Integrative analysis from multi-center studies identities a consensus machine learning-derived lncRNA signature for stage II/III colorectal cancer. EBioMedicine. 2022;75:103750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 91. | Wu J, Liu S, Chen X, Xu H, Tang Y. Machine learning identifies two autophagy-related genes as markers of recurrence in colorectal cancer. J Int Med Res. 2020;48:300060520958808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Xia F, Chen Q, Liu Z, Zhang Q, Guo B, Fan F, Huang Z, Zheng J, Gao H, Xia G, Ren L, Mei H, Chen X, Cheng Q, Zhang B, Zhu P. Machine learning models for predicting postoperative peritoneal metastasis after hepatocellular carcinoma rupture: a multicenter cohort study in China. Oncologist. 2025;30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Zhang XQ, Huang ZN, Wu J, Liu XD, Xie RZ, Wu YX, Zheng CY, Zheng CH, Li P, Xie JW, Wang JB, He QC, Qiu WW, Tang YH, Zhang HX, Zhou YB, Lin JX, Huang CM. Machine Learning Prediction of Early Recurrence in Gastric Cancer: A Nationwide Real-World Study. Ann Surg Oncol. 2025;32:2637-2650. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 94. | Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Machine-learning analysis of contrast-enhanced CT radiomics predicts recurrence of hepatocellular carcinoma after resection: A multi-institutional study. EBioMedicine. 2019;50:156-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 95. | Sundar R, Barr Kumarakulasinghe N, Huak Chan Y, Yoshida K, Yoshikawa T, Miyagi Y, Rino Y, Masuda M, Guan J, Sakamoto J, Tanaka S, Tan AL, Hoppe MM, Jeyasekharan AD, Ng CCY, De Simone M, Grabsch HI, Lee J, Oshima T, Tsuburaya A, Tan P. Machine-learning model derived gene signature predictive of paclitaxel survival benefit in gastric cancer: results from the randomised phase III SAMIT trial. Gut. 2022;71:676-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Guo X, Chen M, Zhou L, Zhu L, Liu S, Zheng L, Chen Y, Li Q, Xia S, Lu C, Chen M, Chen F, Ji J. Predicting early recurrence in locally advanced gastric cancer after gastrectomy using CT-based deep learning model: a multicenter study. Int J Surg. 2025;111:2089-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 97. | Jiang Y, Zhang Z, Yuan Q, Wang W, Wang H, Li T, Huang W, Xie J, Chen C, Sun Z, Yu J, Xu Y, Poultsides GA, Xing L, Zhou Z, Li G, Li R. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. Lancet Digit Health. 2022;4:e340-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 98. | Chen B, Mao Y, Li J, Zhao Z, Chen Q, Yu Y, Yang Y, Dong Y, Lin G, Yao J, Lu M, Wu L, Bo Z, Chen G, Xie X. Predicting very early recurrence in intrahepatic cholangiocarcinoma after curative hepatectomy using machine learning radiomics based on CECT: A multi-institutional study. Comput Biol Med. 2023;167:107612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 99. | Yagi M, Yamamoto T, Iga T, Ogura Y, Suzuki S, Ozaki M, Takahashi Y, Tsuji O, Nagoshi N, Kono H, Ogawa J, Matsumoto M, Nakamura M, Watanabe K; Keio Spine Research Group. Development and Validation of Machine Learning-Based Predictive Model for Prolonged Hospital Stay after Decompression Surgery for Lumbar Spinal Canal Stenosis. Spine Surg Relat Res. 2024;8:315-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 100. | Mazaki J, Katsumata K, Ohno Y, Udo R, Tago T, Kasahara K, Kuwabara H, Enomoto M, Ishizaki T, Nagakawa Y, Tsuchida A. A Novel Predictive Model for Anastomotic Leakage in Colorectal Cancer Using Auto-artificial Intelligence. Anticancer Res. 2021;41:5821-5825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Sunakawa T, Kitaguchi D, Kobayashi S, Aoki K, Kujiraoka M, Sasaki K, Azuma L, Yamada A, Kudo M, Sugimoto M, Hasegawa H, Takeshita N, Gotohda N, Ito M. Deep learning-based automatic bleeding recognition during liver resection in laparoscopic hepatectomy. Surg Endosc. 2024;38:7656-7662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Chen B, Sheng W, Wu Z, Ma B, Cao N, Li X, Yang J, Yuan X, Yan L, Zhu G, Zhou Y, Huang Z, Zhu M, Ding X, Du H, Wan Y, Gao X, Cheng X, Xu P, Zhang T, Tao K, Shuai X, Cheng P, Gao Y, Zhang J. Machine learning based peri-surgical risk calculator for abdominal related emergency general surgery: a multicenter retrospective study. Int J Surg. 2024;110:3527-3535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 103. | Wang J, Tozzi F, Ashraf Ganjouei A, Romero-Hernandez F, Feng J, Calthorpe L, Castro M, Davis G, Withers J, Zhou C, Chaudhary Z, Adam M, Berrevoet F, Alseidi A, Rashidian N. Machine learning improves prediction of postoperative outcomes after gastrointestinal surgery: a systematic review and meta-analysis. J Gastrointest Surg. 2024;28:956-965. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 104. | Endo Y, Tsilimigras DI, Munir MM, Woldesenbet S, Guglielmi A, Ratti F, Marques HP, Cauchy F, Lam V, Poultsides GA, Kitago M, Alexandrescu S, Popescu I, Martel G, Gleisner A, Hugh T, Aldrighetti L, Shen F, Endo I, Pawlik TM. Machine learning models including preoperative and postoperative albumin-bilirubin score: short-term outcomes among patients with hepatocellular carcinoma. HPB (Oxford). 2024;26:1369-1378. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Pera M, Gibert J, Gimeno M, Garsot E, Eizaguirre E, Miró M, Castro S, Miranda C, Reka L, Leturio S, González-Duaigües M, Codony C, Gobbini Y, Luna A, Fernández-Ananín S, Sarriugarte A, Olona C, Rodríguez-Santiago J, Osorio J, Grande L; Spanish EURECCA Esophagogastric Cancer Group. Machine Learning Risk Prediction Model of 90-day Mortality After Gastrectomy for Cancer. Ann Surg. 2022;276:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 106. | Zhu Z, Hu K, Zhao F, Liu W, Zhou H, Zhu Z, Li H. Machine learning-based nomogram for 30-day mortality prediction for patients with unresectable malignant biliary obstruction after ERCP with metal stent: a retrospective observational cohort study. BMC Surg. 2023;23:260. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Jin Y, Li W, Wu Y, Wang Q, Xiang Z, Long Z, Liang H, Zou J, Zhu Z, Dai X. Online interpretable dynamic prediction models for clinically significant posthepatectomy liver failure based on machine learning algorithms: a retrospective cohort study. Int J Surg. 2024;110:7047-7057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 108. | Chen Q, Chen J, Deng Y, Bi X, Zhao J, Zhou J, Huang Z, Cai J, Xing B, Li Y, Li K, Zhao H. Personalized prediction of postoperative complication and survival among Colorectal Liver Metastases Patients Receiving Simultaneous Resection using machine learning approaches: A multi-center study. Cancer Lett. 2024;593:216967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 109. | Kang CM, Ku HJ, Moon HH, Kim SE, Jo JH, Choi YI, Shin DH. Predicting Safe Liver Resection Volume for Major Hepatectomy Using Artificial Intelligence. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Gong M, Jiang Y, Sun Y, Liao R, Liu Y, Yan Z, He A, Zhou M, Yang J, Wu Y, Wu Z, Huang Z, Wu H, Jiang L. Knowledge domain and frontier trends of artificial intelligence applied in solid organ transplantation: A visualization analysis. Int J Med Inform. 2025;195:105782. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 111. | Briceño J, Calleja R, Hervás C. Artificial intelligence and liver transplantation: Looking for the best donor-recipient pairing. Hepatobiliary Pancreat Dis Int. 2022;21:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 112. | Yu YD, Lee KS, Man Kim J, Ryu JH, Lee JG, Lee KW, Kim BW, Kim DS; Korean Organ Transplantation Registry Study Group. Artificial intelligence for predicting survival following deceased donor liver transplantation: Retrospective multi-center study. Int J Surg. 2022;105:106838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 113. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 114. | Jiang L, Wang J, Wang Y, Yang H, Kong L, Wu Z, Shen A, Huang Z, Jiang Y. Bibliometric and LDA analysis of acute rejection in liver transplantation: Emerging trends, immunotherapy challenges, and the role of artificial intelligence. Cell Transplant. 2025;34:9636897251325628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 115. | Sha M, Wang J, Cao J, Zou ZH, Qu XY, Xi ZF, Shen C, Tong Y, Zhang JJ, Jeong S, Xia Q. Criteria and prognostic models for patients with hepatocellular carcinoma undergoing liver transplantation. Clin Mol Hepatol. 2025;31:S285-S300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 116. | Ferrarese A, Sartori G, Orrù G, Frigo AC, Pelizzaro F, Burra P, Senzolo M. Machine learning in liver transplantation: a tool for some unsolved questions? Transpl Int. 2021;34:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 117. | Zabara ML, Popescu I, Burlacu A, Geman O, Dabija RAC, Popa IV, Lupascu C. Machine Learning Model Validated to Predict Outcomes of Liver Transplantation Recipients with Hepatitis C: The Romanian National Transplant Agency Cohort Experience. Sensors (Basel). 2023;23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 118. | Briceño J, Ayllón MD, Ciria R. Machine-learning algorithms for predicting results in liver transplantation: the problem of donor-recipient matching. Curr Opin Organ Transplant. 2020;25:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Morita-Nakagawa M, Okamura K, Nakabayashi K, Inanaga Y, Shimizu S, Guo WZ, Fujino M, Li XK. Supervised machine learning of outbred mouse genotypes to predict hepatic immunological tolerance of individuals. Sci Rep. 2024;14:24399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Wingfield LR, Ceresa C, Thorogood S, Fleuriot J, Knight S. Using Artificial Intelligence for Predicting Survival of Individual Grafts in Liver Transplantation: A Systematic Review. Liver Transpl. 2020;26:922-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 121. | Rashidian N, Abu Hilal M, Frigerio I, Guerra M, Sterckx S, Tozzi F, Capelli G, Verdi D, Spolverato G, Gulla A, Ratti F, Healey AJ, Esposito A, De Pastena M, Belli A, Bouwense SA, Apostolos A, Lang SA, López-López V, Stavrou GA, Aldrighetti L, Strobel O, Croner R, Gumbs AA; E-AHPBA Innovation Committee. Ethics and trustworthiness of artificial intelligence in Hepato-Pancreato-Biliary surgery: a snapshot of insights from the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) survey. HPB (Oxford). 2025;27:502-510. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Ahmed F, Jahagirdar V, Gudapati S, Mouchli M. Three-dimensional visualization and virtual reality simulation role in hepatic surgery: Further research warranted. World J Gastrointest Surg. 2022;14:723-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Parikh M, Tejaswi S, Girotra T, Chopra S, Ramai D, Tabibian JH, Jagannath S, Ofosu A, Barakat MT, Mishra R, Girotra M. Use of Artificial Intelligence in Lower Gastrointestinal and Small Bowel Disorders: An Update Beyond Polyp Detection. J Clin Gastroenterol. 2025;59:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 124. | Shuaib A. Transforming Healthcare with AI: Promises, Pitfalls, and Pathways Forward. Int J Gen Med. 2024;17:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |