Published online Sep 8, 2023. doi: 10.35712/aig.v4.i2.36
Peer-review started: June 4, 2023
First decision: July 28, 2023
Revised: August 18, 2023
Accepted: September 5, 2023
Article in press: September 5, 2023
Published online: September 8, 2023
Processing time: 94 Days and 12.1 Hours
Liver injury is a relevant condition in coronavirus disease 2019 (COVID-19) inpatients. Pathophysiology varies from direct infection by virus, systemic inflammation or drug-induced adverse reaction (DILI). DILI detection and monitoring is clinically relevant, as it may contribute to poor prognosis, prolonged hospitalization and increase indirect healthcare costs. Artificial Intelligence (AI) applied in data mining of electronic medical records combining abnormal liver tests, keyword searching tools, and risk factors analysis is a relevant opportunity for early DILI detection by automated algorithms.
To describe DILI cases in COVID-19 inpatients detected from data mining in electronic medical records (EMR) using AI and the updated Roussel Uclaf Causality Assessment Method (RUCAM).
The study was conducted in March 2021 in a hospital in southern Brazil. The NoHarm© system uses AI to support decision making in clinical pharmacy. Hospital admissions were 100523 during this period, of which 478 met the inclusion criteria. From these, 290 inpatients were excluded due to alternative causes of liver injury and/or due to not having COVID-19. We manually reviewed the EMR of 188 patients for DILI investigation. Absence of clinical information excluded most eligible patients. The DILI assessment causality was possible via the updated RUCAM in 17 patients.
Mean patient age was 53 years (SD ± 18.37; range 22-83), most were male (70%), and admitted to the non-intensive care unit sector (65%). Liver injury pattern was mainly mixed, mean time to normalization of liver markers was 10 d, and mean length of hospitalization was 20.5 d (SD ± 16; range 7-70). Almost all patients recovered from DILI and one patient died of multiple organ failure. There were 31 suspected drugs with the following RUCAM score: Possible (n = 24), probable (n = 5), and unlikely (n = 2). DILI agents in our study were ivermectin, bicalutamide, linezolid, azithromycin, ceftriaxone, amoxicillin-clavulanate, tocilizumab, piperacillin-tazobactam, and albendazole. Lack of essential clinical information excluded most patients. Although rare, DILI is a relevant clinical condition in COVID-19 patients and may contribute to poor prognostics.
The incidence of DILI in COVID-19 inpatients is rare and the absence of relevant clinical information on EMR may underestimate DILI rates. Prospects involve creation and validation of alerts for risk factors in all DILI patients based on RUCAM assessment causality, alterations of liver biomarkers and AI and machine learning.
Core Tip: This is a real-life study that correlated hospital clinical pharmacy data with artificial intelligence (AI) and pharmacovigilance in coronavirus disease 2019 (COVID-19) inpatients. Inpatient screening for liver injury was made with AI and drug-induced liver injury was evaluated with the Roussel Uclaf Causality Assessment Method (RUCAM) algorithm. A total of 17 COVID-19 inpatients were evaluated, there were 31 suspected drugs, RUCAM score: possible (n = 24), probable (n = 5), and unlikely (n = 2). This study contributed to the patient safety and pharmacovigilance database. These results are included in a project of clinical pharmacy using AI tools.
- Citation: Ortiz GX, Ulbrich AHDPS, Lenhart G, dos Santos HDP, Schwambach KH, Becker MW, Blatt CR. Drug-induced liver injury and COVID-19: Use of artificial intelligence and the updated Roussel Uclaf Causality Assessment Method in clinical practice. Artif Intell Gastroenterol 2023; 4(2): 36-47
- URL: https://www.wjgnet.com/2644-3236/full/v4/i2/36.htm
- DOI: https://dx.doi.org/10.35712/aig.v4.i2.36
The coronavirus disease 2019 (COVID-19) pandemic put global health systems at risk of collapse worldwide, with more than 510270667 globally confirmed cases and 6233526 deaths in the past two years[1]. COVID-19 patients may be asymptomatic or develop severe acute respiratory syndrome (SARS) with mild to severe manifestations. As a multiple organ disease, extrapulmonary clinical features range from gastrointestinal to hematological effects. Critically ill patients or those with comorbidities commonly present venous and arterial thromboembolic events, liver injury, secondary bacterial infections including sepsis or cytokine storm, contributing to a poor prognosis and higher mortality rates[2-4]. Current treatment options can be supportive clinical management to drug use such as oxygen, dexamethasone for systemic inflammation, heparin/enoxaparin in prophylaxis of venous thromboembolism, and antiviral or monoclonal antibody such as remdesivir, baricitinib, tofacitinib, and tocilizumab[1,5].
Liver injury is a relevant condition in COVID-19 inpatients. In 2020, liver enzyme abnormalities were estimated to occur in 14% to 53% of patients[6,7]. The liver injury pattern is mild to moderate hepatocellular injury, considering aspartate aminotransferase (AST)/alanine aminotransferase (ALT) <5× the upper limit of normal (ULN) and cholestatic for Delta/Omicron variants[8,9]. High levels of AST and ALT, gamma-glutamyl transferase, and total bilirubin have been associated with severe COVID-19, intensive care unit (ICU) admission, and prolonged hospital stay[10-12]. Pathophysiology possibilities of liver injury in COVID-19 vary from direct infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hypoxic changes, systemic inflammation, exacerbation of underlying disease and adverse drug reactions. Drug-induced liver injury (DILI) may be present in COVID-19 patients due to wide exposure to multiple treatments with antipyretic, antibiotics, corticosteroids, immunomodulators, and antiviral drugs[13,14].
DILI is a rare adverse drug reaction (ADR) that can cause acute liver failure and even the need for liver transplantation in the worst cases[15]. The disease is classified as hepatocellular, cholestatic, or mixed[16]. The diagnosis is made by exclusion of other liver pathologies such as cirrhosis, viral hepatitis, auto-immune hepatitis or other chronic liver diseases. In clinical practice, relevant DILI occurs when patients present ALT>5×ULN with jaundice, hepatomegaly, hyperbilirubinemia, or right-upper-quadrant pain. In most DILI cases, withdrawal of suspected drug(s) and supportive therapy are the standard treatment practices[13,15,17]. DILI detection and monitoring is clinically relevant, as it may contribute to poor prognosis, prolonged hospitalization and increase indirect healthcare costs[12,14,15].
The updated Roussel Uclaf Causality Assessment Method (RUCAM) is a causality assessment tool strongly recommended by specialists worldwide to evaluate and correlate liver damage to drug/herb use due to its hepatic specificity[18]. The updated RUCAM was complemented by additional criteria to establish DILI with a high degree of certainty, such as clarification of ambiguous questions related to alcohol use, exclusion of non-drug causes as a checklist of differential diagnosis[16].
DILI causality is scored in RUCAM by seven domains: time to symptoms onset, ALT course, patient’s risk factors such as alcoholism and age, concomitant drug use, non-drug causes of liver injury, previous knowledge on the hepatotoxicity of the drug, and response to rechallenge. The individual points range from −3 to +3 and the total possible score ranges from −9 to +14. The interpretation of the final score is as follows: 0 or less indicates that the drug is “excluded” as a cause; 1 to 2 that it is “unlikely”; 3 to 5, “possible”; 6 to 8, “probable”; and greater than 8, “highly probable”. Although RUCAM provides an objective scoring system, its use still shows limitations regarding expert interpretation and comorbidities such as COVID-19 itself[13,19]. Lack of good quality evidence-based DILI is a reality in Brazil and its early detection by healthcare professionals is an important challenge[20]. DILI sub notification may be attributed to lack of knowledge on how to properly assess DILI and apply RUCAM.
Recent literature has shown the use of technologies to actively search and assess ADR. Artificial intelligence (AI) algorithm-based applications simulate human decision making based on large datasets and health information patterns[21,22]. In the field of in vitro studies, AI that uses prediction models based on the chemical structure of compounds and the exploration of 2D and 3D in vitro imaging data have demonstrated interesting results in predicting DILI for novel drugs[23]. In clinical practice, a systematic review demonstrated that AI has been used in COVID-19 for diagnosis, clinical decision making, drug discovery, vaccine development and surveillance and chest images classification[21].
Literature has shown interesting AI tools to detect and analyze COVID-19 patients such as rapid classification of medical images (X-ray) through convolutional neural networks using datasets with positive and negative images of COVID-19 inpatients[24]. Specifically for DILI, AI applied in data mining of EMR combining abnormal liver tests, keyword searching tools, and risk factors analysis is a relevant opportunity for automated algorithms to detect possible DILI cases early[25].
Despite global large-scale vaccination slowing the advance of the COVID-19 pandemic, the disease remains a serious concern, demanding clinical investigation and elucidation. Polypharmacy and off label drugs use in COVID-19 are an alert for DILI screening since they can contribute to poor clinical outcomes. This observational study aims to describe DILI cases in COVID-19 inpatients retrospectively detected using AI from data mining in EMR. We intend to discuss the hepatotoxicity profile of drugs used in COVID-19 detected cases assessed by RUCAM, as well as the current prospects and challenges of applying AI and the updated RUCAM in inpatient evaluation that may contribute to patient safety and pharmacovigilance practices.
We conducted a descriptive retrospective study investigating alterations in liver markers in patients diagnosed with COVID-19 who were admitted to a reference COVID-19 hospital complex in southern Brazil during March 2021. The hospital complex comprises 7 hospitals with different specialties such as cardiology, pulmonology, neurology, pediatrics, and general care, with emergencies, ICU, and a surgical center. In March, 100523 patients were admitted.
We included all patients aged 18 years or more who had COVID-19 diagnosed by real-time polymerase chain reaction assays, with at least one complete set of ALT, AST, and alkaline phosphatase (ALP) results during their inpatient stay and with at least one normal and one abnormal ALT value. We excluded patients with liver injuries defined by other etiologies such as viral hepatitis, alcoholic hepatitis, hepatocellular carcinoma, autoimmune hepatitis, cytomegalovirus, leptospirosis, Epstein Barr, hemolytic diseases, among other hepatobiliary disorders.
The hospital is associated with the NoHarm©[26], a system that uses AI to support decision making in clinical pharmacy. It currently develops two algorithms to optimize pharmacist validation for prioritizing non-standard prescriptions and identifying critical patients. The system is linked to hospital data and indicates potential prescription errors, increasing quality of care and hospital efficiency. We used this AI platform to automatically screen EMR of inpatients with ALT>3×ULN who were suspected of having DILI. We accessed each patient in this platform to check inclusion/exclusion criteria. Afterwards, a chart with ALT course during hospitalization was presented to guide investigators in which days relevant clinical information should be collected. The ALT>3×ULN was chosen to be a cut off to pre-analyze patients for DILI.
We applied the updated RUCAM to all suspected cases of DILI[27]. Then, two independent reviewers (GL and GXO) separately assessed the likelihood of altered liver tests being related to drug use during hospital stay. In the case of disagreement, a third pharmacist reviewed the suspected DILI case. If there was still no consensus, the case was discussed with a fourth hepatology specialist. The domains evaluated were liver injury pattern, timing of events, rechallenge, risk factors, comedications, alternative causes, hepatotoxicity previously established in the scientific literature, and response to unintentional rechallenge.
The RUCAM classifies DILI as highly probable (≥ 9), probable (6-8), possible (3-5), unlikely (1-2), or excluded (≤ 0)[16]. Cases scored as highly probable, probable, and possible were considered as DILI. Since this is a retrospective study, not all patients would have the same profile of laboratory tests to rule out other causes of abnormal liver chemistries. The RUCAM is used for DILI diagnosis in individual cases, case series, registries, or epidemiological studies involving any types of drugs, herbal medicines, or dietary supplements. Thus, assessments were only based on information available in EMR. Missed information in EMR may compromise RUCAM scores, decreasing punctuation as well as the likelihood of DILI. Drugs with a well-established pattern of absence of liver toxicity in which they were used at usual doses and treatment times were not included in the RUCAM analysis, unless they were the only drug used and the timeframe of events were highly compatible with DILI. Liver injury pattern is defined by the R (the ratio of ALT and ALP expressed as multiples of the ULN) and corresponds to: (1) Hepatocellular if R≥5, (2) mixed if 2<R<5, and (3) cholestatic if R≤2.
