INTRODUCTION
Since its description for the first time by Leo Kanner in 1943, the rate of autism has been on the rise and steadily increasing[1]. Autism is a neurodevelopmental condition. Autism, Asperger's disorder, pervasive developmental disorder-not otherwise specified, form the autism spectrum disorders (ASD). At the same time, autism spectrum disorders, together with Rett's disorder, childhood disintegrative disorder, and the overactive disorder accompanied with mental retardation and stereotyped movements, are a part of the pervasive developmental disorders[2]. The prevalence of autism varies from one country to another depending on the racial differences and the diagnostic facilities available, with an average of 1% worldwide. The autism incidence in the United States of America may reach up to 1/110, increasing to 1/64 in the United Kingdom[3]. In other parts of the world, the prevalence of autism may be underestimated. For example, in Bahrain, the prevalence of autism is 1/1000, with possible underestimation because of missed diagnosis and no official recording in some cases. Autism is also 4-5 times more common in boys than girls. Autism shows a wide range of prevalence according to the race, being more common in non-Hispanic white children, less in Hispanic and African American/black children, with wide variability in Asian/Pacific Residents[4].
The genesis of autism is still unclear. Nevertheless, we can assert that autism development is due to the complex interaction of several genetic, biological, advanced parental age, environmental, immunological, and psychosocial factors[5]. Recently, genetic studies discovered a wide variety of genetic mutations in most patients. These mutations do not necessarily follow the same pattern with a wide range of variability. However, these mutations can ultimately induce brain changes and inflammation[6]. This neuroinflammation can also occur in utero through defective placenta augmented by the immaturity of the blood-brain barrier of the fetus and the newly delivered baby. This neuroinflammation can be triggered either as a part of the maternal immune response to infection during pregnancy, premature delivery, as a part of postnatal encephalitis, or exposure to a toxic environment[1]. We still need to have more knowledge to understand the different causes and their effects on patients with autism.
The clinical presentation of autism is heterogeneous, formed mainly from a constellation of social, cognitive, motor, and perceptual symptoms, which usually appear before three years of age. Children with autism have a diverse range of behaviors, communications, interactions, and learning ways from most other children. The abnormal social communication and interaction skills are manifested by poor eye contact, a stern facial expression such as happiness or sadness, lack of interest with others, and lack of interest in playing or interacting with others. They also have restricted interests manifested by playing with the same toys the same way every time, getting upset with changing routine or minor changes, and focusing on certain parts of the toys or the body with obsessive interest. Additionally, they have repetitive or stereotyped behaviors such as constantly repeating words or phrases (i.e., echolalia), flapping hands, body rocking, or spinning self in circles). They also suffer from delayed language, movement, and cognitive or learning skills[7,8].
MEDICAL COMORBIDITIES
Besides the classic manifestations of autism, the affected patients may suffer the presence of many other medical comorbidities that are more common in people with ASD than in the general population. The presence of these comorbidities is one of the reasons for the significant increase of early mortality in patients with ASD, with death rates 3-10 times higher than the general population. These comorbidities may increase the risk of death in patients with autism and could affect their quality of life, impair proper diagnosis, interfere with their compromised learning capacity, and impair their ability to retain the acquired learning skills. Early recognition of these comorbidities helps improve the quality of life for both children and their families[9]. These comorbidities may include but are not limited to genetic, inborn errors of metabolism, congenital anomalies of the nervous system, neurologic disorders such as epilepsy and neuroinflammation, gastrointestinal (GI) disorders, and allergic disorders[10]. However, diagnosis of these comorbidities is not easily accessible due to communication impairments, occasional ambiguity of the symptoms, changes of the symptoms over time, and mimicking some of the classic symptoms of autism. A lack of available diagnostic instruments to screen these disorders further augments these difficulties[11].
