Published online Dec 8, 2023. doi: 10.35712/aig.v4.i3.48
Peer-review started: July 27, 2023
First decision: August 31, 2023
Revised: September 11, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: December 8, 2023
Processing time: 132 Days and 17.8 Hours
Artificial intelligence (AI) has been used in various fields of day-to-day life and its role in medicine is immense. Understanding of oncology has been improved with the introduction of AI which helps in diagnosis, treatment planning, management, prognosis, and follow-up. It also helps to identify high-risk groups who can be subjected to timely screening for early detection of malignant conditions. It is more important in pancreatic cancer as it is one of the major causes of cancer-related deaths worldwide and there are no specific early features (clinical and radiological) for diagnosis. With improvement in imaging modalities (computed tomography, magnetic resonance imaging, endoscopic ultrasound), most often clinicians were being challenged with lesions that were difficult to diagnose with human competence. AI has been used in various other branches of medicine to differentiate such indeterminate lesions including the thyroid gland, breast, lungs, liver, adrenal gland, kidney, etc. In the case of pancreatic cancer, the role of AI has been explored and is still ongoing. This review article will focus on how AI can be used to diagnose pancreatic cancer early or differentiate it from benign pancreatic lesions, therefore, management can be planned at an earlier stage.
Core Tip: Surgical management of a pancreatic head lesion usually requires pancreaticoduodenectomy, which is associated with significant morbidity and mortality. For a benign lesion it is unacceptable. The investigation modalities i.e. computed tomography, magnetic resonance imaging, endoscopic ultrasound, positron emission tomography, and biochemical markers are available today to distinguish benign from malignant lesions and have their limitations (human judgmental errors). The application of artificial intelligence (AI) algorithms can minimize human errors and improve the sensitivity and specificity of diagnostic yield. The AI can help with great precision in differentiating benign from malignant lesions, affecting the management strategy and minimizing post-operative complications.
- Citation: Rawlani P, Ghosh NK, Kumar A. Role of artificial intelligence in the characterization of indeterminate pancreatic head mass and its usefulness in preoperative diagnosis. Artif Intell Gastroenterol 2023; 4(3): 48-63
- URL: https://www.wjgnet.com/2644-3236/full/v4/i3/48.htm
- DOI: https://dx.doi.org/10.35712/aig.v4.i3.48
The concept of a machine that can think like a human being was proposed by Mr. Alan Turing in the year 1950 in his book entitled “Computing Machinery and Intelligence” and later, the term “artificial intelligence (AI)” was coined by John McCarthy[1,2]. The applicability of AI ranges from simple tasks to more complex tasks mimicking a human brain. There are six major sub-fields of AI: machine learning (ML), neural network, deep learning (DL), natural language processing (NLP), cognitive computing, and computer vision. ML can learn from data, recognize typical patterns, and make decisions with little or no human interference. A neural network is the field of AI that is inspired by the human brain, where a set of algorithms is used to derive a correlation. Most of the AI models in the medical field use ML and neural networks. NLP is a method where textual data has been used to search, analyze, and comprehend complex information. Computer vision understands visual inputs (radiological or pathological images, surgical videos) and derives desired information. There are many modifications of conventional sub-fields of AI which have been in use. The twentieth century has seen that AI has become an essential part of day-to-day life, including health tracking devices[3], automobiles[4], banking and finances (robo-traders)[5], surveillance, social media, entertainment, education, space exploration, and disaster management, etc[6,7].
AI has been used in various fields of medicine including online appointments and hospital check-ins, medical records digitalization, follow-up, drug dosage reminders, adverse effect warnings, etc. Moreover, its application in the field of oncology is paramount. AI can be useful in cancer detection, screening, diagnosis, classification, prognostication, new drug discovery, etc[8-11]. It has played its role in differentiating various indeterminate lesions in the thyroid gland[12,13], breast[14], lungs[15,16], liver[17], adrenal[18,19], kidneys[20], and indeterminate biliary strictures[21] (Table 1). Various authors have studied the role of AI algorithms to identify pancreatic lesions from imaging modalities computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS), positron emission tomography (PET) scan, etc and thus can differentiate malignant indeterminate pancreatic lesions (IPLs) from benign ones for better management at an early stage.
| No. | Ref. | Number of patients | Organ of interest | Sub-type of AI | Outcome |
| 1 | Ippolito et al[12], 2004 | 453 | Thyroid nodule (benign vs malignant) | ANN | Refinement of risk stratification of FNAB and clinical data |
| 2 | Daniels et al[13], 2020 | 121 | Indeterminant thyroid nodule | ML | ML and ultrasonography can identify genetically high risk lesions |
| 3 | Becker et al[14], 2018 | 632 | Breast lesion (benign vs malignant) | Generic DLS | Aids diagnosing cancer on breast ultrasound images with an accuracy comparable to radiologists |
| 4 | Scott et al[15], 2019 | 125 | Lung GGO (benign vs malignant) | ANN | Improve diagnostic ability using CT scan, PET, and clinical data |
| 5 | Guo et al[16], 2022 | 20 | Indeterminant small lung lesions | DNN | DNN based method may detect small lesions < 10 mm at an effective radiation dose < 0.1 mSv. |
| 6 | Yasaka et al[17], 2018 | 460 | Liver mass (HCC vs others) | CNN | High diagnostic performance in differentiation of liver masses using dynamic CT |
| 7 | Moawad et al[18], 2021 | 40 | Adrenal incidentaloma (benign vs malignant) | ML | Machine learning and CT texture analysis can differentiate between benign and malignant indeterminate adrenal tumors |
| 8 | Stanzione et al[19], 2021 | 55 | Indeterminant solid adrenal lesions | ML | MRI handcrafted radiomics and ML can be used to different adrenal incidentalomas |
| 9 | Massa'a et al[20], 2022 | 160 | Indeterminant solid renal mass (benign vs malignant) | ML | MRI-based radiomics and ML can be useful in differentiation |
| 10 | Saraiva et al[21], 2022 | 85 | Indeterminant biliary strictures | CNN | CNN can accurately differentiate benign strictures from malignant ones |
IPLs are those detected by imaging techniques performed for non-specific abdominal complaints or detected incidentally, otherwise known as pancreatic incidentaloma. With the increase in imaging modalities, the detection of such IPLs has increased[22]. These incidentalomas are mostly detected in other organs, i.e. the thyroid gland, pituitary gland, kidney, lungs, adrenal gland, etc. Though, the incidence of indeterminate lesions is less in the pancreas, however, most of them are malignant compared to other sites[23]. Identification of such lesions creates confusion in clinicians and anxiety among the patients. Moreover, early diagnosis of malignancy can provide reasonably early management and better overall outcomes. Therefore, it is necessary to diagnose such lesions for better patient management.
The overall prevalence of such lesions was reported to be 0.01%–0.6% in 2009, which may be less compared to its true incidence[24]. A review of a series of pancreatic resections shows an asymptomatic neoplastic lesion to be 6%-23% (24% to 50% of them are malignant, and 24% to 47% are considered potentially malignant or pre-malignant)[25,26]. A recently published Leopard-2 trial comparing laparoscopic and open pancreaticoduodenectomy has shown the incidence of benign or pre-malignant lesions to be 12%[27]. Frequently, cystic lesions of the pancreas are detected on MRI and their incidence is up to 20%[28] and recent series shows the incidence to be 49% in the general population[29]. The majority of cystic lesions are benign, however, approximately, 3% are malignant or potentially malignant[30].
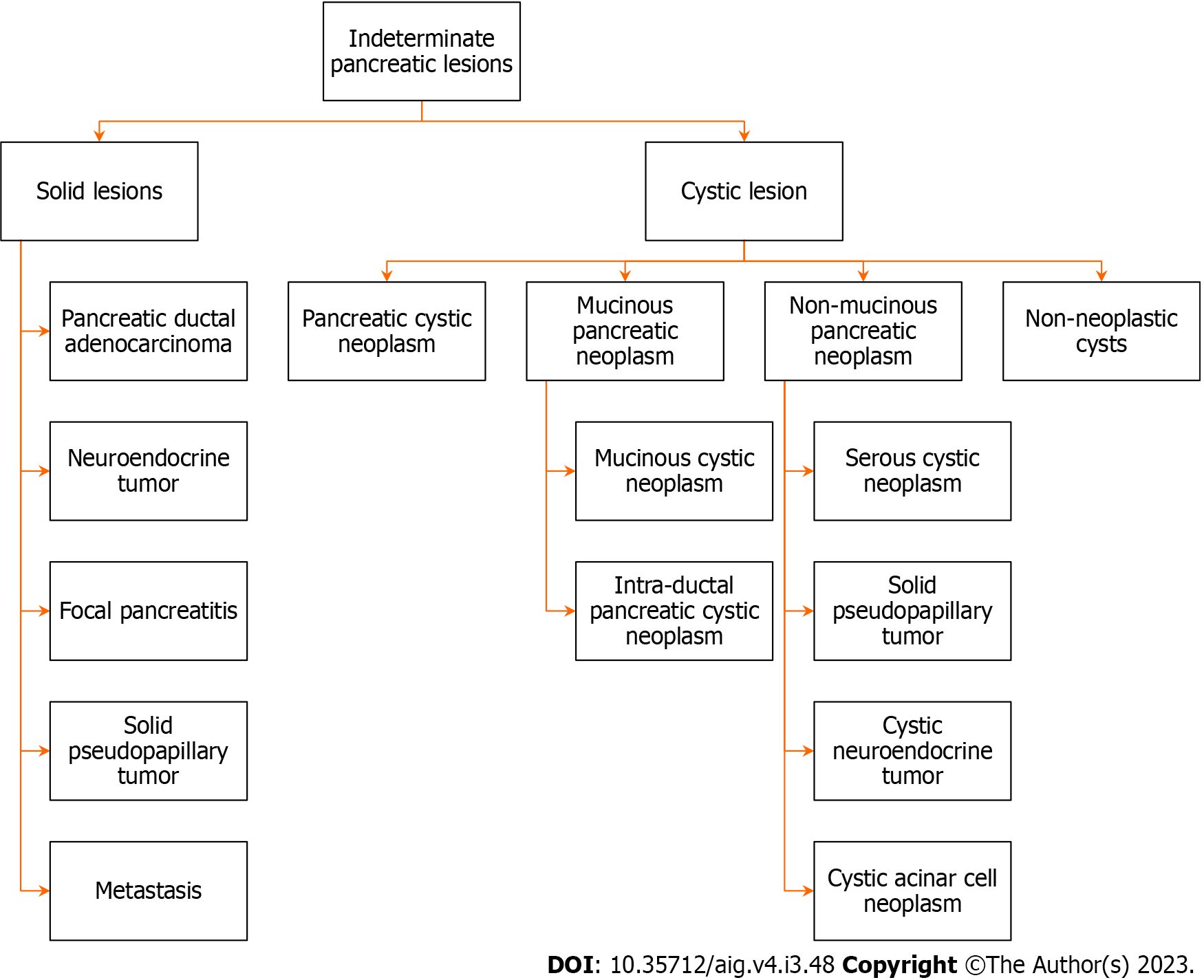
The etiology of such lesions is diverse, benign adenoma to adenocarcinoma, borderline malignant tumors, mesenchymal tumors, neuroendocrine tumors, cysts, congenital changes, metastatic lesions, inflammatory masses etc[23]. These lesions may be broadly divided into benign, pre-malignant, or malignant lesions[24]. Figure 1 shows different pathologies of IPLs[31].
There is a considerable overlap of imaging features of different benign and malignant pancreatic lesions. Cystic degeneration of solid tumors may masquerade as cystic lesions. Various modalities (ultrasonography, contrast-enhanced CT, MRI, EUS, PET, cytopathology, histopathology, and tumor markers) have been used to differentiate the possible etiology, however, there are limitations of each modality intrinsic to the investigation itself or on the operator. Recently, AI has been used to distinguish various indeterminate lesions in the breast, lungs, adrenal gland, kidney, etc. Thus, the use of AI in association with conventional imaging or diagnostic modalities can improve their overall diagnostic yield and therefore, more precise diagnosis and patient care.
This paper reviews the current status of AI in the differentiation of various IPLs and its future implications.
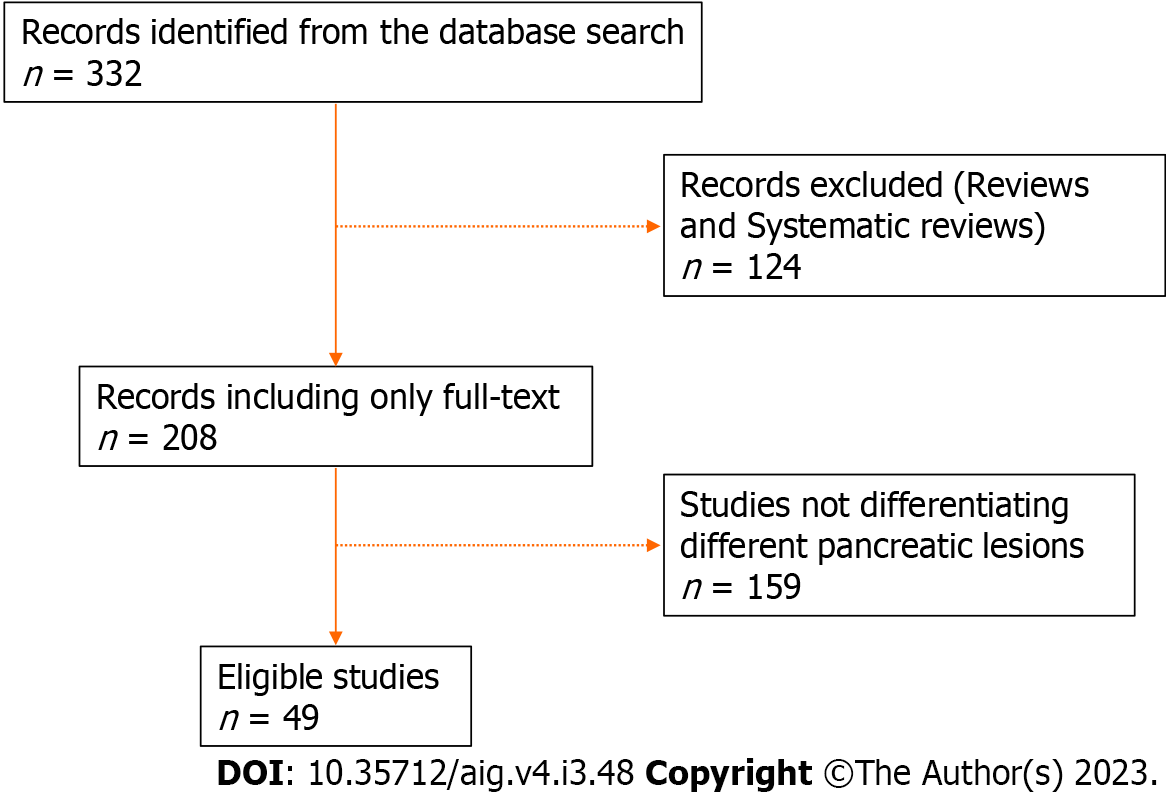
All the relevant articles were searched from PubMed and Google Scholar using the keywords, i.e. “artificial intelligence” AND “pancreatic lesions” OR “cystic lesions”, OR “CT”, OR “MRI”, OR “EUS”, OR “PET” OR “pathology”, OR “biomarkers” between 2005 and 2023, and only full articles were studied. Articles discussing the differentiation of different types of pancreatic lesions were included and screened by all authors. Abstracts and conference presentations were excluded. Studies discussing the differentiation of any pancreatic lesion (benign vs. malignant) were included in relevant sections for discussion. The study flow chart is shown in Figure 2.
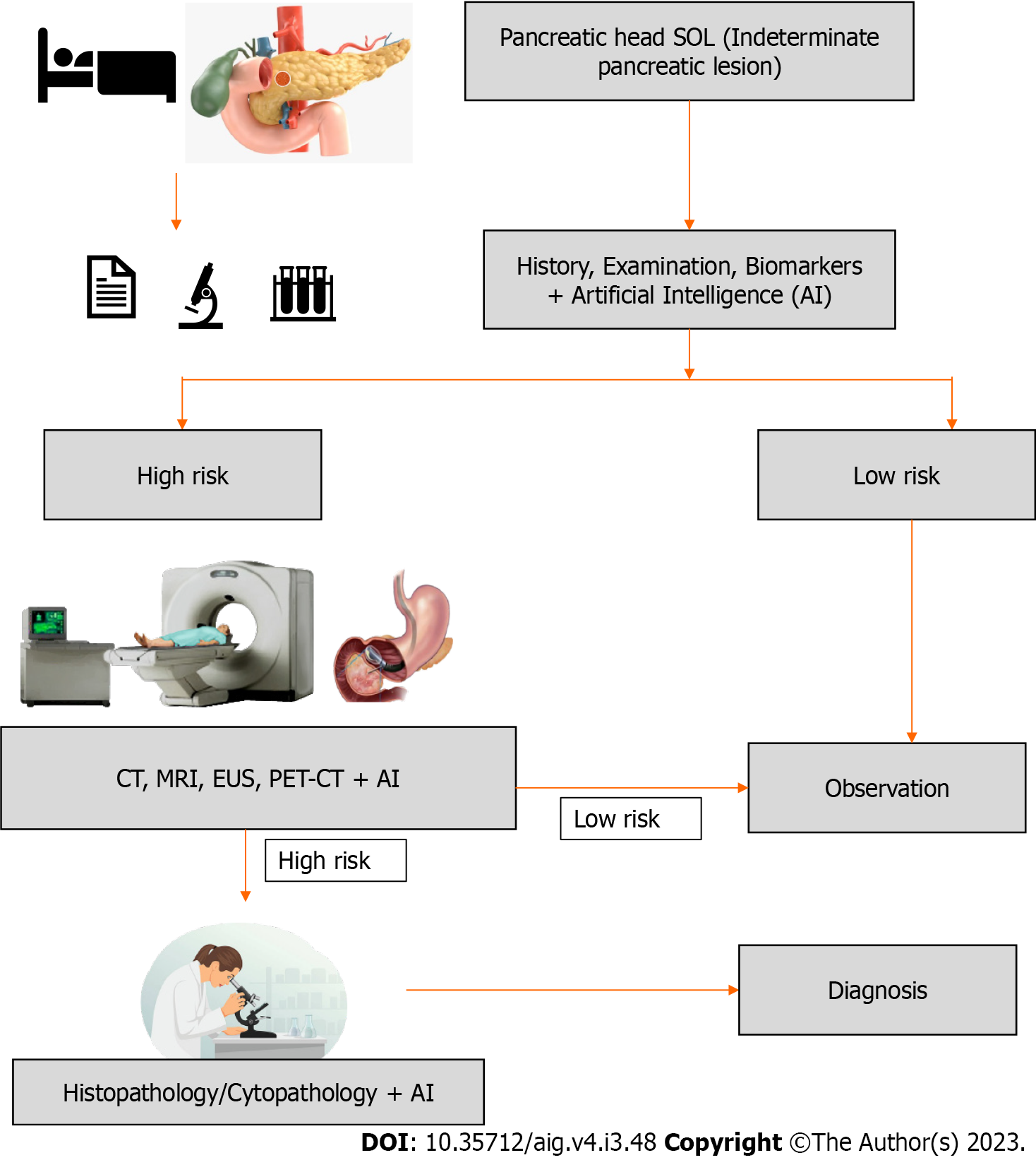
Pancreatic cancer is one of the leading causes of cancer-related death worldwide, thus early diagnosis is crucial for better management. Often, patients are asymptomatic to start with, so presentation is delayed leading to advanced disease at diagnosis. This delay in diagnosis can be minimized by the identification of high-risk groups and the introduction of targeted screening of high-risk populations. Any lesion identified in these patient groups can be subjected to further evaluation using an AI augmented imaging system (CT, MRI, PET, EUS), which will be discussed later. The proposed schema of patient evaluation and management is presented in Figure 3.
Several clinical parameters can be used to predict the future incidence of pancreatic cancer including, symptoms, hereditary factors (BRCA1, BRCA2, PALB2, Hereditary pancreatitis, and Peutz-Jeghers Syndrome), pre-existing clinical conditions (new-onset diabetes mellitus), lifestyle (smoking, alcohol, obesity, nutrient-poor diet), and demographic factors. Elevation of CA 19-9, CEA, and recently developed CEMIP (cell migration-inducing hyaluronan binding protein) can be considered as an early indicator of pancreatic cancer[32-34]. None of these parameters can confirm pancreatic cancer, however, a combined assessment can suggest a possible pancreatic cancer leading to screening of high-risk populations. In a retrospective study from Kaiser Permanente Southern California, an algorithm for risk stratification for pancreatic cancer was generated using imaging (CT/magnetic resonance) and clinical factors[35]. In this study, imaging features used were pancreatic duct dilatation as a predictor of malignancy and other features such as atrophy, calcification, pancreatic cyst, and irregular pancreatic duct. Multi-state prediction model showed a discriminatory index (c-index: 0.825–0.833) between normal individuals and individuals with pancreatic cancer. A study at the Biomedical Imaging Research Institute of Cedars Sinai Medical Center, Los Angeles used ML and CT-based radiomic features as an indicator of pancreatic ductal adenocarcinoma (PDAC)[36]. The scans were obtained in non-pancreatic cancer patients for different purposes, who later developed pancreatic cancer after 6 mo to 3 years. The AI model had an accuracy of 86% in the prediction of PDAC. As CT scans were performed frequently for different purposes, such AI models can identify patients having potential risk for future pancreatic malignancy.
Muhammad et al[37], Placido et al[38], and Chen et al[39] used demographic and clinical parameters with artificial neural networks (ANNs) algorithms to predict pancreatic cancer. In the validation arm, the area under the curve (AUC) was 0.85, and the sensitivity and specificity of diagnosis were 80.7%. Malhotra et al[40] used ML principles to identify symptoms to predict pancreatic cancer. Their algorithm could detect 41.3% of patients with pancreatic cancer < 60 years of age, 20 mo earlier than diagnosis (AUC: 0.66), and 43.2% of patients with pancreatic cancer > 60 years of age, 17 mo earlier than diagnosis (AUC: 0.61). Appelbaum et al[41] used neural network algorithms to identify high-risk groups 1 year in advance. Thus, these AI techniques not only help to detect pancreatic cancer but also, earlier than conventional imaging.
If a mass lesion is detected in the pancreas, the possibility of neoplasm is kept as a differential diagnosis. The most common (85%–95%) among the lesions is pancreatic ductal adenocarcinoma (PDAC) and it has a poor prognosis[42,43]. Ill-defined hypovascular mass is the characteristic of PDAC in contrast-enhanced imaging[44]. Atypical imaging of a solid mass may harbor a malignancy, however, its mimic, an inflammatory mass, can have a better prognosis than PDAC, and management of both these conditions is different.
Among all the imaging modalities, CT is most commonly favored for the investigation of a pancreatic lesion, as it is widely available, quick to acquire, has a high spatial resolution, assesses relationship to vascular structures, and determines surgical planning. Recent advances in CT imaging in the form of multiplanar reformatted images, and three-dimensional (3D) techniques have improved sensitivity by up to 96% in tumor identification[45,46]. However, small tumors or tumors with atypical features may not be visible on CT scans or subtle changes may not be appreciable to the human eye and prone to errors. These limitations of conventional CT imaging can be overcome by the use of AI algorithms.
Among all malignancies, PDAC has the worst overall survival[47]. It is because patients present late at an advanced stage due to late detection of asymptomatic subtle pancreatic lesions on imaging[40]. Zhu et al[48] and Liu et al[49] have used DL to detect pancreatic cancer and in the study by Liu et al[49], malignancy could be detected in just 3 s with an AUC of 0.96. Chu et al[50] could diagnose PDAC with an AUC of 99.9% using ML algorithms.
With the increase in the frequency of cross-sectional imaging, the detection of cystic lesions of the pancreas has increased and it is aptly called “technopathies”. Management of these cystic lesions requires classification of the type of lesion and the risk of malignancy which is sub-optimal with present imaging modalities[51,52]. AI has been used to differentiate the types of cystic lesions into, intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), serous cystic neoplasia (SCN), solid pseudopapillary neoplasia, etc[53,54]. Dmitriev et al[53] used the convolutional neural network (CNN) model (contrast-enhanced CT and clinical data) to differentiate the types of cystic lesions with an accuracy of 84% which is better than radiologists which has an accuracy of less than 70%[53,55]. However, Li et al[54] used only CT images and AI (DL) to differentiate the cystic lesions with an accuracy of 73% compared to radiologists in their study which had an accuracy of only 48%. Differentiation of SCN from other cystic lesions is important as they have a rare chance of being malignant, thus, Wei et al[56] used an ML-based algorithm to distinguish SCN from others based on CT images. Yang et al[57] and Chen et al[58] have used AI algorithms to distinguish SCN from MCN. Chakraborty et al[59] and Polk et al[60] used the RF model to differentiate low-grade IPMN from high-grade IPMN which has management implications. Table 2 summarizes studies on the uses of AI along with CT images in the differentiation of pancreatic lesions.
| No. | Ref. | Number of patients | Primary objective | Sub-type of AI used | Outcome |
| 1 | Qureshi et al[36], 2022 | 108 | Identification of PDAC | ML | Accuracy: 86% |
| 2 | Ebrahimian et al[121], 2022 | 103 | Differentiation of benign vs malignant pancreatic lesions | RF | AUC: 0.94 |
| 3 | Chakraborty et al[59], 2018 | 103 | High risk vs low risk IPMN | RF, SVM | AUC: 0.81 |
| 4 | Polk et al[60], 2020 | 29 | High risk vs low risk IPMN | LR | AUC: 0.90 |
| 5 | Ikeda et al[122], 1997 | 71 | PDAC vs pancreatitis | NN | AUC: 0.916 |
| 6 | Chen et al[58], 2021 | 100 | SCN vs MCN | LASSO and RFE_Linear SVC | AUC: 0.932 |
| 7 | Yang et al[57], 2019 | 53 | SCN vs MCN | LASSO | AUC: 0.66 |
| 8 | Yang et al[123], 2022 | 63 | SCN vs MCN | MMRF-ResNet | AUC: 0.98 |
| 9 | Ren et al[124], 2020 | 112 | PDAC vs pancreatic adenosquamous carcinoma | RF | AUC: 0.98 |
| 10 | Xie et al[125], 2021 | 226 | MCN vs ASCN | RF | AUC: 0.734 |
| 11 | Ziegelmayer et al[126], 2020 | 86 | AIP vs PDAC | CNN, ML | AUC: 0.90 |
| 12 | Li et al[62], 2022 | 97 | Focal-type AIP vs PDAC | LASSO regression | AUC: 0.97 |
| 13 | Gao et al[127], 2021 | 170 | MCN vs SCN | mRMR + LASSO | AUC: 0.91 |
| 14 | Dmitriev et al[53], 2017 | 134 | Classification of pancreatic cyst | RF, CNN | Accuracy: 83.6% |
| 15 | Li et al[54], 2019 | 206 | Classification of pancreatic cysts | DNN (Dense-Net) | Accuracy: 72.8% |
| 16 | Wei et al[56], 2019 | 260 | SCN vs other cystic neoplasms | ML | AUC: 0.767 |
MRI is favored over CT scan due to superior soft tissue delineation and it also helps to detect small lesions, assessment of the vascular relationship, and relationship to the pancreatic duct, lymph node, or distant metastasis[43,61]. Detection of iso-attenuating pancreatic lesions on CT scan is challenging which is observed in approximately 10% of patients. In these situations, indirect evidence of malignancy is used for diagnosis, i.e. convex pancreatic contour, double duct sign, vascular involvement, mass effect, etc[42]. However, MRI can be helpful to diagnose such lesions. Recently, the use of AI algorithms has improved the diagnostic ability of MRI. Li et al[62] and Chen et al[63] used AI algorithms for the identification of PDAC on different phases of MRI (Table 3).