Data were constituted by the patient’s profile, symptoms, drugs, laboratory tests, image and biopsy exams, if available, hospitalization time, and outcomes. Due to the small sample, statistical analysis of independent variables (risk factors) of DILI was not performed. However, it did not compromise the purpose of the study which is to describe possible DILI cases and suspected COVID-19 medications. The analysis evaluated DILI causality.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations checklist was applied to this study to facilitate critical appraisal and interpretation of cross-sectional study results[28]. This study was approved by the Ethics Committee (number 4763390 CAAE 46652521.9.0000.5530).
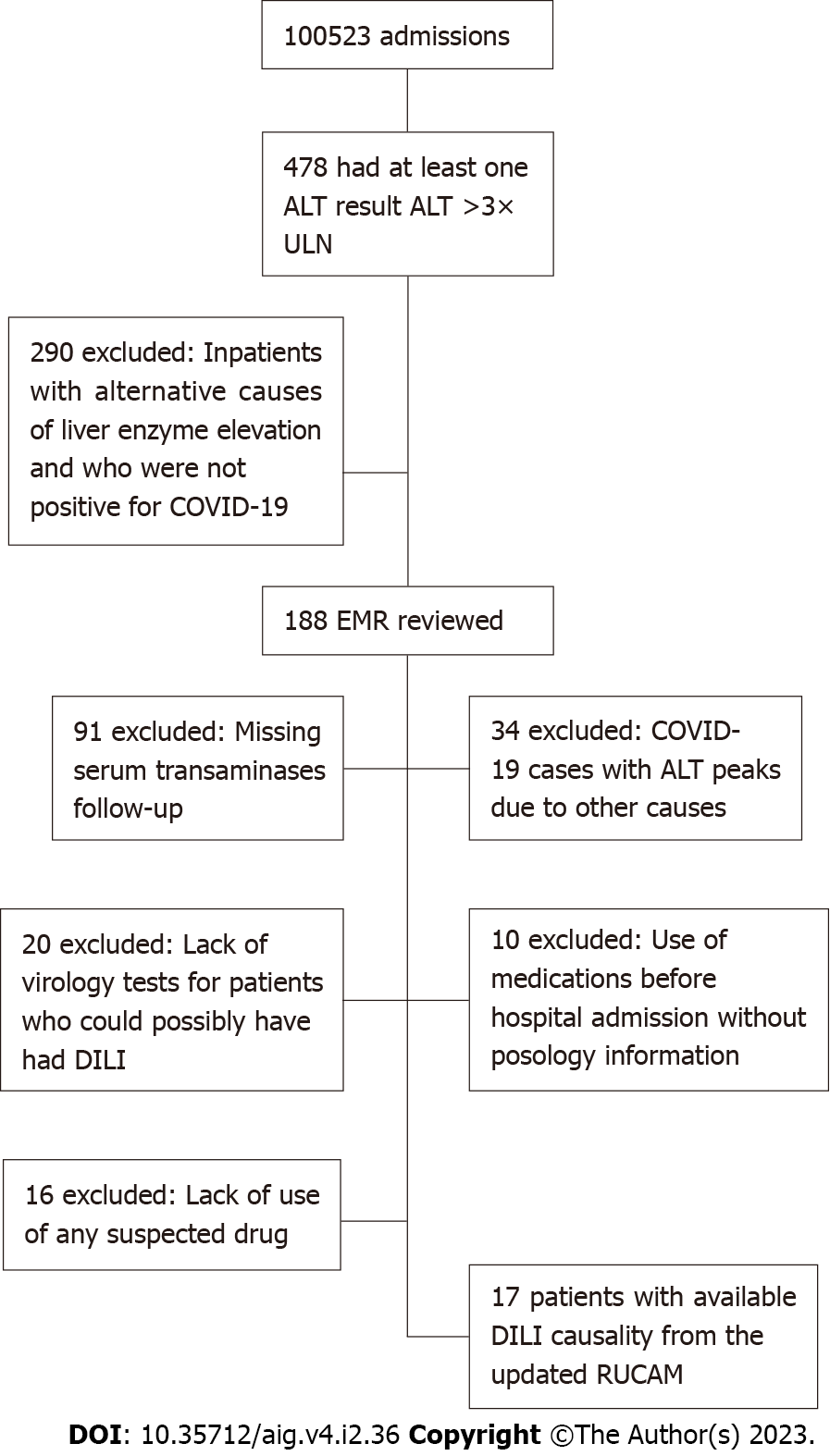
In total, 100523 patients were admitted into the hospital in March 2021, with 478 inpatients showing at least one ALT result >3×ULN detected by AI. Of these, we excluded 290 inpatients with alternative causes of liver enzyme elevation, and who were not positive for COVID-19. We reviewed 188 EMR. From these, absence of essential clinical information excluded most patients. Figure 1 summarizes patients’ inclusion and exclusion criteria. The main missing information was serum transaminases follow-up in 91 patients as the patient was released for outpatient follow-up at the slightest improvement. A total of 34 patients had COVID-19 but the ALT peaks were due to other causes such as sepsis, cardiopulmonary arrest, or hypovolemic shock; 16 patients did not use any suspected drug; and 10 patients used medications before hospital admission with no posology information. Lack of virology tests excluded 20 patients who could possibly have had DILI. Drugs used in those patients were chlorpromazine, azithromycin, ceftriaxone, bicalutamide, and ivermectin (off-label use). All of them are well established common DILI agents. We assessed DILI causality with the updated RUCAM in 17 patients and Table 1 shows the complete RUCAM score.