GASTROINTESTINAL DISORDERS IN CHILDREN WITH AUTISM
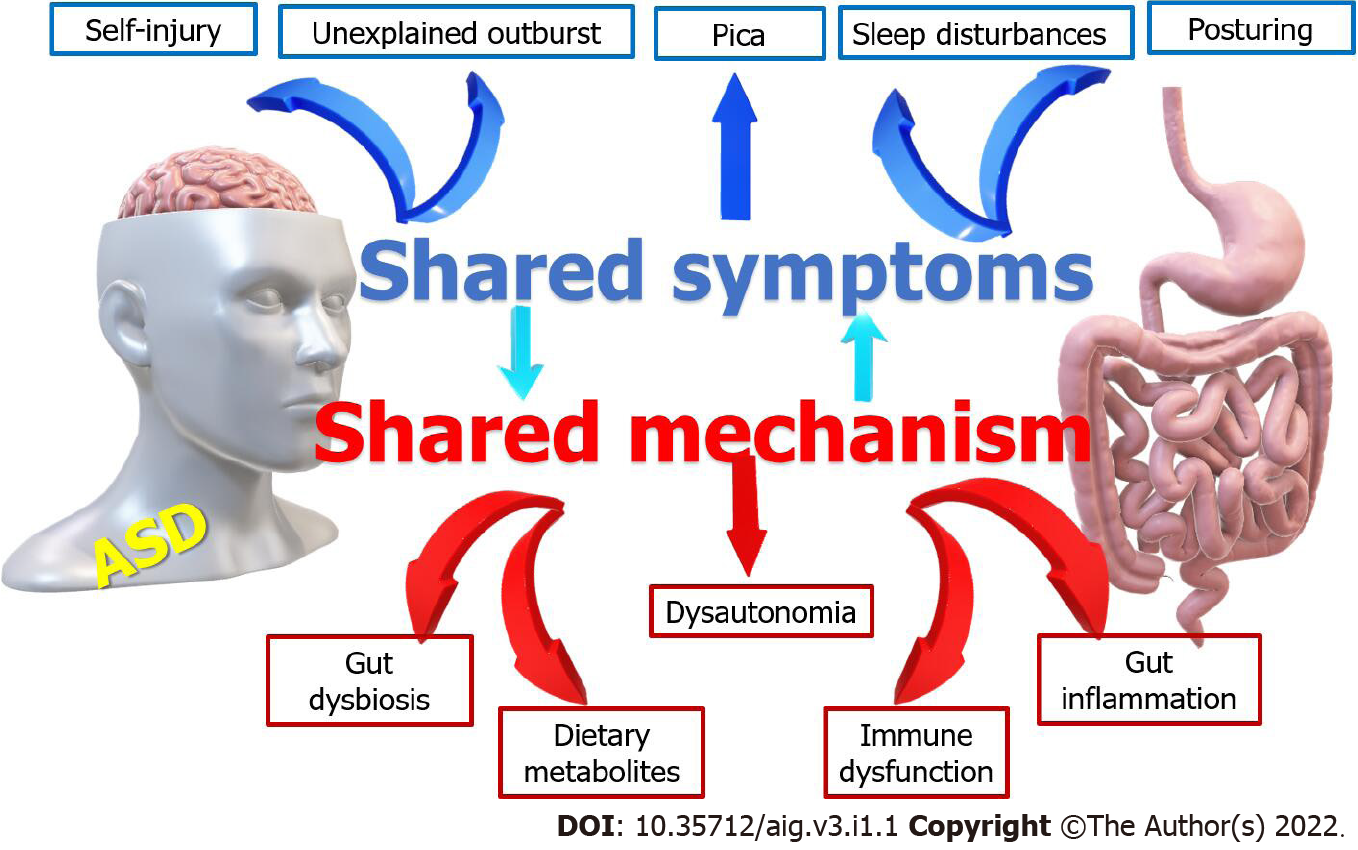
Children with autism have a high prevalence of GI disorders occurring in 46%-84% of them. The interaction between autism and gastrointestinal disorders is shown in Figure 1. Unfortunately, many of these children cannot effectively communicate their symptoms or discomfort to their doctors. Chronic constipation occurs in about 50%. They have a restricted diet with low fibers, abnormal bowel training, increased intestinal transit time, and a high incidence of hypothyroidism, increasing the frequency of constipation. Diarrhea is three times more common in children with autism than the control due to increased prevalence of food sensitivities, gut dysbiosis, immune dysfunction, and the increased infection rate due to increased incidence of pica and abnormal child behavior[12,13]. They have also increased frequency of nausea and/or vomiting, gastroesophageal reflux and/or disease, and chronic flatulence due to various factors such as food allergies gastrointestinal dysmotility[14]. Abdominal pain is also frequent in children with autism which results from simple, functional disorders such as irritable bowel syndrome, or organic causes such as food allergies, food intolerance, parasitic infestations due to pica, colitis, ulcers, or inflammatory bowel diseases[15].
Figure 1
The interaction between autism and gastrointestinal disorders.
Food allergies occur in about one-quarter of children with autism compared to 5%-8% in the general Pediatric population[9,16]. The link between autism and Celiac diseases (CD) is debatable. However, some high-quality studies proved this link even in the absence of GI symptoms[17]. Given that children with autism are more prone to suffer from atopy & food allergies, possible non-coeliac gluten sensitivity (NCGS) or wheat sensitivity in those children needs to be considered, especially when irritable bowel syndrome symptoms are present[18]. Physicians should consider the possibility of NCGS in some patients with ASD, especially those presenting with atopic diseases, migraines, and mood and anxiety disorders. Therefore, investigating CD and non-coeliac gluten sensitivity even in the absence of typical GI symptoms could yield good results for children with autism[19].
Children with ASD are more liable to have various feeding disorders; behavioral, sensory-based, or medically related feeding problems. The behavioral feeding disorders may include aversive eating behaviors (such as food refusal, frequent choking or gagging, the expulsion of the food without a medical reason), and frequent Pica habits. The sensory-based feeding problems include restrictive or selective eating and textural refusal of specific foods, usually involving larger textures. The medically-related feeding disorder may affect oesophageal and swallowing disorders and motor delays[20]. Almost two-thirds of children with autism eat less than 20 types of foods and accept fewer foods from the primary food groups than typically developing children[21]. This high prevalence of feeding problems in ASD may be related to their propensity to concentrate on details, their fear of novelty, their way of perseveration, and impulsivity. The associated sensory impairments and the deficits in social compliance of children with autism augment their feeding disorders. These feeding behaviors could also be aggravated by specific biological food intolerance and parental anxiety, reinforcing negative feeding patterns[22]. These feeding disorders have tremendous effects on both children and their families. They may increase the risk of child abuse and the occurrence of specific nutritional deficiencies, but Weight and height are usually not affected. They also increase parental anxiety and stress, ending with child abuse[23].
IMPORTANCE AND DIFFICULTIES IN DIAGNOSING GI DISORDERS IN CHILDREN WITH AUTISM
It is essential to check for the presence of gastrointestinal disorders in children with autism, as they can cause deterioration of autistic behaviors. For example, Abdominal pain related to especially reflux esophagitis and disaccharide malabsorption can cause irritation and discomfort to children with autism, which may contribute to the aggravation of their behavioral problems. It also could interfere with their learning abilities[24]. Meanwhile, gastrointestinal pain can cause behaviors that might be misdiagnosed as a behavior problem instead of a medical issue. For example, posturing, self-Injury, and/or outbursts without apparent cause could result from gastroesophageal reflux or esophagitis. The symptoms of GI dysfunction could induce sleep disturbances, which further aggravate the autistic manifestations[25].
Primary lactase deficiency that does not cause intestinal inflammation or injury is common in children with autism and may contribute to abdominal discomfort, pain, and observed aberrant behavior. Clinicians should screen for constipation and diarrhea or underwear soiling in children with autism who have prominent rigid–compulsive symptoms. If the GI disorder is recognized and medical treatment is effective, the behavioral problem may improve. When abdominal pain or discomfort is not alleviated, failure of psychotropic medications is more likely to occur. At the same time, these medications may even aggravate the problem if they have adverse gastrointestinal effects[9,26].
There is much evidence that modulation of the gut microbiota may be a manageable strategy for developing innovative therapies for complex CNS disorders, including autism[27]. The strong positive correlation of the gastrointestinal symptoms with the severity of autism indicates that children more severely affected by autism are likely to have severe gastrointestinal symptoms. Healthcare professionals should consider the possibility of gastrointestinal dysfunction in children with ASD, especially in those presenting with strange posturing or movements, sleep disorders, food intolerances, and aggressive or self-injurious behaviors[28]. The symptoms of GI dysfunction are associated with sleep disorders and food intolerance. Thus, it is essential to consider such association when evaluating and treating these comorbidities.
WHY IS IT DIFFICULT TO DIAGNOSE GI DISORDERS IN CHILDREN WITH AUTISM?
It is not always easy to detect GI manifestations in ASD. Children with autism have impaired communication skills, and many of them are nonverbal and cannot adequately express their pain, discomfort, or complaint through speech. Even those who can communicate verbally cannot adequately describe their symptoms. In addition, the symptoms of GI disorders may be missed as one of the classic symptoms and behavior commonly observed in children with autism. For example, toe-walking may be one of the typical stereotyped motor manifestations of autism to reduce feet overstimulation. It could also occur due to abdominal pain or loaded rectum or bladder. At the same time, GI disorders may present in atypical ways[29]. For example, suppose the child has abdominal pain or discomfort. In that case, he/she may touch his/her abdomen in a stereotyped way so that it can be easily missed with other stereotyped behaviors.