| No. | Ref. | Number of patients | Primary objective | Sub-type of AI used | Outcome |
| 1 | Li et al[62], 2022 | 267 | PDAC detection | UDA + meta learning + GCN | DSC (62.08%, T1), (61.35%, T2), (61.88%, DWI), (60.43%, AP) |
| 2 | Chen et al[63], 2022 | 73 | PDAC detection | Spiral-ResUNet | DSC: 65.60%, Jaccard index: 49.64% |
| 3 | Liang Y et al[128], 2020 | 56 | PDAC detection | CNN | DSC: 71% |
| 5 | Cui et al[129], 2021 | 202 | Grading-BD IPMN | LASSO | AUC: 0.903 |
| 6 | Corral et al[67], 2019 | 139 | Classification of IPMN | CNN | AUC: 0.783 |
| 7 | Cheng et al[68], 2022 | 60 | Malignant IPMN | LR, SVM | MRI + SVM: AUC: 0.940, CT + SVM: AUC: 0.864 |
| 8 | Hussein et al[130], 2019 | 171 | Classification of IPMN | SVM, RF, 3D, CNN | Accuracy 84.22% |
Management of cystic lesions depends upon the precise characterization, which indicates its clinical behavior[64]. However, overlapping imaging features make differentiation challenging[64]. The role of imaging is to differentiate benign from malignant cystic neoplasms. MRI uses T2 images to identify ductal communication and post-contrast images to characterize the lesion. It is limited in the detection of calcifications which is better appreciated on a CT image. MRI can differentiate benign from malignant lesions with an accuracy of 73% to 81% compared to a CT scan which has an accuracy of 75% to 78%[52,65,66].
The use of AI has enabled MRI to detect high-grade dysplasia or malignancy in IPMN with a sensitivity and specificity of 75% and 78%, respectively[67]. Corral et al[67] used 3D CNN to classify IPMN into different types with an accuracy of 58%. Interestingly, Cheng et al[68] compared radiomics features of CT and MRI using AL algorithms [LASSO, LR, support vector machine (SVM)] and found out that, the MRI-based model(AUC: 0.940) had better diagnostic ability than the CT based model(AUC: 0.864). Studies on the use of AI with MRI to detect the type of cystic or solid pancreatic lesions are presented in Table 3.
EUS uses a high-frequency transducer at the tip of an endoscope. It helps to obtain high-resolution images of the pancreas through the esophagus, stomach, or duodenum. Various modalities of EUS including contrast-enhanced EUS, EUS-guided fine needle aspiration (FNA), and EUS elastography have been used for the evaluation of pancreatic cancer, detection of small lesions, differentiation of solid from cystic tumors, and assessment of resectability[69]. Most importantly, it helps to obtain tissue for cytopathology or histopathology[70,71]. The main drawback is operator dependency, which may reduce the diagnostic yield[72,73]. AI algorithms have been used in association with EUS to detect pancreatic cancers and to differentiate from other lesions (Table 4). Mass-forming chronic pancreatitis may masquerade as pancreatic malignancy, EUS based AI algorithms can be used to distinguish pancreatic cancer from chronic pancreatitis.
| No. | Ref. | Number of patients | Primary outcome | Sub type of AI used | Outcome |
| 1 | Zhu et al[78], 2013 | 262 | PDAC vs CP | SVM | Accuracy: 94.2% |
| 2 | Zhu et al[131], 2015 | 100 | AIP vs CP | SVM | Accuracy: 89.3% |
| 3 | Zhang et al[74], 2010 | 216 | Normal pancreas vs PDAC | SVM | Accuracy: 97.98% |
| 4 | Ozkan et al[76], 2016 | 332 | Recognition of pancreatic cancer amongst various age group | ANN | Accuracy: Average: 87.5% (all ages), Min: 88.46% (40-60 yr), Max: 92% (< 40 yr) |
| 5 | Kuwahara et al[83], 2019 | 50 | Benign vs malignant IPMN | CNN | Accuracy: 94% |
| 6 | Das et al[75], 2008 | 56 | PDAC vs normal pancreas vs CP | ANN | AUC: 0.93 |
| 7 | Săftoiu et al[80], 2008 | 68 | Benign vs malignant pancreatic lesion | ANN | Accuracy: 89.7% |
| 8 | Tonozuka et al[132], 2021 | 139 | PDAC vs CP | CNN | AUC: 0.94 |
| 9 | Marya et al[133], 2021 | 583 | PDAC vs benign causes of pancreatic SOL | CNN | AUC: 0.976 |
| 10. | Xu et al[134], 2013 | Systemic Analysis of 6 studies | Benign vs malignant pancreatic lesion | - | AUC: 0.962 |
Authors have used ML algorithms to differentiate normal pancreatic tissue from PDAC with more than 93% accuracy[74-76]. Two studies have used AI to distinguish chronic pancreatitis from PDAC on EUS images with an accuracy of more than 80%[77,78]. Săftoiu et al[79] demonstrated better diagnostic ability of contrast-enhanced EUS (94.6% and a specificity of 94.4%) compared to EUS-FNA (87.5% and 92.7%) in differentiating CP from PDAC using AI.
Recently, EUS elastography has been used to diagnose focal pancreatic lesions. Using ANN, it can differentiate benign from malignant lesions with an accuracy of 95%[80]. In another multicenter prospective study using ANN, they demonstrated that EUS elastography (sensitivity (87.6%) and specificity (82.9%)) had better diagnostic ability than two experienced endoscopists combined (sensitivity 80.0%, specificity 50.0%)[81]. Udriştoiu et al[82] used ML principles to distinguish focal pancreatitis from pancreatic mass (neuroendocrine tumor or PDAC) with an accuracy of 98.26%. Differentiation of benign IPMN from malignant IPMN has management implications, Kuwahara et al[83] studied to detect malignant IPMN using CNN (ResNet-50).
PET is a functional imaging technique used for staging malignant lesions and is based on the physiological characteristics of tumor cells[84,85]. However, inflammation may mimic a malignant lesion due to high metabolic activity giving rise to false positive results, conversely, in patients with hyperglycemia, it can give a false negative result[86,87]. PET CT is also useful in the assessment of tumor response to therapy[43]. Li et al[88] used a hybrid feedback-SVM-random forest model to detect pancreatic cancer from a normal pancreas with an accuracy of 96.47%. Liu et al[89] studied the role of dual time PET/CT and SVM model to differentiate PDAC from AIP with an AUC of 0.96 similarly, Xing et al[90] showed a diagnostic performance of 0.93 of AUC.
Often, imaging cannot achieve an accurate diagnosis, requiring a tissue diagnosis-cytology or histology[91,92]. AI can be applied to hematoxylin-eosin-stained slides for the detection of pancreatic cancer[93]. Song et al[94] used AI algorithms to segment epithelial cell nuclei on slide images and extract morphological features and could differentiate SCN from MCN and grading of PDAC[95]. The CNN was used by Kriegsmann et al[96] to localize pancreatic intra-epithelial neoplasm or PDAC in a slide. Niazi et al[97] used DL to detect neuroendocrine tumors from normal tissues on Ki-67 stained biopsy images with a 97.8% sensitivity and 88.8% specificity. Momeni-Boroujeni et al[98] could differentiate benign from malignant pathology using a K-means clustering algorithm from FNA-based slides with an accuracy of 100%. Naito et al[99] used CNN in FNB-based slides to assess PDAC with an AUC of 0.984. Cyst fluid analysis is an essential part of the diagnosis of pancreatic cystic lesions. Kurita et al[100] used a neural network to differentiate benign from malignant cysts taking into consideration biomarkers in cyst fluid, cytology and clinical parameters.
Biomarkers act as an adjunct in diagnosis, prognosis, and screening for recurrence and they can be used for early diagnosis of tumors. However, in the case of pancreatic cancer, it lacks sensitivity and specificity for routine clinical practice[91,101,102]. Liquid biopsy is one of the recent developments in oncology, developed with the intent of detecting tumor cells from blood when biopsy cannot be obtained, or to assess tumor response to therapy (surgery or chemoradiotherapy) and assess genetic mutation. It includes three types of sampling of biological materials; which are circulating tumor cells (CTCs), circulating tumor DNA, and exosomes. CTCs have faced difficulties for years because of very low concentrations in many studies, which is 1–10 cells per 10-mL of blood (much lower than billions of hematopoietic cells) and short half-life (approximately from 1 to 2.4 h) in blood which poses difficulty in further study. AI can be used in the detection of disease from these biomarkers and various studies have explored AI algorithms for biomarkers for diagnosis[91,103]. Studies used exosomes[104-106], cell-free DNA[107], extracellular vesicles long RNA[108], proteins[109-112], and circulating microRNA[113] in association with AI for diagnosis of pancreatic cancer. Table 5 shows studies on the role of biomarkers and AI in the differentiation of pancreatic lesions.