| Patient ID | Age/sex | ALT peak (U/L) | Hospital sector | Type of liver injury | Suspected drug | Posology/time treatment | RUCAM score | Causality | DILI diagnosis in EMR | Outcomes (timeframe) | Hospital length of stay (d) |
| 1 | 83/M | 388 | ICU | Unclassified | Ivermectin | 48 mg daily/6 d | 3 | Possible | No | Recovered (10 d) | 18 |
| Bicalutamide | 150 mg daily/8 d | 2 | Unlikely | ||||||||
| Linezolid | 600 mg 12/12 h/5 d | 4 | Possible | ||||||||
| Azithromycin | 500 mg daily/5 d | 4 | Possible | ||||||||
| Ceftriaxone | 2 g 12/12 h/3 d | 4 | Possible | ||||||||
| 2 | 61/M | 269 | ICU | Mix | Amoxicillin + Clavulanate | 625 mg 8/8 h/3 d | 7 | Probable | No | Recovered (7 d) | 40 |
| Azithromycin | 500 mg daily/7 d | 7 | Probable | ||||||||
| Ceftriaxone | 1 g 12/12 h/5 d | 7 | Probable | ||||||||
| 3 | 62/M | 307 | ICU | Mix | Tocilizumab | 800 mg/1 d | 1 | Unlikely | No | Recovered (12 d) | 31 |
| 4 | 67/M | 222 | Non-ICU | Cholestatic | Azithromycin | 500 mg daily/3 d | 3 | Possible | No | Recovered (25 d) | 28 |
| Ceftriaxone | 1 g 12/12 h/4 d | 3 | Possible | ||||||||
| 5 | 69/F | 588 | ICU | Cholestatic | Azithromycin | 500 mg daily/5 d | 4 | Possible | No | Death | 25 |
| Ceftriaxone | 2 g daily/7 d | 4 | Possible | ||||||||
| 6 | 78/M | 163 | Non-ICU | Unclassified | Ceftriaxone | 2 g daily/7 d | 3 | Possible | No | Recovered (23 d) | 70 |
| 7 | 55/M | 682 | Non-ICU | Hepatocellular | Azithromycin | 500 mg daily/5 d | 3 | Possible | No | Recovered (6 d) | 8 |
| Ivermectin | 18 mg daily/4 d | 4 | Possible | ||||||||
| 8 | 50/F | 157 | Non-ICU | Unclassified | Ivermectin | 18 mg daily/5 d | 3 | Possible | No | Recovered | 7 |
| 9 | 49/F | 127 | ICU | Cholestatic | Azithromycin | 500 mg daily/6 d | 3 | Possible | No | Recovered (2 d) | 17 |
| Ceftriaxone | 2 g daily/9 d | 3 | Possible | ||||||||
| 10 | 57/M | 274 | Non-ICU | Mix | Azithromycin | 500 mg daily/5 d | 6 | Probable | No | Recovered (4 d) | 10 |
| 11 | 22/M | 312 | Non-ICU | Hepatocellular | Azithromycin | 500 mg daily/4 d | 4 | Possible | No | Recovered | 5 |
| Ceftriaxone | 1 g 12/12 h/3 d | 4 | Possible | ||||||||
| 12 | 33/M | 551 | Non-ICU | Hepatocellular | Ceftriaxone | 1 g 12/12 h/53 d | 3 | Possible | No | Recovered | 8 |
| 13 | 61/F | 168 | Non-ICU | Mix | Piperacillin + tazobactam | 4.5 g 8/8 h/9 d | 6 | Probable | No | Recovered | 21 |
| 14 | 32/M | 489 | Non-ICU | Cholestatic | Ceftriaxone | 2 g daily/5 d | 5 | Possible | No | Recovered (10 d) | 10 |
| 15 | 73/M | 160 | Non-ICU | Mix | Ceftriaxone | 1 g daily/8 d | 6 | Possible | No | Recovered | 15 |
| 16 | 29/F | 369 | Non-ICU | Mix | Amoxicillin + clavulanate | 625 mg 8/8 h/3 d | 4 | Possible | Yes | Recovered | 15 |
| 17 | 33/M | 213 | ICU | Mix | Azithromycin | 500 mg daily/5 d | 5 | Possible | No | Recovered (10 d) | 22 |
| Piperacillin + tazobactam | 4.5 g 8/8 h/10 d | 4 | Possible | ||||||||
| Ivermectin | 18 mg daily/1 d | 5 | Possible | ||||||||
| Albendazole | 400 mg daily/2 d | 5 | Possible |
The mean age of patients was 53 years (SD ± 18.37; range 22-83). Most were male (70%), and admitted to the non-ICU sector (65%). The liver injury pattern was mainly mixed with mean normalization of liver injury within 10 d and mean length of hospitalization was 20.5 days (SD ± 16; range 7-70). Almost all patients recovered from DILI and one patient died of multiple organ failure. There were 31 suspected drugs with 2 medications suspected per inpatient. The RUCAM score distribution was as follows: 24 possible cases, 5 probable, and 2 unlikely. The DILI agents in our study were ivermectin, bicalutamide, linezolid, azithromycin, ceftriaxone, amoxicillin-clavulanate, tocilizumab, piperacillin-tazobactam, and albendazole. Antimicrobials were 77% of the suspected agents, followed by antiparasitic 16%, and antineoplastic and immunomodulating agents 7%.
The DILI prevalence was 1.6 to every 10000 inpatients in this study differing from the 9.8% in China[29]. The first review of Brazilian DILI case reports was published in 2019 showing DILI as a rare ADR; however, this is controversial since DILI underreporting is a major concern leading to alarmingly low rates of its suspicion and identification[30]. The DILI prevalence in hospitalized patients tends to be higher than in our study. A prospective study from France reported a DILI prevalence of 6.6 per 1000 per week among hospitalized patients. DILI incidence in Switzerland on hospitalization admission was 0.7% and in Turkey prevalence among inpatients was 3.1%[31]. In Iceland, the crude annual incidence rate of DILI was 19.1 cases per 100000 patients[32]. The discrepancy may be related to the calculation of prevalence since we only entered patients whose causality could be assessed with RUCAM unlike other studies that consider DILI if the patient had all other liver diseases excluded.
Most (77%) of our patients with ALT above 5 times the UNL were mainly men and had mild to moderate DILI. Regarding clinical features, COVID-19 Chinese DILI patients’ median age was 61 years, they were mainly men, the mean (SD) hospital stay was 21.49 (11.89) d, and the mean (SD) days for the first acute liver injury was 9.57 (9.38) after admission[29]. These findings corroborate with our study, demonstrating a set of specific clinical features for patients with DILI and COVID-19.