Moreover, GI disorders may present with non-GI manifestations. For example, sleep problems could be the manifestations of chronic GI disorders. It can be missed as being attributed to autism. The rate of sheep disorders increases from 30% of children with autism without GI disorders to reach 50% in the presence of GI disorders[30]. Children with autism may have hypersensitivity to various stimuli. On the other hand, they could occasionally have pain hyposensitivity with a high-pain threshold, affecting their symptoms[31]. Unfortunately, no clinical practice guidelines exist to diagnose the presence of GI disorders in patients with ASD.
ROLE OF ARTIFICIAL INTELLIGENCE IN CHILDREN WITH AUTISM
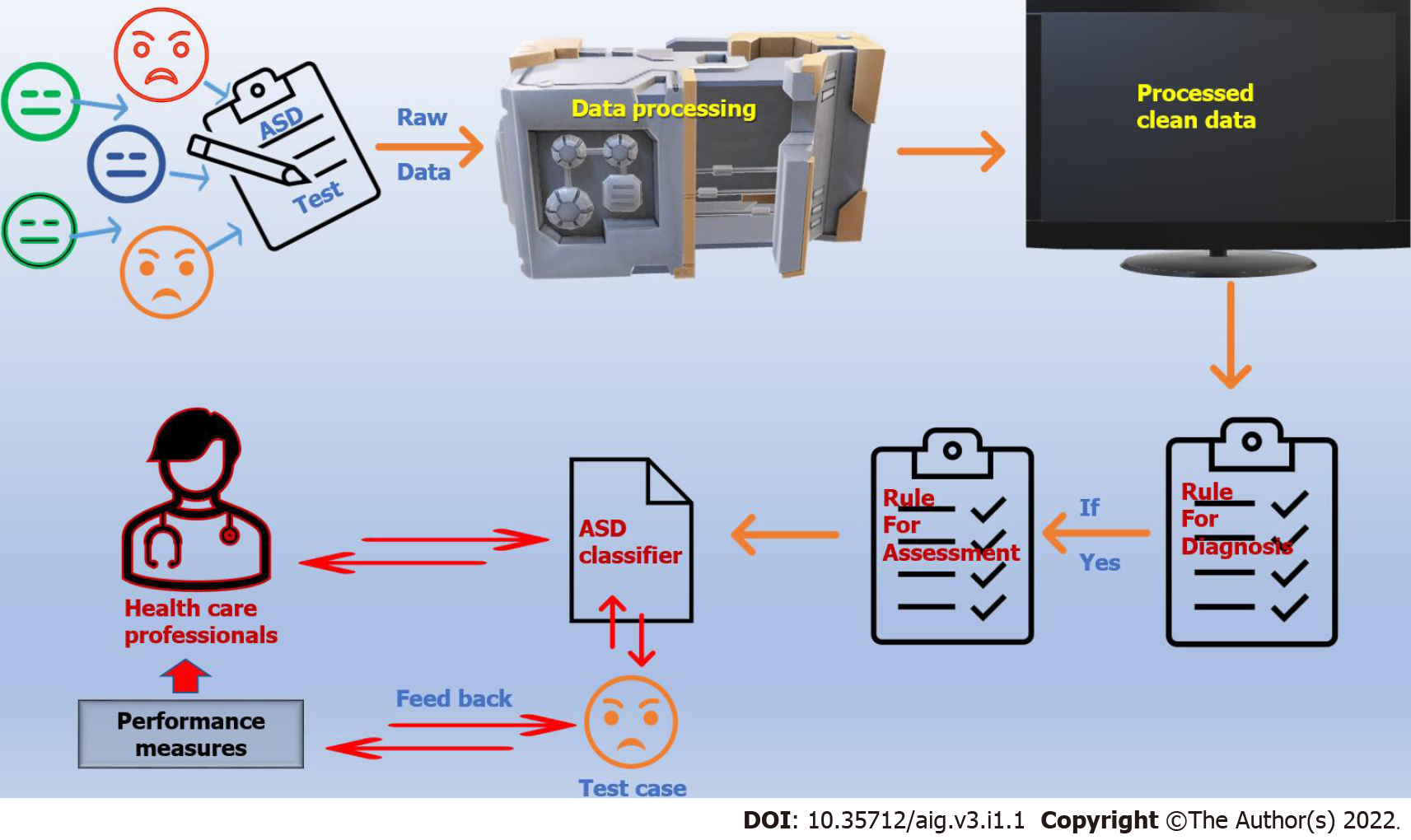
Artificial intelligence (AI) enables a computer or computer-operated robot to perform tasks that humans usually do because they require human intelligence and judgment. The extensive application of artificial intelligence in various areas of life, including health, has begun to bear fruit. Whether we acknowledge it or not, artificial intelligence is inevitable and has a significant role in almost every aspect of our lives. The most important feature of AI is its ability to learn from its interaction, with the interaction-learning-interaction cycle. So, through pre-programmed flexible adaptation, AI can accurately interpret supplied external data, use these data to learn, and reuse the achieved learning to reach specific goals and duties. Machine learning is a part of AI and computer science that focuses on using data and algorithms to imitate how humans learn and gradually improve accuracy (Figure 2).
Figure 2
Machine learning and Artificial intelligence in Autism.
Deep learning, together with supervised and unsupervised learning, is a sub-class of machine learning that combines certain approaches that use specific algorithms to process and interpret data quicker, simpler, and more precisely[32]. In supervised learning, AI uses a computer algorithm to analyze predefined data to train and learn and then accurately names the new, hidden data. In unsupervised learning, the computer learns from massive, unlabelled led data and recognizes similarities and commonalities. Personalized medicine is an example of unsupervised medicine. The computers analyze the medical history, the result of the neck ultrasound or other radiology procedure, and the laboratory results for a patient with thyroid cancer to provide new perceptions for the treatment and the prognosis[33]. Medical sciences have greatly benefited from artificial intelligence, whether in diagnosing diseases, inventing appropriate medicines and treatments, or improving communication between doctors and patients[34]. There is much promise that AI will help improve healthcare services in many ways, including patient diagnosis, patient outcome, and drug invention, and assist the physician assistant and provide a better and more patient-tailored experience. This hope is driven by some of the emerging successful AI applications in healthcare[35].
It was a dream that AI could help diagnose and manage autism by improving children's communication, social, and emotional skills for a long time. However, this dream starts to convert into reality despite not being the norm yet. Diagnosis of autism is subjective. Consequently, it becomes a real challenge in many situations. Parents and physicians may miss children with mild symptoms, while the more severe cases can simulate many other developmental disorders. Diagnosis of autism can be achieved using machine learning to provide a rapid, simple, and easy technique to provide for autism early diagnosis[36]. Machine learning can also help in improving the efficacy of behavioral health screening. The addition of machine learning techniques to complement the conventional methods in diagnosing autism helps fasten the diagnosis and reduce its cost[37]. An example of machine learning recognizes abnormal behavior using video monitor and artificial intelligence analysis of the body movement and behavior in children to detect early children with autism. Alcañiz Raya et al[38] used machine-learning techniques to detect stereotyped and repetitive behaviors biomarkers, characteristics for autism. They used a depth sensor camera to track the body movements of the examined children. Consecutively, they exposed the children to different visual, auditory, and olfactory stimuli. They found that children with ASD had more significant body movements than typically developed children, especially in the head, trunk, and feet and for visual, followed by visual-auditive, and lastly for visual-auditive-olfactory stimuli.
An exciting study by Rahman et al[39] aimed to study the ability of machine learning to predict the risk of autism during the neonatal period. They combined the machine learning techniques with electronic medical records using parental sociodemographic information and medical histories and the prescribed medications data to create features to train various machine learning algorithms. They succeeded in capturing early-life features that increase the risk of ASD. They were also able to uncover previously unknown features linked with increased ASD risk. An additional exciting study used fetal ultrasound features by a computer program to predict the child's autism from the first day after birth. The fetal features included the baby's head and stomach size, thighs length, and the time of acquiring a vertex presentation in preparation for delivery. The program also used the peri-labor data such as heart rate and body temperature and followed the children up to 6 years. Then the program can independently recognize the associations between different fetal characteristics and outcomes[40]. Artificial intelligence can obtain data on a large scale from all over the world, then re-study it and extract data used to increase the accuracy of autism diagnosis. Many applications are used in diagnosing autism. Artificial intelligence can use the data collected by these applications and process it, so we get accurate results that represent helpful diagnostic tools for their application in different parts of the world. It is also possible to determine the criteria of autism for each race according to its culture and customs. One of the widely used applications to diagnose autism worldwide was created by Dr. Fadi Fayez and Dr. Reza Shahamiri (Nelson Marlborough Institute of Technology, New Zealand). It uses ten questions for the four age groups, from toddlerhood, childhood, and adolescence to adulthood. They used 70% of the data to identify the presence of autism and 30% to ensure that AI has appropriately learned the autistic features[41].