| No. | Ref. | Number of samples | Type of biomarker used | Sub-type of AI used | Conclusion |
| 1 | Chen et al[104], 2019 | 28 | Exosomes | LDA | Accuracy: 100% |
| 2 | Zheng et al[105], 2022 | 220 | Exosomes | ANN | AUC: 0.86 |
| 3 | Ko et al[106], 2017 | 28 | Exosomes | LDA | Accuracy: 100% |
| 4 | Cristiano et al[107], 2019 | 34 | Cell-free DNA | GBM | AUC: 0.86 |
| 5 | Yu et al[108], 2020 | 501 | extracellular vesicles long RNA | SVM | AUC: 0.96 |
| 6 | Gao et al[109], 2012 | 199 | Proteomes | SVM, KNN, ANN | AUC: 0.971 |
| 7 | Yu et al[110], 2005 | 100 | Proteomes | DT | Sensitivity: 88.9%, specificity: 74.1% |
| 8 | Qiao et al[112], 2022 | 136 | Proteomes | CNN | Accuracy: 87.63% |
| 9 | Alizadeh et al[113], 2020 | 671 | Circulating micro RNA | ANN | Accuracy: 0.86 |
This review has shown that AI can be used in routine investigation modalities (CT, MRI, EUS, PET, biomarkers) to improve diagnostic and differentiating potential; however, it is still in progress. In the beginning, studies have trained and validated AI algorithms, in the future it is a challenge to implement such studies at different geographical locations, ethnicity, genetic makeup, etc. The majority of studies have explored the potential to differentiate, chronic pancreatitis from pancreatic ductal adenocarcinoma, SCN from MCN, and high-risk vs. low-risk IPMN, however, there can be other differential diagnoses in a clinical scenario.
Surgery for malignant pancreatic head lesions was standardized by Whipple et al[114] which is acceptable worldwide. It includes a complex single-stage procedure of pancreaticoduodenectomy, which is associated with morbidity (25%) and mortality (0%-9.3%) even in high-volume centers[115-117]. Professor Whipple[118] reported a mortality of 29.2% in his series of patients who underwent pancreaticoduodenectomy. Though, recent series have reported reduced mortality following pancreaticoduodenectomy, morbidity of the procedure continues to be high. Recently, many modifications have been made to reduce morbidity, however, none of the measures appeared to be successful. Are et al[119] reported a historical perspective where 7 out of 37 pancreaticoduodenectomies performed by Prof Whipple AO turned out to be chronic pancreatitis (18.9%), where such a morbid procedure could have been avoided. Recent series have also supported these findings of incidence of benign pathology following pancreaticoduodenectomy in the range of 5%-10%[117,120]. Hence, there is an unmet need to differentiate benign pancreatic lesions from malignant ones. Multiple imaging modalities have been used to distinguish benign from malignant lesions, however, each investigation modality has its limitations which are compounded by human errors. The application of AI has minimized those errors and can make diagnoses earlier. Table 6 shows how AI increases the yield of different imaging modalities for predicting a malignant pancreatic head lesion. We have proposed an algorithm for the diagnosis of such entities. Whenever a patient presents to a clinician, history and clinical examination precede imaging. Hence, AI can be used to develop algorithms to predict malignancy[32-34]. In a patient with a high risk of pancreatic malignancy, a pancreatic indeterminate lesion should be investigated further with imaging or biopsy to rule out malignancy. Studies have reported the usefulness of biomarkers in the diagnosis of pancreatic cancer[107-110]. Hence, all non-invasive markers (clinical, biochemical) can be used to develop an algorithm that can predict pancreatic cancer before imaging has been performed and it can differentiate malignant pancreatic lesions. As shown in Table 6, AI has an added advantage over conventional imaging in differentiating pancreatic cancer from benign conditions. So, those high-risk patients marked on non-invasive pancreatic cancer detection models can be subjected to AI-enhanced imaging for better diagnosis. Further in line, to clarify the final tissue diagnosis, AI can help to detect subtle markers that can be ignored by human error. Therefore, AI can be used in every step of the diagnosis of an indeterminate pancreatic head mass, to detect malignant lesions early thus, availing proper oncological management.
| No. | Ref. | Objective | Modality | Sensitivity | Specificity | Accuracy |
| 1 | Corral et al[67], 2019 | Differentiate cystic SOL of pancreas | Fukuoka guideline | 62% | 77 | 77.5% |
| Deep learning | 75% | 78% | 78.3% | |||
| 2 | Kuwahara et al[83], 2019 | Detection of malignant IPMN | Human pre-operative diagnosis (Clinical + lab + imaging) | 95.7% | 22.2% | 56% |
| Artificial intelligence | 95.7% | 92.66 | 94% | |||
| 3 | Gao et al[135], 2020 | Ability to differentiate pancreatic disease | CE-MR | NA | NA | 83.93% |
| GAN | NA | NA | 76.79% | |||
| 4 | Rigiroli et al[136], 2021 | Detection of pancreatic cancer and SMA involvement | CT scan | NA | NA | 71% |
| Artificial intelligence | 62% | 77% | 54% | |||
| 5 | Chen et al[137], 2023 | Detection of pancreatic cancer | CT scan | 89.9% | 95.9% | AUC: 0.96 |
| CNN | 90% | 93% | NA | |||
| 6 | Tang et al[138], 2023 | Pancreatic mass diagnosis | EUS FNA | 81.6% | 100% | 87.9% |
| CE EUS Master-guided FNA | 90.9% | 100% | 93.8% |
Pancreatic incidentalomas or indeterminate lesions are on the rise due to the plethora of cross-sectional imaging performed to diagnose non-specific abdominal complaints. Though plenty of studies have been made in the fields of breast cancer, lung cancer, hepatocellular carcinoma, renal cell carcinoma, and adrenal tumors, there is a dearth of literature discussing how to differentiate benign pancreatic lesions from benign ones. The current literature included studies comparing individual pancreatic lesions, i.e. serous cystadenoma vs. mucinous cystadenoma, autoimmune pancreatitis vs. pancreatic adenocarcinoma, low-grade vs. high-grade IPMN, etc. However, a comprehensive review discussing how to differentiate various malignant pancreatic lesions (both cystic and solid) from benign lesions with the help of AI is lacking. Hence, in this review, we have discussed how to differentiate different pancreatic lesions encountered in day-to-day clinical practice using different algorithms of AI. We have discussed individually about different diagnostic modalities and different types of pancreatic lesions. There are more studies available in the field of radiological investigations and fewer studies available for the histopathological diagnosis or intra-operative differentiation of malignant from benign lesions. As the understanding of the usefulness of AI is increasing, these limitations can be curtailed in the near future.
There is a surge in the number of medical imaging for different indications leading to the identification of many indeterminate pancreatic lesions (IPLs), which help to diagnose a disease earlier or can lead to a plethora of other investigations, psychological stress, clinical dilemmas, etc. Human judgment is prone to errors as subtle differences in these small or atypical lesions are challenging to discern leading to inter-observer and intra-observer variations which can be minimized with the use of AI.