Almost all patients’ normalized liver markers showed a time frame from drug suspension to recovery ranging from 2 to 25 d. Our findings were consistent with the scientific literature of DILI for COVID-19 and non-COVID-19, suggesting a moderate severity of liver injury, rarely progressing to severe and spontaneously recovering. Even so, clinically monitoring DILI is recommended since it can contribute to a reserved prognosis and prolong length of hospital stay (LOS)[15,33]. Prolonged LOS is associated with negative patient experience and inpatient complications such as infections and falls[34]. None of the patients received drug treatment specifically for DILI besides clinical monitoring and suspected drug suspension.
Serum transaminase elevation presented no determined cause for 171 of our patients and DILI could neither be confirmed nor excluded. This finding suggests that DILI diagnosis remains a challenge due to its multifactorial characteristics and many confounders. Proper diagnosis will depend on the healthcare professional’s familiarity with DILI[30]. The fifth RUCAM domain decreases DILI causality if there is no information for any of the following viruses: hepatitis C virus (HCV), hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis E virus (HEV). In our cases, if virology results were available and negative, drugs scored as “Unlikely” would change to “Possible”, suggesting underestimation of DILI prevalence. Medication reconciliation was incomplete for 10 patients, making it infeasible to apply RUCAM. Drugs used before hospital admissions were ivermectin, azithromycin, cefuroxime, hydroxychloroquine, dexamethasone, and prednisone, but posology and duration of treatment were not available in EMR.
Another study evaluating the association between drug treatments and incidence of liver injury and DILI in inpatients with COVID-19 presented the same limitation, since 10% of the suspected cases were excluded due to incomplete EMR[29]. The more incomplete the relevant clinical information, such as the presence of serologies to exclude other possible causes of liver injury, the less likely it is to assign adequate scores to drugs that may be associated with DILI. Considering this scenario and our findings, quality data on DILI cases assessed by RUCAM in COVID-19 patients are scarce[33].
Literature demonstrates that suspected DILI agents in COVID-19 are mostly antiviral, antibacterial, antifungal drugs, hydroxychloroquine/chloroquine, corticosteroids, and immunotherapy[29]. In a systematic review of 996 DILI cases published in 2020/2021 based on RUCAM as a causality assessment method, antiviral drugs given empirically were mainly responsible for DILI. Liver injury pattern was mainly hepatocellular, differently from our prevalence of mixed pattern. This may be associated with different types of drugs used, as they have diverse DILI pathology mechanisms. Most patients had a positive outcome most likely due to quick cessation of drug treatment, even though DILI was fatal in 19 cases. The ALT and AST peak values were 1.541 U/L and 1.076 U/L, respectively[33]. In contrast, our study showed a maximum ALT of 682 U/L and AST of 556 U/L.
In active search for DILI cases in COVID-19 patients, using lower cutoff points such as ALT one to three times the ULN instead of five times the ULN is preferable, so that no case is missed, since many medicines are used off label and DILI data could demonstrate different hepatotoxicity profiles. Comparison of different liver test thresholds for DILI in hospital patients demonstrate that higher cut-offs, such as ALT levels greater than 5 × the ULN on two consecutive occasions and/or ALP levels greater than 2 × the ULN on two consecutive occasions, as proposed by the DILI Network, are more effective in detecting relevant cases; however, it misses positive cases that were found when the National Medical Products Administration of China, using ALT levels greater than 1 × the ULN on two consecutive occasions or ALT levels greater than 2 × the ULN. In other words, much more labor is required to detect DILI at lower thresholds, despite higher negative predictive values[35]. Automated algorithms to detect DILI using AI may overcome the time-consuming limitation of lower cut-offs, such as when it uses ALT > 3× ULN, as it performs better in sensitivity, a desirable characteristic to detect rare events and avoid missing cases[36]. ALT values 5×> ULN are considered clinically significant, as they will be the best signal allied to symptoms for physicians to consider stop suspected drug treatment[13].
The DILI agents in our study were ivermectin, bicalutamide, linezolid, azithromycin, ceftriaxone, amoxicillin-clavulanate, tocilizumab, piperacillin-tazobactam, and albendazole. The updated Brazilian report of clinical guidelines for inpatient treatment of COVID-19[37] indicates: (1) Anticoagulants: Unfractionated heparin, enoxaparin, or fondaparinux; (2) corticosteroids: Dexamethasone, methylprednisolone, or hydrocortisone; and (3) antimicrobials according to institutional protocols only in the suspected presence of associated bacterial infection. The report demonstrates the lack of evidence for clinical benefit in the hospitalized patient regarding the use of: Azithromycin, chloroquine, colchicine, hydroxychloroquine, ivermectin, and lopinavir/ritonavir. Attention is needed to the irrational use of azithromycin and ivermectin since those drugs have no evidence of clinical benefits in patients with COVID-19 and, as our findings suggest, may even worsen liver injury[38-40].
Antibiotics were the most common agents both in this study and the international literature[17,41]. Ceftriaxone was the main DILI suspected causative agent (10 cases) followed by azithromycin (9 cases). Ceftriaxone is very likely causative of cholestatic injury with minimal symptoms due to crystallization of ceftriaxone in bile present in the gallbladder[42]. Azithromycin is a well-known but rare cause of clinically apparent self-limited cholestatic hepatitis with rapid resolution of symptoms whether the drug is stopped or not[43]. Amoxicillin-clavulanate is the main one of the top-ranking drugs implicated in RUCAM-based DILI cases retrieved from large international medical centers[41]. It causes mostly self-limited cholestatic or mixed idiosyncratic DILI. The onset of injury is a few days to 8 wk after therapy initiation[44,45]. It appeared in only one case probably related to prescription pattern rather than prevalence ratio. Piperacillin-tazobactam rarely causes self-limited cholestatic idiosyncratic DILI[46].
Ivermectin and albendazole are antiparasitic agents used off-label for COVID-19 treatment. Ivermectin presented in vitro antiviral effects and a few studies suggested clinical benefits against COVID-19 in the early stages of the pandemic[47]. However, medical centers and governmental organizations such as the Food and Drug Administration and the World Health Organization (WHO) rapidly identified the poor quality data of the publications and ivermectin use was not recommended[48]. The DILI caused by ivermectin and albendazole is extremely rare, with one case report[49]. Ivermectin ADR with COVID-19 reported in the WHO’s pharmacovigilance database evidenced a considerable increase (> 50%) in ivermectin related reports since May 2020. Among 53 serious cases, eight cases presented gastrointestinal ADR including one death[50]. Ivermectin is not a potential hepatotoxicity agent; however, in our study, dosages were considerably higher than recommendations leading to causality assessment of “possible” DILI agent.
The COVID-19 patients with DILI presented polypharmacy with acetaminophen, azithromycin, ceftriaxone, dexketoprofen, doxycycline, enoxaparin, hydroxychloroquine, interferon, levofloxacin, lopinavir, metamizole, omeprazole, pantoprazole, piperacillin-tazobactam, remdesivir, ritonavir, and tocilizumab[51]. Domain four of the updated RUCAM[16] regarding concomitant use of drugs lowers scores of a specific drug assessed if the patient is also using another hepatotoxic drug. In the COVID-19 polypharmacy scenario, this is a controversial concern since suspected cases previously scored as “possible” or “probable” would change to high causality association if hepatotoxicity synergism was considered.
It is controversial that the combination of hepatotoxic drugs may increase the risk of DILI. Evidence exists that rifampicin increases the risk of hepatotoxicity when combined with isoniazid for tuberculosis treatment due to a synergistic effect. Anti-tuberculosis treatment combined with non-nucleoside reverse transcriptase inhibitors and protease inhibitors is more likely to cause DILI than both treatments alone[52]. Concomitant administration of drugs metabolized by the liver (via CYP450) modulates active metabolites via induction, inhibition, or substrate competition and may increase DILI risk[53]. On the other hand, RUCAM decreases an individual medication’s score when concomitant hepatotoxic drugs are co-administered, since sufficient evidence regarding synergistic hepatotoxicity of drugs beyond antiretroviral and anti-tuberculostatic is still lacking[16]. We found only one article summarizing possible ADR worsening by drug-drug interactions in COVID-19, which specifically mentioned remdesivir vs rifampicin and ribavirin and human immunodeficiency virus antiviral treatment contraindication due to hepatotoxicity[54]. As a result, especially for COVID-19, cases of DILI could be missed or sub classified after a RUCAM score considering the fourth domain.
Intrinsic DILI is drug dose-dependent, thus the risk of developing liver injury may increase accordingly when the potential safety range of the drug dose is exceeded. In addition, lipophilicity molecules may enter hepatocytes and hepatic metabolism, which hypothetically could increase DILI risk[31]. Alcohol consumption is included in the RUCAM causality assessment scale as a risk factor due to liver metabolization, especially regarding acetaminophen, isoniazid, methotrexate, and halothane[55]. In our study, none of the patients were heavy alcohol consumers excluding this risk factor and probable confounder. The role of pre-existing liver disease on DILI is yet to be completely understood.
There is no consensus if chronic hepatic diseases, such as non-alcoholic fatty liver disease, hepatocellular carcinoma, or viral hepatitis, could worsen DILI severity outcomes[31]. Currently, RUCAM decreases DILI causality if the patient has HAV (type A viral hepatitis), HBV (type B viral hepatitis), HCV (type C viral hepatitis), HEV (type E viral hepatitis), ultrasound alterations of the hepatobiliary tract, acute hypotension (especially cardiac arrest), sepsis, malignant metastatic disease, autoimmune hepatitis, chronic hepatitis, primary biliary cholangitis, sclerosing cholangitis, genetic liver diseases, Cytomegalovirus infection, Epstein-Barr Virus infection, Herpes Simplex Virus infection, and Varicella Zoster Virus infection[16].
Idiosyncratic DILI occurs independently of drug dose, route, duration of treatment, or administration. The RUCAM score increases if patients are 65 years or older, and older age is considered a risk factor. The underlying mechanisms include declination in liver capacity, underlying diseases and alterations in pharmacokinetics features[56]. Data from the WHO Safety Report Database revealed that elderly patients were much more likely to develop cholestatic DILI[32]. Even though gender is not independently associated with DILI, cases are preponderantly in females, especially severe and immune-mediated DILI. Nowadays, literature demonstrates that polymorphisms of genes involved in drug metabolism and transport and human leukocyte antigen are risk factors for DILI.
COVID-19 itself has been associated with transaminase elevation either caused by viral direct damage, hypoxemia, or multisystem inflammatory syndrome. Thus, we suggest reviewing the updated RUCAM’s domain number five and adding COVID-19 as an alternative cause of increased ALT/AST. The systematic review of 966 DILI cases in COVID-19 patients also evidenced barriers to properly determine the quantitative contribution of DILI and COVID-19 in abnormal liver tests, suggesting COVID-19 as a DILI confounder[31]. Although we have identified these possible weaknesses in applying the RUCAM to assess DILI in COVID-19 patients, the algorithm remains the best choice to assist health care professionals in the diagnosis and causality assessment of liver injury when alternative causes are excluded[57]. We strongly recommend the prospective use of RUCAM in healthcare services to overcome poor DILI clinical assistance.
The main challenges in the DILI field refer to early detection and diagnosis by healthcare professionals. Using AI and machine learning may be the key to overcoming this scenario. Literature has focused on AI on quantitative structure activity relationship analysis to predict hepatotoxicity substances in drug development with interesting contributions[58,59]. However, relevant clinical application of AI would consist of machine learning algorithms automatically and prospectively tracing DILI in EMR and patients’ hospitals datasets by crosslinking DILI threshold criteria, risk factors, liver injury International Classification of Diseases (ICD-10) codes, liver tests, and data mining[60,61]. In the next step, an alert would be triggered in electronic systems to physicians and clinical pharmacists informing of potential DILI cases for clinical follow-up[62].
Research that used EMR algorithms for DILI screening found low positive predictive values (PPV)[13]. A recent meta-analysis showed that PPV was only 14.6% for machine learning and AI in EMR. Divergences in liver tests reference values and DILI threshold criteria among different studies decrease comparisons and evaluations of sensitivity and specificity[25]. In this study, we used AI to automatically detect COVID-19 patients with abnormal liver markers and compiled data with dashboards to identify key days patients should be investigated. However, the updated RUCAM application was still manually performed. Prospects in our work involves creating and validating signals for pharmacists generated from automated scores for all DILI patients based on RUCAM assessment causality, AI, and machine learning.
This study has the limitations of a retrospective study design. Incomplete case datasets in EMR that could impact different RUCAM scores may underestimate the prevalence of DILI as well as the hepatotoxicity profile of the suspected drug. No statistical analysis was performed due to the small and non-homogenous sample. Therefore, these findings may only suggest associations, propose insight into DILI, and guide further investigations since the results lack external validation. The assessment of DILI and vaccines was not performed as it was out of the study scope.
Our study shows that DILI has a rare incidence in COVID-19 inpatients and the absence of relevant clinical information on EMR may underestimate DILI rates. Abnormal liver tests such as ALT and AST are important triggers to detect DILI, but since they lack specificity a complete evaluation of the patient is necessary for a proper diagnosis. The DILI features in COVID-19 inpatients are provided by age, gender, patients, suspected drugs, type of injury, laboratory data, and clinical outcomes and these findings are consistent with DILI literature of non-COVID-19 cases.
The DILI diagnosis is still a challenge due to its multifactorial character and many confounding factors, including COVID-19, and its early detection by health professionals is an important challenge. The updated RUCAM is the standard tool to assess hepatotoxicity and future research must focus on its prospective applicability to improve DILI quality data. The use of AI in clinical pharmacy decision support in conjunction with RUCAM can contribute to patient safety and pharmacovigilance practices, improving clinical outcomes.
Liver injury is a relevant condition in coronavirus disease 2019 (COVID-19) inpatients. Drug-induced liver injury (DILI) may be present in COVID-19 patients due to wide exposure to multiple treatments. Artificial intelligence (AI) applications are interesting tools for early detection of DILI cases in hospitals using electronic medical records.
DILI detection and monitoring is clinically relevant, as DILI may contribute to poor prognosis, prolonged hospitalization and increase indirect healthcare costs.
To demonstrate the use of AI and the updated Roussel Uclaf Causality Assessment Method (RUCAM) to detect DILI cases from data mining in electronic medical records (EMR) of COVID-19 inpatients.
The study was conducted in March 2021 in a hospital in southern Brazil. Hospital admissions were 100523 during this period. The NoHarm© system uses AI to support decision making in clinical pharmacy. 478 cases met the inclusion criteria and from these, 290 inpatients were excluded due to alternative causes of liver injury and/or due to not having COVID-19. We manually reviewed the EMR of 188 patients for DILI investigation. Absence of clinical information excluded most eligible patients. The updated RUCAM was applied to all suspected cases of DILI.
In total, 17 COVID-19 inpatients were evaluated and there were 31 suspected drugs with the following RUCAM score: possible (n = 24), probable (n = 5), and unlikely (n = 2). DILI agents were ivermectin, bicalutamide, linezolid, azithromycin, ceftriaxone, amoxicillin-clavulanate, tocilizumab, piperacillin-tazobactam, and albendazole. Lack of essential clinical information excluded most patients.
These results are included in a project of clinical pharmacy using AI tools. Future research must focus on the prospective applicability of the updated RUCAM to improve DILI quality data. The use of AI in clinical pharmacy decision support in conjunction with RUCAM can contribute to patient safety and pharmacovigilance practices, improving clinical outcomes.
These results are included in a project of clinical pharmacy using AI tools. Future research must focus on the prospective applicability of the updated RUCAM to improve DILI quality data. The use of AI in clinical pharmacy decision support in conjunction with RUCAM can contribute to patient safety and pharmacovigilance practices, improving clinical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Mijwil MM, Iraq S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | World Health Organization. COVID-19 Dashboard. Geneva: World Health Organization, 2020 [cited 30 April 2022]. Available from: https://covid19.who.int/. |
| 2. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2049] [Article Influence: 409.8] [Reference Citation Analysis (2)] |
| 3. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 4. | Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health, 2022 [cited April 30, 2022]. Available from: https://www.covid19treatmentguidelines.nih.gov/. |
| 6. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18881] [Article Influence: 3776.2] [Reference Citation Analysis (7)] |
| 7. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 8. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30124] [Article Influence: 6024.8] [Reference Citation Analysis (3)] |
| 9. | Deng H, Lin H, Mai Y, Liu H, Chen W. Clinical features and predictive factors related to liver injury in SARS-CoV-2 Delta and Omicron variant-infected patients. Eur J Gastroenterol Hepatol. 2022;34:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2021;45:101556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Clinton JW, Kiparizoska S, Aggarwal S, Woo S, Davis W, Lewis JH. Drug-Induced Liver Injury: Highlights and Controversies in the Recent Literature. Drug Saf. 2021;44:1125-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Council for International Organizations of Medical Sciences. Drug-induced liver injury (DILI): Current status and future directions for drug development and the post-market setting. A consensus by a CIOMS Working Group. 2020; Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS).. [DOI] [Full Text] |
| 16. | Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 17. | Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019;42:365-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Danan G, Teschke R. Roussel Uclaf Causality Assessment Method for Drug-Induced Liver Injury: Present and Future. Front Pharmacol. 2019;10:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Ghabril M, Gu J, Yoder L, Corbito L, Dakhoul L, Ringel A, Beyer CD, Vuppalanchi R, Barnhart H, Hayashi PH, Chalasani N. Significant Medical Comorbidities Are Associated With Lower Causality Scores in Patients Presenting With Suspected Drug-Induced Liver Injury. Clin Transl Gastroenterol. 2020;11:e00141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Becker MW, Fontoura LN, Blatt C R. (2020). Pharmacovigilance of drug-induced liver injury in search for frequency and outcomes in a Brazilian hospital: Challenges in future cases using a robust causality assessment method such as the updated RUCAM. JMMC. 2020;8:65-73. [DOI] [Full Text] |
| 21. | Wang L, Zhang Y, Wang D, Tong X, Liu T, Zhang S, Huang J, Zhang L, Chen L, Fan H, Clarke M. Artificial Intelligence for COVID-19: A Systematic Review. Front Med (Lausanne). 2021;8:704256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Mijwil MM, Abttan RA, Alkhazraji A. Artificial intelligence for COVID-19: A Short Article. AJPNMS. 2022;10. [DOI] [Full Text] |