AI is promising in treating children with autism, despite currently being costly. Robots can train and interact with the advantage of showing different facial expressions, proper social interaction, and response to different social cues with unlimited patience and the ability to repeat the cues in the same manner, unlimited times without variation. Some robots can show social expression by changing their eye color, raising their arms, or changing their voice tone[42]. Some children with autism have a better response to the robot than a human therapist. Some robots can incorporate data about individual children using video, audio, and measurements of vital signs such as heart rate and temperature and presence or absence of skin sweat to personalize their response to the child's behaviors[43]. Despite being promising and effective, robotic intervention needs more wide-scale research, especially cost-effectiveness.
ROLE OF AI IN DIAGNOSIS OF GI DISORDERS
Diagnostic and therapeutic endoscopies have provided significant help in managing Pediatric gastrointestinal disorders for decades. Endoscopy with small bowel sampling is the gold standard to diagnose coeliac disease. Endoscopy also provides excellent assistance in diagnosing various GI diseases such as gastro-oesophageal reflux disease, eosinophilic oesophagitis, and inflammatory bowel disease. It is also used to stop GI bleeding, insert a gastrostomy tube, dilate a stricture, and remove a polyp. The recent marvelous endoscopic field achievements helped us reach previously non-reachable areas of the mid-small intestine using the wireless capsule video-endoscopy[44]. There was a broad jump in the endoscopic industry from the white light to the blue light endoscopy and recently endocytoscopy and endomicroscopy. These recent modalities helped visualize the mucosal structure at the cellular level with adequate histopathology determination. It helps gather a vast amount of data that needs many hours of interpretation by a highly experienced physician[45]. AI helps process these vast amounts of data and allows rapid and precious interpretation.
Gastroesophageal reflux disease (GERD) is a principal reason for abnormal behaviors in children with autism. Upper gastrointestinal endoscopy is one of the preferred modalities to diagnose and detect complications of GERD, including Barret esophagitis, by evaluating the oesophageal mucosa. It also can rule out other possible causes of the child's symptoms, such as eosinophilic esophagitis[46]. AI helps improve the mucosal images' quality and detect their exact anatomical location. Changes in the oesophageal mucosa such as Micro-erosions, Changes in intrapapillary capillary loops, and increased vascularity are landmarks for GERD detected by narrow-band imaging with the help of AI model using convolutional neural networks (CNNs)[47].
Meanwhile, Takiyama et al[48] used CNNs to precisely recognize the anatomical location of esophagogastroduodenoscopy images. Pace et al[49] developed an artificial neural networks (ANN) model that can predict the presence of GERD without the need for invasive diagnostic techniques in patients with GERD symptoms. We hope that AI will help classify patients with GERD provide personalized therapeutic approaches[50]. However, the ability of ANN to expect GERD diagnosis depending on the symptoms still requires more verifications in different clinical settings. Considering that patients with autism may have other ways of expressing GER- or GERD-related symptoms, they may need specific and additional protocols to be applied with AI.
Coeliac disease is a common but underdiagnosed autoimmune disorder affecting 1/100 people worldwide with a relatively higher incidence in children with autism. The presence of villous atrophic histology in duodenal mucosal biopsy samples obtained by endoscopy is the gold standard for diagnosis. Endoscopy can also detect unsuspected cases of coeliac disease by meticulous analysis of the small bowel mucosa and identification of subtle findings of villous atrophy. However, it needs multiple biopsies not to miss the lesion as it is patchy[51]. New endoscopic techniques such as the modified immersion technique under traditional white-light or narrow-band imaging significantly improve the visual confirmation of coeliac disease during endoscopy[52,53]. Video capsule endoscopy is reasonably well-tolerated and safe in children. It can identify mucosal lesions in the bowel, especially in the small intestine, with the risk of radiation or sedation[54]. Video capsule imaging could also help identify the coeliac disease[55]. Augmentation techniques using AI can help augment the obtained original mucosal images to avoid the effects of conditions that could affect the quality of images, such as the rotation of the endoscope or the effects of distant viewpoint from the mucosal wall changes. However, a patency capsule test should be done before video capsule endoscopy, especially in infants and young children[56]. Recent AI modalities using deep learning techniques such as convolutional neural network (CNN), Bayesian inference, or support vector machines are innovative computer technology that can aid computerized coeliac disease diagnosis[57]. Foers et al[58] used machine learning methods to classify intestinal T-cell receptor repertoires to detect patients with coeliac disease irrespective of their dietary gluten status.