AI is an evolving technical advancement in the field of medicine and can play a significant role in differentiating IPLs into benign or malignant, by enhancing the diagnostic yield of conventional imaging (CT, MRI, PET), EUS, tissue diagnosis (cytopathology, histopathology), and biomarkers (liquid biopsy). An early and accurate diagnosis may lead to timely intervention, thereby improving the patient outcome. The current literature on this is still limited and sparse, therefore, more studies are required to reach a standard approach for the application of AI in IPLs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain S-Editor: Lin C L-Editor: Filipodia P-Editor: Zhao S
| 1. | Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (1)] |
| 2. | Hamamoto R, Suvarna K, Yamada M, Kobayashi K, Shinkai N, Miyake M, Takahashi M, Jinnai S, Shimoyama R, Sakai A, Takasawa K, Bolatkan A, Shozu K, Dozen A, Machino H, Takahashi S, Asada K, Komatsu M, Sese J, Kaneko S. Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 3. | Meng Y, Speier W, Shufelt C, Joung S, E Van Eyk J, Bairey Merz CN, Lopez M, Spiegel B, Arnold CW. A Machine Learning Approach to Classifying Self-Reported Health Status in a Cohort of Patients With Heart Disease Using Activity Tracker Data. IEEE J Biomed Health Inform. 2020;24:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Kirsch D. Autopilot and algorithms: accidents, errors, and the current need for human oversight. J Clin Sleep Med. 2020;16:1651-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bredt S. Artificial Intelligence (AI) in the Financial Sector-Potential and Public Strategies. Front Artif Intell. 2019;2:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 6. | Goli A, Malmir B. A Covering Tour Approach for Disaster Relief Locating and Routing with Fuzzy Demand. Int J Intell Transp Syst Res. 2019;18: 140-152. [DOI] [Full Text] |
| 7. | Goli A, Mohammadi H. Developing a sustainable operational management system using hybrid Shapley value and Multimoora method: case study petrochemical supply chain. Environ Dev Sustain. 2021;24: 10540-10569. [DOI] [Full Text] |
| 8. | Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021;11:900-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 312] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 9. | Kann BH, Hosny A, Aerts HJWL. Artificial intelligence for clinical oncology. Cancer Cell. 2021;39:916-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 10. | Huynh E, Hosny A, Guthier C, Bitterman DS, Petit SF, Haas-Kogan DA, Kann B, Aerts HJWL, Mak RH. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | Benzekry S. Artificial Intelligence and Mechanistic Modeling for Clinical Decision Making in Oncology. Clin Pharmacol Ther. 2020;108:471-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Ippolito AM, De Laurentiis M, La Rosa GL, Eleuteri A, Tagliaferri R, De Placido S, Vigneri R, Belfiore A. Neural network analysis for evaluating cancer risk in thyroid nodules with an indeterminate diagnosis at aspiration cytology: identification of a low-risk subgroup. Thyroid. 2004;14:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Daniels K, Gummadi S, Zhu Z, Wang S, Patel J, Swendseid B, Lyshchik A, Curry J, Cottrill E, Eisenbrey J. Machine Learning by Ultrasonography for Genetic Risk Stratification of Thyroid Nodules. JAMA Otolaryngol Head Neck Surg. 2020;146:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Becker AS, Mueller M, Stoffel E, Marcon M, Ghafoor S, Boss A. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Scott JA, McDermott S, Kilcoyne A, Wang Y, Halpern EF, Ackman JB. Comparison of (18)F-FDG avidity at PET of benign and malignant pure ground-glass opacities: a paradox? Part II: artificial neural network integration of the PET/CT characteristics of ground-glass opacities to predict their likelihood of malignancy. Clin Radiol. 2019;74:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Guo H, Wu J, Xie Z, Tham IWK, Zhou L, Yan J. Investigation of small lung lesion detection for lung cancer screening in low dose FDG PET imaging by deep neural networks. Front Public Health. 2022;10:1047714. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 389] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 18. | Moawad AW, Ahmed A, Fuentes DT, Hazle JD, Habra MA, Elsayes KM. Machine learning-based texture analysis for differentiation of radiologically indeterminate small adrenal tumors on adrenal protocol CT scans. Abdom Radiol (NY). 2021;46:4853-4863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Stanzione A, Cuocolo R, Verde F, Galatola R, Romeo V, Mainenti PP, Aprea G, Guadagno E, Del Basso De Caro M, Maurea S. Handcrafted MRI radiomics and machine learning: Classification of indeterminate solid adrenal lesions. Magn Reson Imaging. 2021;79:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Massa'a RN, Stoeckl EM, Lubner MG, Smith D, Mao L, Shapiro DD, Abel EJ, Wentland AL. Differentiation of benign from malignant solid renal lesions with MRI-based radiomics and machine learning. Abdom Radiol (NY). 2022;47:2896-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Saraiva MM, Ribeiro T, Ferreira JPS, Boas FV, Afonso J, Santos AL, Parente MPL, Jorge RN, Pereira P, Macedo G. Artificial intelligence for automatic diagnosis of biliary stricture malignancy status in single-operator cholangioscopy: a pilot study. Gastrointest Endosc. 2022;95:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Rosenkrantz AB, Hanna TN, Babb JS, Duszak R Jr. Changes in Emergency Department Imaging: Perspectives From National Patient Surveys Over Two Decades. J Am Coll Radiol. 2017;14:1282-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Del Chiaro M, Torphy RJ, Schulick RD. Pancreatic incidentalomas: Investigation and management. J Intern Med. 2021;290:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Herrera MF, Pantoja JP, Salazar MS, Velázquez-Fernández, D. Pancreatic Incidentaloma. In: Hubbard J, Inabnet W, Lo CY, editor. Endocrine Surgery. London: Springer, 2009. [DOI] [Full Text] |
| 25. | Karatzas T, Dimitroulis D, Charalampoudis P, Misiakos EP, Vasileiadis I, Kouraklis G. Management of cystic and solid pancreatic incidentalomas: a review analysis. J BUON. 2013;18:17-24. [PubMed] |
| 26. | Herrera MF, Åkerström G, Angelos P, Grant CS, Hoff AO, Pantoja JP, Pérez-Johnston R, Sahani DV, Wong RJ, Randolph G. AACE/ACE disease state clinical review: pancreatic neuroendocrine incidentalomas. Endocr Pract. 2015;21:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, Gerhards MF, de Hingh IH, Karsten TM, Lips DJ, Luyer MD, Busch OR, Festen S, Besselink MG; Dutch Pancreatic Cancer Group. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 28. | Udare A, Agarwal M, Alabousi M, McInnes M, Rubino JG, Marcaccio M, van der Pol CB. Diagnostic Accuracy of MRI for Differentiation of Benign and Malignant Pancreatic Cystic Lesions Compared to CT and Endoscopic Ultrasound: Systematic Review and Meta-analysis. J Magn Reson Imaging. 2021;54:1126-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, Völzke H, Mayerle J, Kühn JP. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 30. | Kobayashi G, Fujita N, Maguchi H, Tanno S, Mizuno N, Hanada K, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R, Yanagisawa A, Tanaka M; Working Group for the Natural History of IPMN of the Japan Pancreas Society. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Caban M, Małecka-Wojciesko E. Pancreatic Incidentaloma. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Meng Q, Shi S, Liang C, Liang D, Xu W, Ji S, Zhang B, Ni Q, Xu J, Yu X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:4591-4598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 33. | van Manen L, Groen JV, Putter H, Vahrmeijer AL, Swijnenburg RJ, Bonsing BA, Mieog JSD. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers. 2020;25:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 34. | Lee HS, Jang CY, Kim SA, Park SB, Jung DE, Kim BO, Kim HY, Chung MJ, Park JY, Bang S, Park SW, Song SY. Combined use of CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic cancer. Sci Rep. 2018;8:3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Chen W, Butler RK, Zhou Y, Parker RA, Jeon CY, Wu BU. Prediction of Pancreatic Cancer Based on Imaging Features in Patients With Duct Abnormalities. Pancreas. 2020;49:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Qureshi TA, Gaddam S, Wachsman AM, Wang L, Azab L, Asadpour V, Chen W, Xie Y, Wu B, Pandol SJ, Li D. Predicting pancreatic ductal adenocarcinoma using artificial intelligence analysis of pre-diagnostic computed tomography images. Cancer Biomark. 2022;33:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Muhammad W, Hart GR, Nartowt B, Farrell JJ, Johung K, Liang Y, Deng J. Pancreatic Cancer Prediction Through an Artificial Neural Network. Front Artif Intell. 2019;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Placido D, Yuan B, Hjaltelin JX, Zheng C, Haue AD, Chmura PJ, Yuan C, Kim J, Umeton R, Antell G, Chowdhury A, Franz A, Brais L, Andrews E, Marks DS, Regev A, Ayandeh S, Brophy MT, Do NV, Kraft P, Wolpin BM, Rosenthal MH, Fillmore NR, Brunak S, Sander C. A deep learning algorithm to predict risk of pancreatic cancer from disease trajectories. Nat Med. 2023;29:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 39. | Chen Q, Cherry DR, Nalawade V, Qiao EM, Kumar A, Lowy AM, Simpson DR, Murphy JD. Clinical Data Prediction Model to Identify Patients With Early-Stage Pancreatic Cancer. JCO Clin Cancer Inform. 2021;5:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Malhotra A, Rachet B, Bonaventure A, Pereira SP, Woods LM. Can we screen for pancreatic cancer? Identifying a sub-population of patients at high risk of subsequent diagnosis using machine learning techniques applied to primary care data. PLoS One. 2021;16:e0251876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Appelbaum L, Cambronero JP, Stevens JP, Horng S, Pollick K, Silva G, Haneuse S, Piatkowski G, Benhaga N, Duey S, Stevenson MA, Mamon H, Kaplan ID, Rinard MC. Development and validation of a pancreatic cancer risk model for the general population using electronic health records: An observational study. Eur J Cancer. 2021;143:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | de la Santa LG, Retortillo JA, Miguel AC, Klein LM. Radiology of pancreatic neoplasms: An update. World J Gastrointest Oncol. 2014;6:330-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864-7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (4)] |
| 44. | Gandhi NS, Feldman MK, Le O, Morris-Stiff G. Imaging mimics of pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2018;43:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1648] [Article Influence: 329.6] [Reference Citation Analysis (1)] |