| 23. | Vall A, Sabnis Y, Shi J, Class R, Hochreiter S, Klambauer G. The Promise of AI for DILI Prediction. Front Artif Intell. 2021;4:638410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Mukherjee H, Ghosh S, Dhar A, Obaidullah SM, Santosh KC, Roy K. Deep neural network to detect COVID-19: one architecture for both CT Scans and Chest X-rays. Appl Intell. 2021;51:2777-2789. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 25. | Tan EH, Low EXS, Dan YY, Tai BC. Systematic review and meta-analysis of algorithms used to identify drug-induced liver injury (DILI) in health record databases. Liver Int. 2018;38:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | NoHarm®. [cited June 12 2022]. Available from: https://noharm.ai/en#funcionalidades. |
| 27. | Lunardelli MJM, Becker MW, Blatt CR. Tradução e validação de algoritmo para identificação de lesão hepática induzida por medicamentos. Rev Cont Saúde. 2020;20:226-235. [DOI] [Full Text] |
| 28. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3559] [Cited by in RCA: 5843] [Article Influence: 324.6] [Reference Citation Analysis (0)] |
| 29. | Gao S, Yang Q, Wang X, Hu W, Lu Y, Yang K, Jiang Q, Li W, Song H, Sun F, Cheng H. Association Between Drug Treatments and the Incidence of Liver Injury in Hospitalized Patients With COVID-19. Front Pharmacol. 2022;13:799338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 30. | Becker MW, Lunardelli MJM, Tovo CV, Blatt CR. Drug and herb-induced liver injury: A critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann Hepatol. 2019;18:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Li X, Tang J, Mao Y. Incidence and risk factors of drug-induced liver injury. Liver Int. 2022;42:1999-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 32. | Ahmad J, Odin JA. Epidemiology and Genetic Risk Factors of Drug Hepatotoxicity. Clin Liver Dis. 2017;21:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Teschke R, Méndez-Sánchez N, Eickhoff A. Liver Injury in COVID-19 Patients with Drugs as Causatives: A Systematic Review of 996 DILI Cases Published 2020/2021 Based on RUCAM as Causality Assessment Method. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: A mixed-studies systematic review. Health Expect. 2018;21:41-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 35. | Yang H, Guo D, Xu Y, Zhu M, Yao C, Chen C, Jia W. Comparison of Different Liver Test Thresholds for Drug-Induced Liver Injury: Updated RUCAM versus Other Methods. Front Pharmacol. 2019;10:816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Tan EH, Ling ZJ, Ang PS, Sung C, Dan YY, Tai BC. Comparison of laboratory threshold criteria in drug-induced liver injury detection algorithms for use in pharmacovigilance. Pharmacoepidemiol Drug Saf. 2020;29:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. |
|
| 38. | Ortiz GX, Lenhart G, Becker MW, Schwambach KH, Tovo CV, Blatt CR. Drug-induced liver injury and COVID-19: A review for clinical practice. World J Hepatol. 2021;13:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Gyselinck I, Janssens W, Verhamme P, Vos R. Rationale for azithromycin in COVID-19: an overview of existing evidence. BMJ Open Respir Res. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 41. | Teschke R. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin Drug Metab Toxicol. 2018;14:1169-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. Ceftriaxone. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [cited May 15 2022] Available from: https://www.ncbi.nlm.nih.gov/books/NBK548258/. |
| 43. | LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. Azithromycin. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases [cited May 15 2022] Available from: https://www.ncbi.nlm.nih.gov/books/NBK548434/. |
| 44. | Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J; United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340-52.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 45. | LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. Amoxicillin-Clavulanate. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [cited May 2022] Available from: https://www.ncbi.nlm.nih.gov/books/NBK548517/. |
| 46. | LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. Piperacillin-Tazobactam. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [cited May 15 2022] Available from: https://www.ncbi.nlm.nih.gov/books/NBK548825/. |
| 47. | Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo). 2020;73:593-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 48. | Lawrence JM, Meyerowitz-Katz G, Heathers JAJ, Brown NJL, Sheldrick KA. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med. 2021;27:1853-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Veit O, Beck B, Steuerwald M, Hatz C. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg. 2006;100:795-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 50. | Campillo JT, Faillie JL. Adverse drug reactions associated with ivermectin use for COVID-19 reported in the World Health Organization's pharmacovigilance database. Therapie. 2022;77:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Delgado A, Stewart S, Urroz M, Rodríguez A, Borobia AM, Akatbach-Bousaid I, González-Muñoz M, Ramírez E. Characterisation of Drug-Induced Liver Injury in Patients with COVID-19 Detected by a Proactive Pharmacovigilance Program from Laboratory Signals. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Ortega-Alonso A, Stephens C, Lucena MI, Andrade RJ. Case Characterization, Clinical Features and Risk Factors in Drug-Induced Liver Injury. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Yu K, Geng X, Chen M, Zhang J, Wang B, Ilic K, Tong W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab Dispos. 2014;42:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Rezaee H, Pourkarim F, Pourtaghi-Anvarian S, Entezari-Maleki T, Asvadi-Kermani T, Nouri-Vaskeh M. Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol Res Perspect. 2021;9:e00705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 56. | Zhong XB, Lai Y. Special Section On Drug Metabolism in Liver Injury and Repair-Editorial. Drug Metab Dispos. 2022;50:634-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 57. | Teschke R, Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis. Medicines (Basel). 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 58. | Chierici M, Francescatto M, Bussola N, Jurman G, Furlanello C. Predictability of drug-induced liver injury by machine learning. Biol Direct. 2020;15:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Liew CY, Lim YC, Yap CW. Mixed learning algorithms and features ensemble in hepatotoxicity prediction. J Comput Aided Mol Des. 2011;25:855-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Minerali E, Foil DH, Zorn KM, Lane TR, Ekins S. Comparing Machine Learning Algorithms for Predicting Drug-Induced Liver Injury (DILI). Mol Pharm. 2020;17:2628-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 61. | Yeboah-Korang A, Louissaint J, Tsung I, Prabhu S, Fontana RJ. Utility of a Computerized ICD-10 Algorithm to Identify Idiosyncratic Drug-Induced Liver Injury Cases in the Electronic Medical Record. Drug Saf. 2020;43:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Teschke R. DILI, HILI, RUCAM algorithm and AI, the artificial intelligence: provocative issues, progress, and proposals. Arch Gastroenterol Res. 2020;1:4-11. [DOI] [Full Text] |