Pediatric colonoscopy needs a high experience not to miss lesions and detect colonic lesions as early as possible. The significant progress in developing computerized vision during gastrointestinal endoscopy allowed the gathering and annotation of high-quality video information. The addition of AI to real-time endoscopy significantly improves the automated detection of colonic or rectal polyps, such as in juvenile polyp or familial adenomatous polyposis. As mentioned before, children with autism have an increased risk to develop inflammatory bowel disease (IBD). This increased risk is due to multifactorial pathogenesis, including an overactive immune system and disturbances of the brain-gut-microbiota axis[59]. A massive flow of data about IBD is currently available using electronic medical records, genetic analysis, and imaging modalities. Analysis, interpretation, and integration of these data with the help of AI can aid to build models that can predict the risk of IBD and increase its detection accuracy[60].
Ozawa et al[61] succeeded to develop a neural network trained on colonoscopy images from patients with ulcerative colitis. With the help of a computer-assisted system, this network was able to identify the normal mucosa, mucosal healing states, mucosa on remission, and mucosa in severe degrees of inflammation with high sensitivity and specificity. These findings will help the physicians personalize the treatment according to the patients' conditions. In children, Mossotto et al[62] classified Crohn's and ulcerative colitis activity at diagnosis, using machine learning with integrating both endoscopic and histologic imaging. They were able to subtype the patients using this model with an accuracy reaching 80%, which significantly improves the diagnostic accuracy and permits a good option for targeted therapy. Dhaliwal et al[63] developed an algorithm using Random Forest Supervised and Unsupervised Machine learning in children to identify features that could help discriminate between ulcerative colitis and colonic Crohn's disease. They have a correct classification in 98% and 95% of children with ulcerative colitis and colonic Crohn disease, respectively.
Video capsule endoscopy is a safe, non-invasive procedure that can help diagnose IBD, especially for the patchy intestinal lesion of Crohn's disease. Nemeth et al[64] examined the accuracy and safety of video capsule endoscopy in 154 children and adolescents with suspected or established Crohn's disease. They found that video capsule endoscopy was safe and able to confirm the diagnosis of Crohn's disease with a significant impact on clinical management. However, interpretation of images obtained, and diagnosis based on Video capsule endoscopy is reader-dependant. As a result of the human concentration limitation, the lesion miss rate in capsule endoscopy ranges between 0.5% to 19% depending on the nature of the lesion[65]. AI can improve the accuracy of capsule endoscopy diagnosis by identifying distinct lesions and areas of interest with ease. However, there are many limitations to providing reliable classifications due to insufficient accuracy[66]. A convolutional neural network is used to analyze the large number of images obtained by capsule endoscopy to overcome these limitations. The convolutional neural network can differentiate normal intestinal mucosa, ulcers, erosion, polyps, and even worms with high accuracy reaching up to 96%[67,68].
Li et al[69] developed an AI system to automatically distinguish colorectal cancer early signs during colonoscopy with high sensitivity and specificity. Aguilar et al[70] found that transabdominal ultrasound augmented with a preceding AI model allows precise, fast, and non-invasive diagnosis of Buried bumper syndrome, complicating percutaneous endoscopic gastrostomy in children. Urban et al[71] successfully identified and removed rectal polyp using a deep neural network with a real-time accuracy rate of 96.4%. They used convolutional neural networks with an ordinary desktop machine with a contemporary graphics processing unit. They concluded that their trained model could identify and locate polyps in real-time with high accuracy. However, caution should be taken when using convolutional neural networks as we cannot generalize the results of these studies to other situations, and we do not know the exact effect of using convolutional neural networks on the endoscopists inspection behavior with overreliance on the technology. In addition, the direct and the indirect cost of these technologies and their acceptance to be a part of the diagnostic tools by the physician should also be considered[72].
ROLE OF AI IN MANAGING GI DISORDERS IN CHILDREN WITH AUTISM
Children with autism have a three-fold increase in the risk of gastrointestinal disorders than the typically developed children. However, the rate of parental reporting of these disorders is less in children with autism than in the typically developed ones. The wide varieties of GI manifestations in children with autism are related to the general heterogeneity of autism disorder and the underlying neurobiological mechanisms and disturbances of the neurotransmitters in both brain and gut[73]. Some GI symptoms are evident as diarrhea and constipation, while the others may vague and challenging to be recognized and can be missed as behavioral changes. Artificial intelligence can help detect and classify autism early, even in young infants.
Meanwhile, AI can help detect and classify gastrointestinal disorders in children. Regrettably, according to the best of our knowledge, there are no currently available specific AI models to detect gastrointestinal disorders in children with autism. AI models designed to detect GI disorders in patients with autism should consider the differences in the symptomatology from the typically developed children and should design algorithms to detect these disorders.