| 47. | Stupple A, Singerman D, Celi LA. The reproducibility crisis in the age of digital medicine. NPJ Digit Med. 2019;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 48. | Zhu Z, Xia Y, Xie L, Fishman EK, Yuille AL. Multi-scale coarse-to-fine segmentation for screening pancreatic ductal adenocarcinoma. In: Shen D, Liu T, Peters TM, Staib LH, Essert C, Zhou S, Yap PT. Ali Khan Medical Image Computing and Computer Assisted Intervention - MICCAI 2019. 22nd International Conference; October 13-17; Shenzhen, China. Cham: Springer, 2019. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Liu SL, Li S, Guo YT, Zhou YP, Zhang ZD, Lu Y. Establishment and application of an artificial intelligence diagnosis system for pancreatic cancer with a faster region-based convolutional neural network. Chin Med J (Engl). 2019;132:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Chu LC, Park S, Kawamoto S, Fouladi DF, Shayesteh S, Zinreich ES, Graves JS, Horton KM, Hruban RH, Yuille AL, Kinzler KW, Vogelstein B, Fishman EK. Utility of CT Radiomics Features in Differentiation of Pancreatic Ductal Adenocarcinoma From Normal Pancreatic Tissue. AJR Am J Roentgenol. 2019;213:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 51. | Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, Lee SH, Kim YT, Lee JY. Preoperative Diagnosis of Pancreatic Cystic Lesions: The Accuracy of Endoscopic Ultrasound and Cross-Sectional Imaging. Pancreas. 2015;44:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Lee HJ, Kim MJ, Choi JY, Hong HS, Kim KA. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol. 2011;66:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Dmitriev K, Kaufman AE, Javed AA, Hruban RH, Fishman EK, Lennon AM, Saltz JH. Classification of Pancreatic Cysts in Computed Tomography Images Using a Random Forest and Convolutional Neural Network Ensemble. Med Image Comput Comput Assist Interv. 2017;10435:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Li H, Shi K, Reichert M, Lin K, Tselousov N, Braren R, Fu D, Schmid R, Li J, Menze B. Differential Diagnosis for Pancreatic Cysts in CT Scans Using Densely-Connected Convolutional Networks. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:2095-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M, del-Castillo CF. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol. 2011;197:W53-W61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Wei R, Lin K, Yan W, Guo Y, Wang Y, Li J, Zhu J. Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol Cancer Res Treat. 2019;18:1533033818824339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 57. | Yang J, Guo X, Ou X, Zhang W, Ma X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front Oncol. 2019;9:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 58. | Chen HY, Deng XY, Pan Y, Chen JY, Liu YY, Chen WJ, Yang H, Zheng Y, Yang YB, Liu C, Shao GL, Yu RS. Pancreatic Serous Cystic Neoplasms and Mucinous Cystic Neoplasms: Differential Diagnosis by Combining Imaging Features and Enhanced CT Texture Analysis. Front Oncol. 2021;11:745001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Chakraborty J, Midya A, Gazit L, Attiyeh M, Langdon-Embry L, Allen PJ, Do RKG, Simpson AL. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys. 2018;45:5019-5029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 60. | Polk SL, Choi JW, McGettigan MJ, Rose T, Ahmed A, Kim J, Jiang K, Balagurunathan Y, Qi J, Farah PT, Rathi A, Permuth JB, Jeong D. Multiphase computed tomography radiomics of pancreatic intraductal papillary mucinous neoplasms to predict malignancy. World J Gastroenterol. 2020;26:3458-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Li J, Feng C, Lin X, Qian X. Utilizing GCN and Meta-Learning Strategy in Unsupervised Domain Adaptation for Pancreatic Cancer Segmentation. IEEE J Biomed Health Inform. 2022;26:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Reference Citation Analysis (0)] |
| 63. | Chen X, Chen Z, Li J, Zhang YD, Lin X, Qian X. Model-Driven Deep Learning Method for Pancreatic Cancer Segmentation Based on Spiral-Transformation. IEEE Trans Med Imaging. 2022;41:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 65. | Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Kim JH, Eun HW, Park HJ, Hong SS, Kim YJ. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol. 2012;81:2927-2935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Corral JE, Hussein S, Kandel P, Bolan CW, Bagci U, Wallace MB. Deep Learning to Classify Intraductal Papillary Mucinous Neoplasms Using Magnetic Resonance Imaging. Pancreas. 2019;48:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Cheng S, Shi H, Lu M, Wang C, Duan S, Xu Q. Radiomics Analysis for Predicting Malignant Potential of Intraductal Papillary Mucinous Neoplasms of the Pancreas: Comparison of CT and MRI. Acad Radiol. 2022;29:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 70. | Moutinho-Ribeiro P, Iglesias-Garcia J, Gaspar R, Macedo G. Early pancreatic cancer - The role of endoscopic ultrasound with or without tissue acquisition in diagnosis and staging. Dig Liver Dis. 2019;51:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Bhutani MS, Koduru P, Joshi V, Saxena P, Suzuki R, Irisawa A, Yamao K. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | ASGE Standards of Practice Committee; Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Saltzman JR, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 73. | Tummers WS, Willmann JK, Bonsing BA, Vahrmeijer AL, Gambhir SS, Swijnenburg RJ. Advances in Diagnostic and Intraoperative Molecular Imaging of Pancreatic Cancer. Pancreas. 2018;47:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Zhang MM, Yang H, Jin ZD, Yu JG, Cai ZY, Li ZS. Differential diagnosis of pancreatic cancer from normal tissue with digital imaging processing and pattern recognition based on a support vector machine of EUS images. Gastrointest Endosc. 2010;72:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Das A, Nguyen CC, Li F, Li B. Digital image analysis of EUS images accurately differentiates pancreatic cancer from chronic pancreatitis and normal tissue. Gastrointest Endosc. 2008;67:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Ozkan M, Cakiroglu M, Kocaman O, Kurt M, Yilmaz B, Can G, Korkmaz U, Dandil E, Eksi Z. Age-based computer-aided diagnosis approach for pancreatic cancer on endoscopic ultrasound images. Endosc Ultrasound. 2016;5:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Zhu M, Xu C, Yu J, Wu Y, Li C, Zhang M, Jin Z, Li Z. Differentiation of pancreatic cancer and chronic pancreatitis using computer-aided diagnosis of endoscopic ultrasound (EUS) images: a diagnostic test. PLoS One. 2013;8:e63820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 79. | Săftoiu A, Vilmann P, Dietrich CF, Iglesias-Garcia J, Hocke M, Seicean A, Ignee A, Hassan H, Streba CT, Ioncică AM, Gheonea DI, Ciurea T. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2015;82:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 80. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 81. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M, Dietrich CF, Havre R, Gheorghe C, McKay C, Gheonea DI, Ciurea T; European EUS Elastography Multicentric Study Group. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 82. | Udriștoiu AL, Cazacu IM, Gruionu LG, Gruionu G, Iacob AV, Burtea DE, Ungureanu BS, Costache MI, Constantin A, Popescu CF, Udriștoiu Ș, Săftoiu A. Real-time computer-aided diagnosis of focal pancreatic masses from endoscopic ultrasound imaging based on a hybrid convolutional and long short-term memory neural network model. PLoS One. 2021;16:e0251701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Kurita Y, Koda H, Toriyama K, Onishi S, Ishihara M, Tanaka T, Tajika M, Niwa Y. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 84. | Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol. 2019;46:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 85. | Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242:360-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 86. | Yokoyama Y, Nagino M, Hiromatsu T, Yuasa N, Oda K, Arai T, Nishio H, Ebata T, Nimura Y. Intense PET signal in the degenerative necrosis superimposed on chronic pancreatitis. Pancreas. 2005;31:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Feldman MK, Gandhi NS. Imaging Evaluation of Pancreatic Cancer. Surg Clin North Am. 2016;96:1235-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Li S, Jiang H, Wang Z, Zhang G, Yao YD. An effective computer aided diagnosis model for pancreas cancer on PET/CT images. Comput Methods Programs Biomed. 2018;165:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Liu Z, Li M, Zuo C, Yang Z, Yang X, Ren S, Peng Y, Sun G, Shen J, Cheng C. Radiomics model of dual-time 2-[(18)F]FDG PET/CT imaging to distinguish between pancreatic ductal adenocarcinoma and autoimmune pancreatitis. Eur Radiol. 2021;31:6983-6991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 90. | Xing H, Hao Z, Zhu W, Sun D, Ding J, Zhang H, Liu Y, Huo L. Preoperative prediction of pathological grade in pancreatic ductal adenocarcinoma based on (18)F-FDG PET/CT radiomics. EJNMMI Res. 2021;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond). 2021;41:1257-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 92. | Nicola M, Onorati M, Albertoni MM, Bianchi CL, De Nucci G, Mandelli ED, Nicola L, Di Nuovo F. Fine Needle Aspiration versus Fine Needle Biopsy of Biliopancreatic Lesions: Are They Really Opposing Techniques or Can They Be Complementary? Our Experience in a Large Cohort of Cases from a Single Institution. Acta Cytol. 2021;65:40-47. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 93. | Elemento O, Leslie C, Lundin J, Tourassi G. Artificial intelligence in cancer research, diagnosis and therapy. Nat Rev Cancer. 2021;21:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 94. | Song JW, Lee JH, Choi JH, Chun SJ. Automatic differential diagnosis of pancreatic serous and mucinous cystadenomas based on morphological features. Comput Biol Med. 2013;43:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Song JW, Lee JH. New morphological features for grading pancreatic ductal adenocarcinomas. Biomed Res Int. 2013;2013:175271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Kriegsmann M, Kriegsmann K, Steinbuss G, Zgorzelski C, Kraft A, Gaida MM. Deep Learning in Pancreatic Tissue: Identification of Anatomical Structures, Pancreatic Intraepithelial Neoplasia, and Ductal Adenocarcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Niazi MKK, Tavolara TE, Arole V, Hartman DJ, Pantanowitz L, Gurcan MN. Identifying tumor in pancreatic neuroendocrine neoplasms from Ki67 images using transfer learning. PLoS One. 2018;13:e0195621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Momeni-Boroujeni A, Yousefi E, Somma J. Computer-assisted cytologic diagnosis in pancreatic FNA: An application of neural networks to image analysis. Cancer Cytopathol. 2017;125:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Naito Y, Tsuneki M, Fukushima N, Koga Y, Higashi M, Notohara K, Aishima S, Ohike N, Tajiri T, Yamaguchi H, Fukumura Y, Kojima M, Hirabayashi K, Hamada Y, Norose T, Kai K, Omori Y, Sukeda A, Noguchi H, Uchino K, Itakura J, Okabe Y, Yamada Y, Akiba J, Kanavati F, Oda Y, Furukawa T, Yano H. A deep learning model to detect pancreatic ductal adenocarcinoma on endoscopic ultrasound-guided fine-needle biopsy. Sci Rep. 2021;11:8454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 100. | Kurita Y, Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Koda H, Tajika M, Shimizu Y, Nakajima A, Kubota K, Niwa Y. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci Rep. 2019;9:6893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 102. | Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (1)] |