Fascinating use of AI in children with autism is the use of 'SMART TOILET' to monitor bowel health and to help to detect irritable bowel syndrome and inflammatory bowel disease. An artificial intelligence tool with a camera and microcomputer are attached to the traditional toilet to help evaluate patients' stool, including form, defecation time, urination, and the presence or absence of blood[74]. This' SMART TOILET' will significantly help manage toilet disorders, common in children with autism, as they cannot correctly report their bowel habits, dysfunction, or defecatory disorders. The use of microbiota transfer therapy (MTT) showed significant potential in alleviating the symptoms associated with GI complications and reducing the severity of behavioral symptoms in children with autism[75]. Children with autism who had MTT also showed changes in their plasma metabolite profile to be nearly similar to the typically developing peers[76]. Qureshi et al[77] examined the differences in gut microbial metabolites between children with autism and GI disorders vs the typically developing children without GI disorders and determined the effects of gut MTT on the fecal metabolites of the group with autism. They used machine learning to create 5-metabolite fecal models for classification, which showed significant changes before and after MTT. The developed multivariate metabolite models showed the potential of fecal metabolite panels to effectively categorize children with autism from the typically developed. Similar machine learning models can diagnose children with autism using their gut microbiome data compared to subjects with and without autism.
About 10% of children with autism are on a special diet. Despite no diet specific for autism, children with autism are frequently put on a gluten-free, casein-free diet. However, children should not start a special diet except when it is evidence-based. Dietary management can help alleviate many of the functional gastrointestinal symptoms in patients with irritable bowel syndrome, which is relatively common in children with autism. One of these dietary managements is restoring the imbalance in gut microbiota. Karakan et al[78] studied the efficacy of artificial intelligence-based personalized modification of the dietary microbiome in patients with IBS. They designed an AI-based diet to optimize a personalized nutritional strategy using an algorithm about the individual gut microbiome features. According to the IBS index score, they developed the algorithm to design the diet. The algorithm assessing an IBS index score used the microbiome composition to design the optimized diets based on the microbiome modification to match the observed with the healthy scores.
Gluten sensitivity is common in children with autism. AI can help produce allergen-free gluten in plants with high gluten content, such as wheat and corn. This new gluten retains its unique beneficial quality regarding texture, taste, and nutritional value without the ability to stimulate the autoimmune response and cascade of gluten sensitivity or coeliac disease. Another way to overcome coeliac disease and gluten sensitivity is to create an oral enzyme able to degrade the ingested gluten. The proposed enzyme should be stable and active in both stomach and duodenum, rapidly neutralize the gluten-peptides that can activate T-cell, and be safe to be ingested by humans. Many enzymes, including cysteine proteases, prolyl endopeptidases, and subtilisin's, could split the non-digestible gluten peptides in vivo and vitro. AI can help develop new techniques like enteric coating to protect the enzyme or genetic modification, increasing its production and enhancing its stability in the GI tract[79].
LIMITATION FOR THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has many obstacles in treating digestive diseases, especially in children with autism. Among these obstacles are the ethical aspects and the confidence of the medical staff in the mechanisms of artificial intelligence. The development of artificial intelligence also requires a robust infrastructure with enhanced patient confidentiality controls. Also, committees must be established to control the work of artificial intelligence to avoid the inappropriate and unethical use of artificial intelligence. Another significant limitation is the difference in symptoms of GI disorders in children with autism than the typically developed children. Children with autism may need a minimum level of communication abilities and cognitive function to use AI-directed models. When building an algorithm, it should be tailored to children with autism.
CONCLUSION
Autism is a neurodevelopmental condition with multiple comorbidities. Besides the classic manifestations of autism, the affected patients may suffer the presence of many other medical comorbidities that are more common in people with ASD than in the general population. Children with autism have a high prevalence of GI disorders occurring in 46%-84% of them with a bilateral mutual pathway between autism and GI disorders. Children with autism have an increased frequency of diarrhea, nausea and/or vomiting, gastroesophageal reflux and/or disease, abdominal pain, chronic flatulence due to various factors as food allergies, gastrointestinal dysmotility, IBS, and IBD. AI could help diagnose and manage autism by improving children's communication, social, and emotional skills for a long time. AI is an effective method to enhance early detection of GI disorders, including GI bleeding, gastroesophageal reflux disease, Coeliac disease, food allergies, IBS, IBD, and rectal polyps. AI can also help personalize the diet for children with autism by microbiome modification. AI can help to provide modified gluten without the ability to initiate an immune response. However, AI has many obstacles in treating digestive diseases, especially in children with autism. There is a need to do more studies and adapt specific algorithms for children with autism.
ACKNOWLEDGEMENTS
We thank the anonymous referees for their valuable suggestions.