| 103. | Iovanna J. Implementing biological markers as a tool to guide clinical care of patients with pancreatic cancer. Transl Oncol. 2021;14:100965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Chen C, Zong S, Liu Y, Wang Z, Zhang Y, Chen B, Cui Y. Profiling of Exosomal Biomarkers for Accurate Cancer Identification: Combining DNA-PAINT with Machine- Learning-Based Classification. Small. 2019;15:e1901014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 105. | Zheng H, Zhao J, Wang X, Yan S, Chu H, Gao M, Zhang X. Integrated Pipeline of Rapid Isolation and Analysis of Human Plasma Exosomes for Cancer Discrimination Based on Deep Learning of MALDI-TOF MS Fingerprints. Anal Chem. 2022;94:1831-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Ko J, Bhagwat N, Yee SS, Ortiz N, Sahmoud A, Black T, Aiello NM, McKenzie L, O'Hara M, Redlinger C, Romeo J, Carpenter EL, Stanger BZ, Issadore D. Combining Machine Learning and Nanofluidic Technology To Diagnose Pancreatic Cancer Using Exosomes. ACS Nano. 2017;11:11182-11193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 107. | Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SØ, Medina JE, Hruban C, White JR, Palsgrove DN, Niknafs N, Anagnostou V, Forde P, Naidoo J, Marrone K, Brahmer J, Woodward BD, Husain H, van Rooijen KL, Ørntoft MW, Madsen AH, van de Velde CJH, Verheij M, Cats A, Punt CJA, Vink GR, van Grieken NCT, Koopman M, Fijneman RJA, Johansen JS, Nielsen HJ, Meijer GA, Andersen CL, Scharpf RB, Velculescu VE. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 797] [Cited by in RCA: 849] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 108. | Yu S, Li Y, Liao Z, Wang Z, Qian L, Zhao J, Zong H, Kang B, Zou WB, Chen K, He X, Meng Z, Chen Z, Huang S, Wang P. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 109. | Gao H, Zheng Z, Yue Z, Liu F, Zhou L, Zhao X. Evaluation of serum diagnosis of pancreatic cancer by using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Int J Mol Med. 2012;30:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Yu Y, Chen S, Wang LS, Chen WL, Guo WJ, Yan H, Zhang WH, Peng CH, Zhang SD, Li HW, Chen GQ. Prediction of pancreatic cancer by serum biomarkers using surface-enhanced laser desorption/ionization-based decision tree classification. Oncology. 2005;68:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Yang Y, Chen H, Wang D, Luo W, Zhu B, Zhang Z. Diagnosis of pancreatic carcinoma based on combined measurement of multiple serum tumor markers using artificial neural network analysis. Chin Med J (Engl). 2014;127:1891-1896. [PubMed] |
| 112. | Qiao Z, Ge J, He W, Xu X, He J. Artificial Intelligence Algorithm-Based Computerized Tomography Image Features Combined with Serum Tumor Markers for Diagnosis of Pancreatic Cancer. Comput Math Methods Med. 2022;2022:8979404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Alizadeh Savareh B, Asadzadeh Aghdaie H, Behmanesh A, Bashiri A, Sadeghi A, Zali M, Shams R. A machine learning approach identified a diagnostic model for pancreatic cancer through using circulating microRNA signatures. Pancreatology. 2020;20:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Whipple AO, Parsons WB, Mullins CR. TREATMENT OF CARCINOMA OF THE AMPULLA OF VATER. Ann Surg. 1935;102:763-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 893] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 115. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (34)] |
| 116. | Wojcicki J, Zen Y, Peddu P, Jain R, Patel AG, Atkinson S, Srinivasan P, Rela M, Heaton N, Prachalias A. Benign histology after pancreaticoduodenectomy for suspected malignancy. Lessons to be learned--a single centre experience. Pol Przegl Chir. 2015;87:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 117. | Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430-5; discussion 435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 565] [Article Influence: 17.7] [Reference Citation Analysis (33)] |
| 118. | Whipple AO. Radical surgery in the treatment of cancer. Ann Surg. 1950;131:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB (Oxford). 2011;13:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Yarandi SS, Runge T, Wang L, Liu Z, Jiang Y, Chawla S, Woods KE, Keilin S, Willingham FF, Xu H, Cai Q. Increased Incidence of Benign Pancreatic Pathology following Pancreaticoduodenectomy for Presumed Malignancy over 10 Years despite Increased Use of Endoscopic Ultrasound. Diagn Ther Endosc. 2014;2014:701535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Ebrahimian S, Singh R, Netaji A, Madhusudhan KS, Homayounieh F, Primak A, Lades F, Saini S, Kalra MK, Sharma S. Characterization of Benign and Malignant Pancreatic Lesions with DECT Quantitative Metrics and Radiomics. Acad Radiol. 2022;29:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Ikeda M, Ito S, Ishigaki T, Yamauchi K. Evaluation of a neural network classifier for pancreatic masses based on CT findings. Comput Med Imaging Graph. 1997;21:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Yang R, Chen Y, Sa G, Li K, Hu H, Zhou J, Guan Q, Chen F. CT classification model of pancreatic serous cystic neoplasms and mucinous cystic neoplasms based on a deep neural network. Abdom Radiol (NY). 2022;47:232-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Ren S, Zhao R, Cui W, Qiu W, Guo K, Cao Y, Duan S, Wang Z, Chen R. Computed Tomography-Based Radiomics Signature for the Preoperative Differentiation of Pancreatic Adenosquamous Carcinoma From Pancreatic Ductal Adenocarcinoma. Front Oncol. 2020;10:1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 125. | Xie T, Wang X, Zhang Z, Zhou Z. CT-Based Radiomics Analysis for Preoperative Diagnosis of Pancreatic Mucinous Cystic Neoplasm and Atypical Serous Cystadenomas. Front Oncol. 2021;11:621520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 126. | Ziegelmayer S, Kaissis G, Harder F, Jungmann F, Müller T, Makowski M, Braren R. Deep Convolutional Neural Network-Assisted Feature Extraction for Diagnostic Discrimination and Feature Visualization in Pancreatic Ductal Adenocarcinoma (PDAC) versus Autoimmune Pancreatitis (AIP). J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Gao J, Han F, Wang X, Duan S, Zhang J. Multi-Phase CT-Based Radiomics Nomogram for Discrimination Between Pancreatic Serous Cystic Neoplasm From Mucinous Cystic Neoplasm. Front Oncol. 2021;11:699812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | Liang Y, Schott D, Zhang Y, Wang Z, Nasief H, Paulson E, Hall W, Knechtges P, Erickson B, Li XA. Auto-segmentation of pancreatic tumor in multi-parametric MRI using deep convolutional neural networks. Radiother Oncol. 2020;145:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 129. | Cui S, Tang T, Su Q, Wang Y, Shu Z, Yang W, Gong X. Radiomic nomogram based on MRI to predict grade of branching type intraductal papillary mucinous neoplasms of the pancreas: a multicenter study. Cancer Imaging. 2021;21:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 130. | Hussein S, Kandel P, Bolan CW, Wallace MB, Bagci U. Lung and Pancreatic Tumor Characterization in the Deep Learning Era: Novel Supervised and Unsupervised Learning Approaches. IEEE Trans Med Imaging. 2019;38:1777-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 131. | Zhu J, Wang L, Chu Y, Hou X, Xing L, Kong F, Zhou Y, Wang Y, Jin Z, Li Z. A new descriptor for computer-aided diagnosis of EUS imaging to distinguish autoimmune pancreatitis from chronic pancreatitis. Gastrointest Endosc. 2015;82:831-836.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Tonozuka R, Itoi T, Nagata N, Kojima H, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Nagakawa Y, Mukai S. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci. 2021;28:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 133. | Marya NB, Powers PD, Chari ST, Gleeson FC, Leggett CL, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Majumder S, Pearson RK, Petersen BT, Rajan E, Sawas T, Storm AC, Vege SS, Chen S, Long Z, Hough DM, Mara K, Levy MJ. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut. 2021;70:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 134. | Xu W, Shi J, Li X, Zeng X, Lin Y. Endoscopic ultrasound elastography for differentiation of benign and malignant pancreatic masses: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 135. | Gao X, Wang X. Performance of deep learning for differentiating pancreatic diseases on contrast-enhanced magnetic resonance imaging: A preliminary study. Diagn Interv Imaging. 2020;101:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | Rigiroli F, Hoye J, Lerebours R, Lafata KJ, Li C, Meyer M, Lyu P, Ding Y, Schwartz FR, Mettu NB, Zani S Jr, Luo S, Morgan DE, Samei E, Marin D. CT Radiomic Features of Superior Mesenteric Artery Involvement in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Radiology. 2021;301:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 137. | Chen PT, Wu T, Wang P, Chang D, Liu KL, Wu MS, Roth HR, Lee PC, Liao WC, Wang W. Pancreatic Cancer Detection on CT Scans with Deep Learning: A Nationwide Population-based Study. Radiology. 2023;306:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 138. | Tang A, Gong P, Fang N, Ye M, Hu S, Liu J, Wang W, Gao K, Wang X, Tian L. Endoscopic ultrasound diagnosis system based on deep learning in images capture and segmentation training of solid pancreatic masses. Med Phys. 2023;50:4197-4205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |