Published online Aug 28, 2021. doi: 10.35712/aig.v2.i4.111
Peer-review started: May 28, 2021
First decision: June 16, 2021
Revised: June 19, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: August 28, 2021
Processing time: 93 Days and 3.9 Hours
It is estimated in Western industrialized countries that inflammatory bowel disease (IBD) has a prevalence of 1 for every 200 inhabitants. In the past, the fat mass disproportionate increase in relation to the fat-free mass was considered uncommon in patients with IBD, due to the observation of the disease being more common with weight loss and malnutrition. However, more in-depth investigations demonstrate that the fat/lean mass disproportion stands out both in prevalence in patients with new diagnoses of ulcerative colitis or Crohn's disease as well as a factor of poor prognosis to the natural evolution of the disease or to the therapeutic response. Another important aspect associated with obesity in IBD is the increased risk of drug clearance [including anti-tumor necrosis factor (TNF) and anti-integrin agents], resulting in short half-life and low trough drug concentrations, since the levels of TNF secreted by adipocytes sequester anti-TNF agents, which could result in suboptimal response to biologics. In view of these characteristic aspects of the inflammatory process of IBD, the identification of cellular functioning is necessary, which can be associated with the staging of the underlying disease, biochemical parameters, and body composition, helping as an indicator for a more accurate clinical and nutritional conduct.
Core Tip: Inflammatory bowel disease (IBD) patients have a severe inflammatory process that negatively reflects their absorption of vitamins and minerals, resulting in poor nutritional status. Even though it has already been described that these patients need a greater supply of calories and proteins, how will we know if the cells of this patient will be able to metabolize and absorb these nutrients to avoid worsening their nutritional status by overfeeding? Having a tool that serves as a guide for cellular functionality and integrity, such as the phase angle through electrical bioimpedance, is of great relevance in the clinical and nutritional management of patients with IBD.
- Citation: Fernandes SA, Rossoni C, Koch VW, Imbrizi M, Evangelista-Poderoso R, Pinto LP, Magro DO. Phase angle through electrical bioimpedance as a predictor of cellularity in inflammatory bowel disease. Artif Intell Gastroenterol 2021; 2(4): 111-123
- URL: https://www.wjgnet.com/2644-3236/full/v2/i4/111.htm
- DOI: https://dx.doi.org/10.35712/aig.v2.i4.111
Inflammatory bowel diseases (IBD) are systemic diseases that affect the gastro
Important tools recommended for assessing nutritional status are software-based anthropometric analyzes, such as bioelectrical impedance analysis (BIA), computed tomography (CT), and dual X-ray absorptiometry (DEXA)[5]. However, the easiest method and access to clinical practice ends up being bioimpedance, but it is not indicated in cases of changes in body composition (BC), such as changes in body fluids. On the other hand, the BIA not only provides the composition distribution within the classic model of compartmentalization of the human body, that is, fat mass and lean mass, but also provides a parameter called phase angle (PA), which through a mathematical formula, using the values of resistance and reactance, being these parameters of evaluation of the vitality and the integrality of the cell. Values above 6 indicate preserved cellular activity[6], in addition to being currently considered an important predictor of morbidity and mortality, taking into account inflammatory processes and nutritional status[4,7-9].
These diseases had their first phase of acceleration of incidence in the middle of the 19th century, a period in line with a change in life habits brought about by the Industrial Revolution. Subsequently, a new period of increased incidence occurred in the new industrialized countries, in the mid-20th century. Since the 2000s, it has been estimated that in Western industrialized countries, IBDs have a prevalence of 1 for every 200 inhabitants[10,11].
CD can directly affect the gastrointestinal tract in all its extension, and it is traditionally subdivided into phenotypes considering: (1) Non-penetrating, non-stenosing inflammatory involvement; (2) Stenosing involvement, resulting from fibrosis; and (3) Penetrating disease, characterized by abscesses or fistula formations between bowel loops or intestines and other organs. The disease occurs in cycles of inflammatory outbreaks and may cause the progression of its structural data[1].
UC occurs through the inflammatory affection of the mucosa, starting in the rectum, being able to progress continuously to the other segments of the colon. As in CD, the exact pathogenesis of the disease is not completely clarified, but four factors are related: Genetic susceptibility, intestinal microbial flora, uncontrolled immune response, and external environmental factors[2].
There are several environmental factors related both to the genesis of the disease and to the exacerbation of the inflammatory condition, among them smoking, low consumption of vitamin D, use of non-steroidal anti-inflammatory drugs, use of antibiotics, depression and psychosocial stress, low dietary fiber consumption, and high dietary consumption of fats and proteins[1,10].
Eating habits have been changing over time and may have been a crucial factor in the higher prevalence of IBD in Europe and North America. The dynamic changes in the diet of industrialized countries may be related to the increased incidence of IBD in these countries. It is worth remembering that when highlighting the diet in the pathogenesis of these diseases, the interaction between diet, microbiome, and mucosal barrier integrity must be emphasized, which are interconnected factors. The breakdown of hemostasis between such components can increase the chances of developing the disease or controlling the disease in those patients already diagnosed[12].
In 1988, Sonnenberg[13] published his pioneering study associating increased consumption of sugar and margarine with the highest incidence of CD in Europe. Since then, several other studies have corroborated that the high-fat diet is a risk factor for the development of IBD as well as for the disease control. It is important to highlight the differences between the types of fat and their impact on disease: The pro-inflammatory potential of ω-6 polyunsaturated essential fatty acids, the association between long-chain triglycerides, and the stimulation of the proliferation of intestinal lymphocytes as well as the pro-inflammatory mediators and the action of the high-fat diet capable of reducing intestinal permeability and increasing serum levels of endotoxins[14].
In the past, the disproportionate increase in fat mass in relation to the fat-free mass (FFM) was considered uncommon in patients with IBD, due to the observation of the disease being more common with weight loss and malnutrition. However, more in-depth investigations demonstrate that the fat/lean mass disproportion stands out both in prevalence in patients with new diagnoses of UC or CD, as well as a factor of poor prognosis to the natural evolution of the disease or to the therapeutic response[15].
It is important to highlight the pathogenic mechanism of IBD, as its aggression process significantly compromises the proper cellular functioning and, consequently, the individual's homeostasis.
The pathogenesis of IBD-CD and UC-remains unclear. We know that intestinal inflammation results from a dysregulation of the immune system in response to changes in the commensal intestinal microbiota (non-pathogenic). Genetic studies have shown that interactions between microbiota and host have a prominent role in the pathogenesis of IBD and involve genomic regions that regulate defense against microorganisms and intestinal inflammation[16].
Among the genetic findings, some of the most cited are involving nucleotide oligomerization domain 2, autophagy genes, and components of the interleukin route 23 - helper T cell 17 (IL23/Th17), which regulate intestinal immune mechanisms[17].
The gut microbiota, in its role of modulating the intestinal inflammatory response, when altered by environmental factors such as diet, obesity, exposure to helminths, and the use of antibiotics, can lead to an increased risk of developing IBD. In patients with IBD, changes in the diversity and density of bacteria (and even viruses and fungi), and in the functions of the bacteria present (oxidative stress, nutritional regulation) have been described[18-20]. It is still unclear what exactly are the microorganisms involved, but recent studies show the importance of the phyla Firmicutes and Proteobacteria in the pathogenesis of IBD[19].
Other environmental factors such as geography (higher incidence in industrialized countries), diet high in fat and sugar and poor in fruits and vegetables, smoking, psychological stress, appendectomy, and medications also alter the risk for IBD[21].
Immune dysregulation in IBDs is characterized by epithelial damage (abnormal mucus production and inadequate cell repair), inflammatory increase via microbiota, and cell infiltration in the lamina propria, including T cells, B cells, macrophages, dendritic cells, and neutrophils, causing a failure immune regulation in the face of the inflammatory process. The cells activated in the lamina propria produce high levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1-β, interferon-gamma, and IL23/Th17[21].
The immune system is divided into innate immunity and adaptive immunity. Innate immunity includes the function of the epithelial barrier of the intestinal mucosa, antibacterial proteins, pH of the stomach limiting microbial growth, innate immunity cells such as neutrophils, macrophages, dendritic cells, and natural killer T cells, in addition to cytokines and innate molecules (IL-1, TNF, denfensins). Adaptive immunity is pathogen-specific and usually initiated in circumstances in which innate immunity is not effective in isolation from the pathogen's aggression. After exposure to the pathogen, it usually takes several days to activate finally the adaptive immune response, including T and B cells. The microbiome's immune response is regulated in response to the aggressor's action, and it is this regulation that determines the immune protection of the microbiota or exacerbated inflammatory response with significant cellular damage. This regulatory compromise of immune action causes IBD[21].
By understanding the inflammatory routes involved in IBD, we can analyze some of the most used drugs for its treatment. The first to be used were corticosteroids, which manage to induce remission in most cases but have important adverse effects in the long term[22], not being indicated for the period of maintenance and control of the disease. Drugs such as azathioprine and methotrexate are also indicated for their immunosuppressive effects, including in combination with anti-TNF drugs[23-26]. Anti-TNF (infliximab, adalimumab, certolizumab), anti-interleukin 12/23 (uste
As previously stated, the inflammatory process of IBD and the effective immune response to this inflammation have a direct connection with the patient's food intake as well as his nutritional status[21].
Patients with UC and CD may be affected by malnutrition (6% and 22%)[3,30,31], but the prevalence is greater in CD, given its capacity to affect one or more parts of the gastrointestinal tract, reducing the absorption of macro and micronutrients[4]. Lack of treatment response, fistulizing and stenotic phenotypes, and previous bowel resections in CD are typical aspects of patients with higher risk of malnutrition[30].
Malnutrition in IBD is five times higher when compared with non-IBD patients[3]. According to the European Crohn's and Colitis Organization, IBD subjects should be routinely screened for malnutrition. Body mass index (BMI) and involuntary weight change should be assessed[30].
There are significant differences in nutritional status in IBD. CD patients remain malnourished for longer periods, with higher protein-energy malnutrition[5] and impaired absorption of micronutrients. On the other hand, UC patients have more protein-energy malnutrition during disease activity or hospitalization[30,32-34].
Symptoms that cause weight loss with depletion of body fat deposit, muscle mass, and fluid loss are diarrhea, high-output fistulas, decreased appetite, and restrictive diets often imposed in structuring disease during a flare[3,4,30,34]. Patients with active disease commonly have nausea, vomiting, abdominal pain, anorexia due to inflammation, and medication6. Inflammatory response mediated by pro-inflammatory cytokines such as TNF and IL-1 and 6, increasing energy expenditure, and anorexigenic hormones contribute to undernutrition[7]. Active inflammation leads to chronic anemia and protein loss within the intestinal lumen[34].
Chronic bowel inflammation or intestinal surgery may accelerate the intestinal transit resulting in increased stool volume and diarrhea, as well as the loss of epithelial integrity and small intestine bacterial overgrowth. Increased motility can cause malabsorption, altered BC, and micronutrient deficiencies[6,9]. CD patients with ileal involvement frequently have reduced nutrient absorption, mainly vitamins C, B12, D, K, folate, and magnesium[30,33].
Micronutrient deficiency in IBD is often associated with disease complications2,4 as well as the use of certain medications. Glucocorticoids can reduce calcium, zinc, vitamin D, and phosphorus contributing to osteoporosis. Methotrexate and sulfasalazine therapies, used for long periods, might impair the absorption of folic acid causing anemia[32,34].
Malnutrition in patients with IBD should be treated adequately because it worsens prognosis, increases complication rates and mortality, and decreases quality of life[4]. Micronutrient deficiency should be corrected and is best achieved by a multidisciplinary team[2]. There is low staff awareness on the role of nutrition in patient care, and this can be the main barrier for nutrition recognition and optimization[30,35].
While in Europe and Asia CD patients usually gave have a lower BMI ≤ 18.5 kg/m2, in the United States, obesity (BMI ≥ 30.0 kg/m2) is more common in IBD patients, probably associated with local dietary habits[30]. The prevalence of obesity in IBD is 15%-40%, and an additional 20%-40% are overweight[31,36]. An important feature in obese patients with CD is the loss of lean mass4 and sarcopenia (low muscle strength combined with low muscle mass or quality)[30,33,34].
Disproportional accumulation of visceral fat (VF) can be observed in CD patients[34,37,38] regardless of nutritional status and may be associated with the maintenance of disease activity due to an overexpression of pro-inflammatory adipokines[31] and increased levels of lipopolysaccharides (LPS). Serum levels of LPS were correlated with the severity of the disease and were observed an increase 6-fold and 2-fold in activity CD and remission CD, respectively, when compared to controls[39].
The ratio of VF/BMI (expressed in grams of fat per BMI) was increased both in malnourished and obese CD patients when compared to controls, indicating the possible presence of an adiposopathy by a higher VF tissue volume[30]. The degree of VF may be caused by several factors, including corticosteroid use, prior abdominal surgery, structuring disease or penetrating complications, and CD activity[40].
Another important aspect associated with obesity in IBD is the increased risk of drug clearance (including anti-TNF and anti-integrin agents), resulting in short half-life and low trough drug concentrations since the levels of TNF secreted by adipocytes sequester anti-TNF agents, which could result in suboptimal response to biologics[7,36].
The risk of complications, hospitalizations, and infections might be increased in obese patients with IBD, and nutritional therapy for obesity could be a potential adjunct therapeutic target in patients with IBD[36,37].
The type and distribution of abdominal fat were associated with complicated disease in patients with IBD[41]. The use of corticosteroids increases body fat and decreases lean mass. Loss of muscle mass can occur during IBD and has been associated with increased morbidity and risk of infectious complications[42].
A systematic review demonstrated that approximately one-third of CD patients have altered BC, with reduced BMI, FFM, and fatty mass when compared with controls, despite only 5% being underweight by BMI criteria[7]. Taken alone, BMI is inaccurate for assessing BC[8]. CD patients have lower lean mass when compared to UC. Body fat decreases with increasing disease severity and FFM decreases with longer duration of the disease in both CD and UC[43].
Muscle loss is a key marker of malnutrition or sarcopenia, although the ability to monitor accurately lean tissue in clinical is limited7. Important recommended tools to evaluate the nutritional status are software-based analysis anthropometries such as BIA, CT, and DEXA[5].
The compartmentalization of the human body, not only in the classic model usually used in clinical practice, in which it is evaluated only the BC in fat mass and FFM, but also the cellular analysis as proposed by Ellis[44], has been applied studied.
BIA provides us with data on the evaluated substrate in relation to its physical dimensions or changes in its conductive properties, where these properties may change due to changes in electrochemical processes, temperature, pH, hydration status, and viscosity of the fluid or biological tissue analyzed. With this information, it is feasible to monitor possible physiological changes in different living beings[45].
In different disease situations, the evaluation of the composition of the cellular structure and whether it has functioned has shown very important indexes in the patient's prognosis, becoming an independent factor of mortality[46,47]
Compared to other methods of assessing BC with independent measurement by the observer, the BIA method is characterized by making a quick, non-invasive, low-cost, and portable measurement without presenting any risk to the patient[48]. Its electrical current is imperceptible, as it has a low amplitude (800 µA) and a high frequency (50 kHz), enough to generate resistance to non-energy-conducting tissues and at the same time evaluate cell viability. In this body evaluation, there are two parameters of great importance: Body resistance (R) and reactance (Xc). R is the opposition offered by the body to the passage of electrical current, being inversely related to water and electrolytes contained in body tissues. Xc is the capacitance (viability) of the cell membrane properties, which may vary due to its integrity, function, and composition[49].
Tissues of increased fluid and electrolytic composition such as cerebrospinal fluid, blood, muscles, are high electrical conductors. Fatty tissues, bones, and the air that fills some spaces in the body, such as the lungs, are highly resistant to electric current[50]. The conductivity of biological tissues is practically ionic, that is, the electrical charges are transferred by the ionization of salts, bases, and acids dissolved in the body fluid[51]. Therefore, biological conductivity is directly proportional to the amount of body fluid volume. For this reason, in the patient who is in a state of hyperhydration, the value of lean mass is overestimated, with changes in the result of the body evaluation being one of the limitations of this method[50]
Bearing in mind that BIA is based on the theory of body symmetry, where the level of hydration and the percentage of fat are constant, when we are faced with different realities, with age group, ethnic group, body shape, or different clinical conditions, we do not have "universal" equations used in all situations, requiring another parameter as a reference point[52].
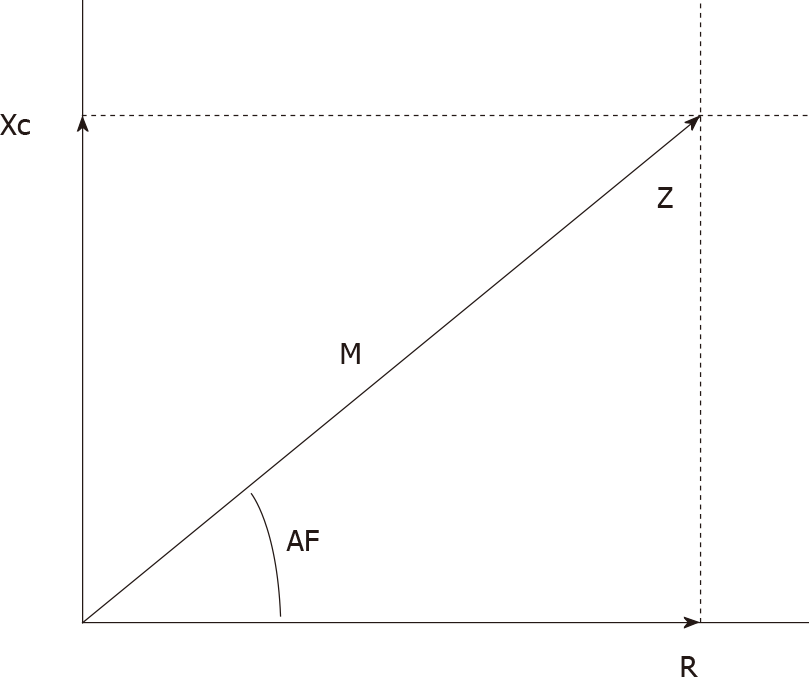
In view of these diversities, the clinically established bioimpedance parameter is the PA. The PA is calculated from the mathematical formula PA = tangent arc (Xc/R) x 180, which considers R and Xc. In addition, the relationship of these components of the current results in a geometric graph, where the relationship of R and Xc will result in an angle then defined as PA (Figure 1)[53,54].
PA has gained popularity in recent years as it is a fast method, applicable in the clinic and that reflects cell vitality and integrality, where the higher values indicate preserved cell activity[6,53,55,56]. In healthy individuals, PA can vary between 6° and 7°[53].
Because it is considered an important predictor of health status including inflammation, malnutrition, and disease, low PA values are associated with apoptosis or alteration in the selective permeability of membranes, compromising their integrity and metabolic functions[4,7-9]. High PA indicates intact cell membranes and high body cell mass, showing a good relationship also with the skeletal muscle structure preserved in its volume and/or functionality. Thus, PA can be one of the markers for monitoring nutritional status[9].
There are still few published studies regarding the use of PA and assessment of nutritional status in IBD[3,8].
Emerenziani et al[34] evaluated the nutritional status and PA in CD patients who received conventional therapy and anti-TNF therapy and concluded that mean values of PA and FFM were significantly lower in patients under conventional therapy when compared with controls and patients with infliximab therapy. Mean PA value increased from 4.6 ± 0.3 to 6.2 ± 0.4 (P < 0.05), after the induction therapy with infliximab (12 ± 2 wk)[7]. Conversely, another study that also assessed the impact of biological therapy on BC of patients with CD did not observed difference between the PA values after 6 mo of infliximab therapy (6.2 vs 6.8; P = 0.94)[42].
Back et al[57] compared BC in patients with CD and UC and found that patients with CD have more impaired nutritional status when compared to patients with UC (PA 6.46 ± 0.76 and 6.83 ± 0.080; P = 0.006)[3]. In another UC study, with 59 patients in clinical remission (94.9%), the PA had a negative correlation with inflammatory markers, C-reactive protein (CRP) (r = 0.59; P < 0.001), and erythrocyte sedimentation rate (r = 0.46; P < 0.001) and positive correlation with lean mass[58].
Mentella et al[38] investigated the association of disease activity, BMI, and PA with vitamin D deficiency in patients with IBD and reported a negative association between BMI and vitamin D serum levels in both CD and UC patients (P < 0.01), and PA was associated to hypovitaminosis D in both groups (CD: Odds ratio = 0.64, P < 0.05; UC: Odds ratio = 0.49, P < 0.01).
Recently, Cioffi et al[8] showed that BIA-derived PA is a valid indicator of nutritional status in CD patients, the values decrease with increasing disease activity, and PA was slightly better in patients receiving biologic therapy (infliximab).
PA might be considered a valid tool to assess nutritional status in IBD patients, as supported by nutritional biomarker evaluation[8] or as a complement of the other nutrition assessment methods. Its measurement in isolation may not be sensitive enough to capture all factors that can influence nutritional status[9].
It is possible to associate laboratory parameters with PA so that their values show greater reliability to the actual clinical condition of the patient with IBD. When analyzing the association between PA and biochemical parameters, previous studies have shown that hemoglobin was lower in patients with CD with active disease, compared to those in remission. Albumin, CRP, and total protein did not differ between groups with active and remission CD and, interestingly, all serum protein parameters, such as albumin, pre-albumin, and total protein, were directly correlated to PA. However, fibrinogen and CRP were inversely associated[8].
The cellular nutrition for its preservation of functionality and structure is very important for a good response to treatment of IBD, including the surgical approach in the pre, peri, and post-operative period[4,8,42].
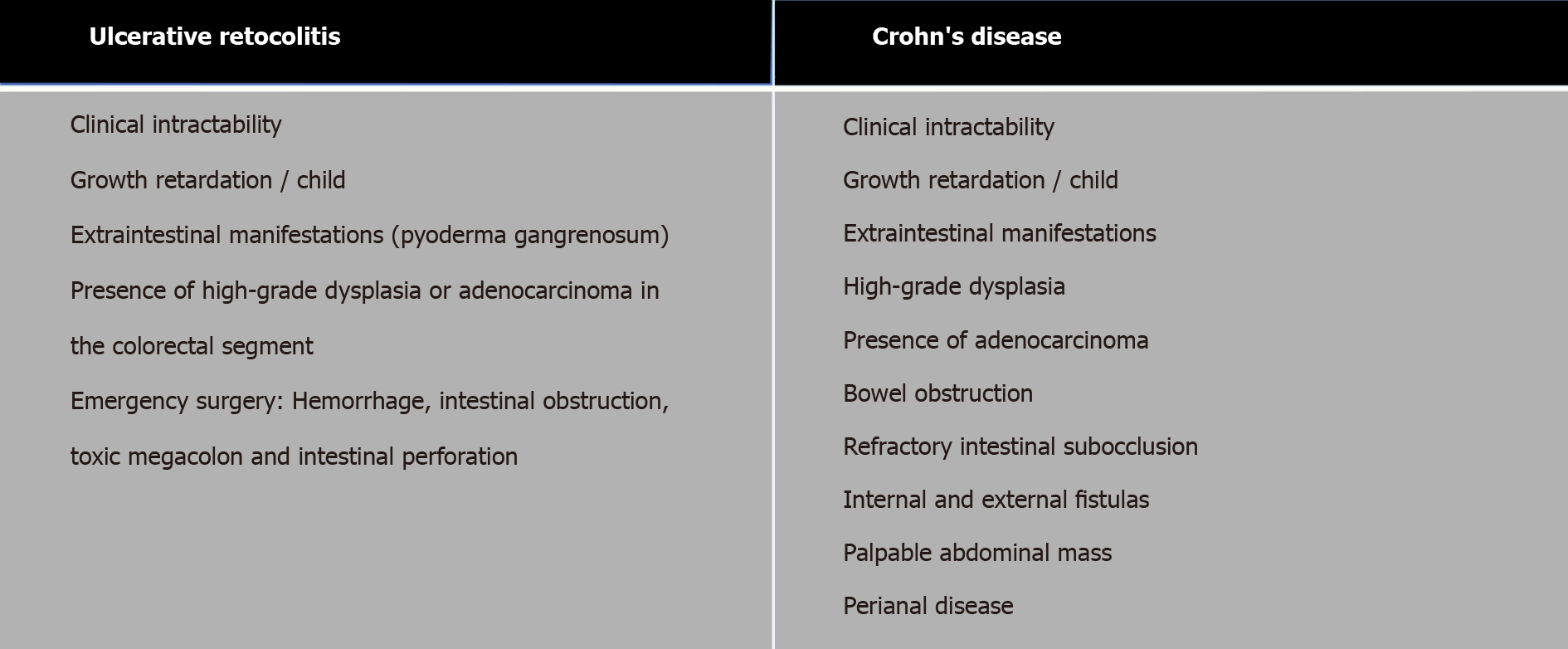
The clinical intractability of IBDs is one of the main factors for surgical indication (Figure 2)[59,30]. People with CD may need at least one surgery throughout their lives, that is about 80% to 90%, of which 50% will need the second surgery and 25% a third surgery. Patients with CD and UC will undergo one or more surgical procedures during their lifetime, 47% and 16%, respectively[61-64].
In CD, the location of the disease (ileal, colonic, ileocolic), the severity of symptoms (disease activity), the history of previous surgeries, the presence of very complex diseases, and the nutritional status are conditioning factors in the definition of the surgical procedure (colectomy total proctocolectomy, total recto colectomy). A weight loss of ≥ 15% in 3 mo and hypoalbuminemia (< 2.5 g/dL) are risk factors for surgical complications, which can be increased in patients who received biological therapy preoperatively[30,64-66].
Some data related to nutritional aspects are noteworthy when considering surgical intervention. At an outpatient level, the nutritional deficit of patients with CD is between 50% and 60% and in UC between 50% and 60%[64]. This malnutrition condition increases, when hospitalized, that is, about 80% to 90% and 60% to 70% of CD and UC, respectively. Considering this scenario, nutritional screening for the identification of patients at risk should be performed routinely, since it is an indication of the need for early nutritional intervention. The following tools are recommended: Perioperative nutrition screen score or nutritional risk screening 2002[30], associated with the analysis of the percentage of weight loss, biochemistry, food intake, and BC, since these changes are directly related to postoperative[30,67,68] complications, due to nutritional deficit and immunological[68].
With the advances of studies that seek to evaluate changes in BC and their impact on IBD, the presence of sarcopenia stands out. Ryan et al[69] demonstrated that 52% of patients with CD and 37% with UC had sarcopenia. The impact of changes in BC in IBD promotes undesirable consequences such as bone demineralization (osteopenia and osteoporosis), inadequate response to therapy, impaired surgical response, and poor quality of life[69-71]. In addition to these, Erős et al[72], identified sarcopenia, through meta-analysis, as an independent predictor of surgical complications. These showed reduced fat and reduced body fat mass, according to the increase in the severity and duration of IBD, respectively.
Fiorindi et al[73] conducted an intervention study with 61 IBD patients (45 CD and 16 UC) that sought to analyze the effect of long-term nutritional pre-rehabilitation on the postoperative result in elective surgery for IBD. In the initial assessment, muscle mass reduction was present in 28% of the cases and significantly associated with the presence of ileostomy and a previously performed IBD surgery. During the preoperative, intervention phase, there was an improvement in body weight, BMI, fat free mass, fat free mass index, and the PA[43].
Despite this, PA derived from electrical bioimpedance is a valid indicator of nutritional status in patients with CD, since its values decrease with the increase in disease activity, a clinical condition that is decisive in the decision of the surgical procedure in IBD. Thus, the assessment of BC should be recommended in clinical practice for the screening, monitoring, and determination of nutritional intervention, even in the preoperative period of patients with IBD[4,8,42,74].
This nutritional intervention when performed early, with the objective of nutritional rehabilitation in the preoperative of elective surgeries, ERAS Principles, demonstrates positive responses in the modulation of the BC of individuals with IBD. And so, it represents an important strategy to mitigate the response to surgical stress in lean tissue, even more evident in patients who are at high nutritional risk. Likewise, the use of early nutritional therapy in the postoperative period will provide a significantly reduced hospital stay and a faster recovery of intestinal function[8,30,63].
Still within the context of artificial intelligence and the improvement in the assessment of BC, proposals for the use of raw BIA measures can be an interesting alternative for populations where the use of regression formulas does not apply. In addition to using the isolated PA as a prognostic marker, R and Xc have been used graphically in a method called the electrical bioimpedance vector (BIVA)[75].
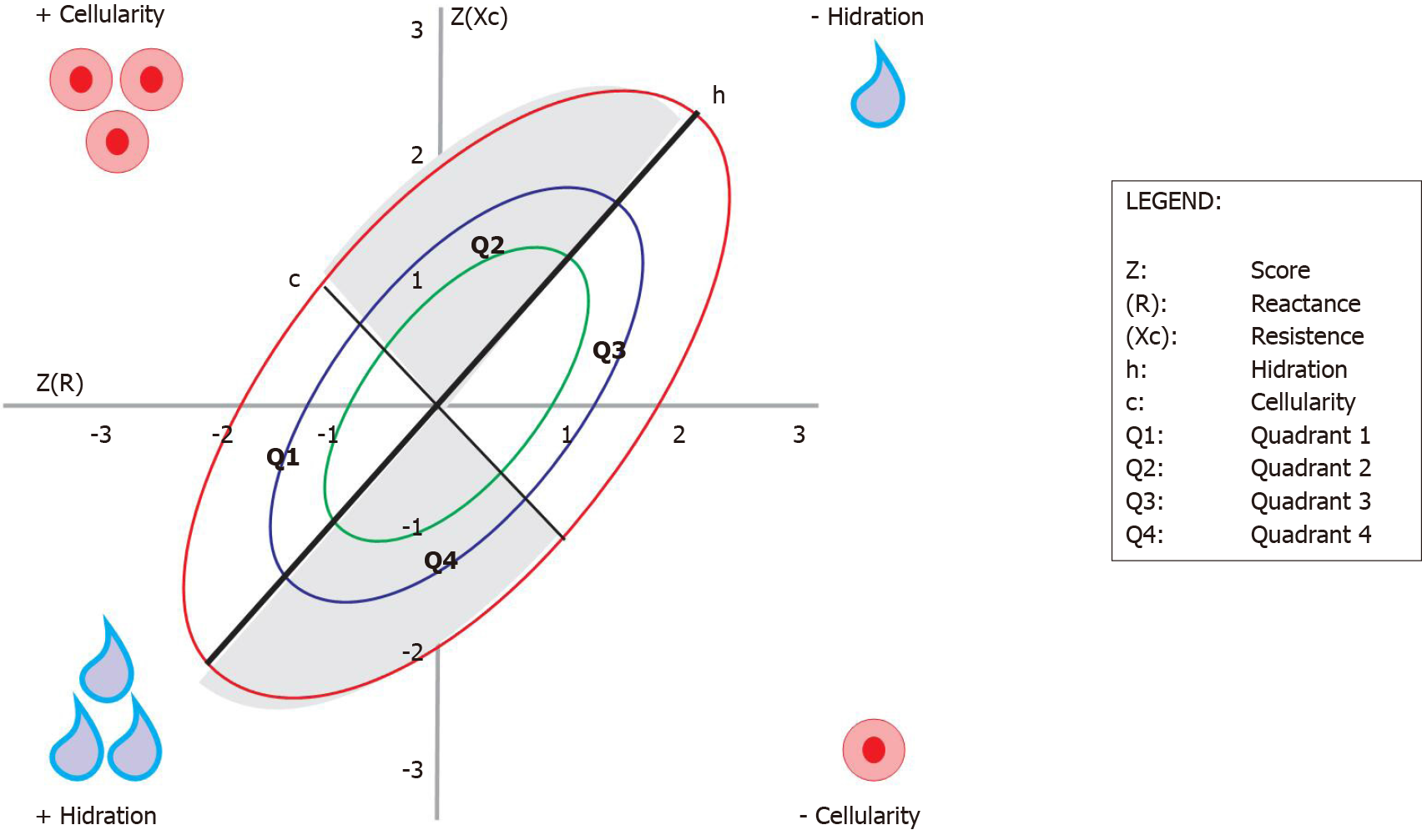
This method consists of the direct analysis of the R and Xc vectors, where their clinical applicability depends on a healthy reference population for comparison. The measurements must be adjusted by the height (H) of each individual and recorded in a Cartesian plane where the horizontal axis represents the standardized resistance for the height (R/H) and the vertical axis represents the reactance standardized by the height (Xc/H). Ellipses of tolerance of 50%, 75%, and 95% (or Z score), of the reference population, are drawn from a centralized mean vector[76,77].
The vector of the studied population will be compared with that of the reference population, determining within which Z score range it is for hydration information, cell body mass, and cell integrity[77].
From validation studies with different populations and diseases, the position of the vector on the graph brought clinical significance to the method. Not only as a classification of a single measure, the method also allows the monitoring and change of BC status[78].
The upward or downward displacement of vectors parallel to the largest axis of the ellipse indicates a progressive change in tissue hydration (dehydration towards the upper pole; hyperhydration with apparent edema towards the lower pole). Vectors migrating parallel to the smallest axis, above on the left, indicate more cell mass and, below on the right, indicate less body cell mass (Figure 3)[79].
When changes in nutritional status and hydration occur simultaneously, the vectors migrate in the two main directions. In the context of IBD, to date, there are no studies using the BIVA to study the degrees of hydration and cellularity of these pediatric or adult patients.
Assessing the individual's cellular condition has shown to be a watershed in the therapeutic planning of patients, whether in the clinical and/or nutritional approach. Given this knowledge, the PA is shown to be a very important parameter in this context of cellularity, because in addition to informing the integrality and cellular functionality, it is a method independent of the observer. In different populations, the PA is an independent marker of mortality and is indicated as a parameter for monitoring clinical and nutritional prognosis. We show in this bibliographic review that there are some studies on IBD and PA and their importance in the management of these patients, making the therapeutic approach more accurate and expanding a long-term vision for the result of sustained remission of the disease. It should be noted that there are still few studies that address the PA and IBD and none using the BIVA method in this population, which indicates an area of research to be explored.
Our consideration to the GEDIIB - Study group on inflammatory bowel disease in Brazil.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wen XL S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu JH
| 1. | Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 2. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 988] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 3. | Schreiner P, Martinho-Grueber M, Studerus D, Vavricka SR, Tilg H, Biedermann L; on behalf of Swiss IBDnet; an official working group of the Swiss Society of Gastroenterology. Nutrition in Inflammatory Bowel Disease. Digestion. 2020;101 Suppl 1:120-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Emerenziani S, Biancone L, Guarino MPL, Balestrieri P, Stasi E, Ribolsi M, Rescio MP, Altomare A, Cocca S, Pallone F, Cicala M. Nutritional status and bioelectrical phase angle assessment in adult Crohn disease patients receiving anti-TNFα therapy. Dig Liver Dis. 2017;49:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1653] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 6. | Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Cioffi I, Marra M, Imperatore N, Pagano MC, Santarpia L, Alfonsi L, Testa A, Sammarco R, Contaldo F, Castiglione F, Pasanisi F. Assessment of bioelectrical phase angle as a predictor of nutritional status in patients with Crohn's disease: A cross sectional study. Clin Nutr. 2020;39:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Rinaldi S, Gilliland J, O'Connor C, Chesworth B, Madill J. Is phase angle an appropriate indicator of malnutrition in different disease states? Clin Nutr ESPEN. 2019;29:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Gearry RB. IBD and Environment: Are There Differences between East and West. Dig Dis. 2016;34:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313-321.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 815] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 12. | Green N, Miller T, Suskind D, Lee D. A Review of Dietary Therapy for IBD and a Vision for the Future. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Sonnenberg A. Geographic and temporal variations of sugar and margarine consumption in relation to Crohn's disease. Digestion. 1988;41:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. Nutrition, IBD and Gut Microbiota: A Review. Nutrients. 2020;12:944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 15. | Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22:7868-7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (13)] |
| 17. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2202] [Article Influence: 137.6] [Reference Citation Analysis (6)] |
| 18. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1677] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 19. | Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 2063] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 20. | Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, Kambal A, Monaco CL, Zhao G, Fleshner P, Stappenbeck TS, McGovern DP, Keshavarzian A, Mutlu EA, Sauk J, Gevers D, Xavier RJ, Wang D, Parkes M, Virgin HW. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 916] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 21. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 628] [Article Influence: 104.7] [Reference Citation Analysis (1)] |
| 22. | Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;2008:CD006792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT; AGA Institute Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2013;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (1)] |
| 27. | Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 873] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 29. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R, Landau SB, Vandervoort MK, Parikh A. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, Doherty G, El-Hussuna A, Ellul P, Fiorino G, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gisbert JP, Gomollon F, González Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Kucharzik T, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Stassen L, Torres J, Uzzan M, Vavricka S, Verstockt B, Zmora O. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 31. | Magro DO, Barreto MRL, Cazzo E, Camargo MG, Kotze PG, Coy CSR. Visceral fat is increased in individuals with crohn's disease: A comparative analysis with healthy controls. Arq Gastroenterol. 2018;55:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 32. | Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 33. | Bamba S, Sasaki M, Takaoka A, Takahashi K, Imaeda H, Nishida A, Inatomi O, Sugimoto M, Andoh A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn's disease. PLoS One. 2017;12:e0180036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare A, Cicala M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients. 2020;12:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 35. | Sáez-González E, Mateos B, López-Muñoz P, Iborra M, Moret I, Nos P, Beltrán B. Bases for the Adequate Development of Nutritional Recommendations for Patients with Inflammatory Bowel Disease. Nutrients. 2019;11:1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 37. | Swanson SM, Harper J, Zisman TL. Obesity and inflammatory bowel disease: diagnostic and therapeutic implications. Curr Opin Gastroenterol. 2018;34:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients. 2019;11:2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Magro DO, Kotze PG, Martinez CAR, Camargo MG, Guadagnini D, Calixto AR, Vasques ACJ, Ayrizono MLS, Geloneze B, Pareja JC, Saad MJ, Coy CSR. Changes in serum levels of lipopolysaccharides and CD26 in patients with Crohn's disease. Intest Res. 2017;15:352-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Stidham RW, Waljee AK. Editorial: visceral fat as a predictor of post-operative recurrence of Crohn's disease. Aliment Pharmacol Ther. 2017;45:1551-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Barroso T, Conway F, Emel S, McMillan D, Young D, Karteszi H, Gaya DR, Gerasimidis K. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann Gastroenterol. 2018;31:566-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Santos JCD, Malaguti C, Lucca FA, Cabalzar AL, Ribeiro TCDR, Gaburri PD, Chebli LA, Chebli JMF. Impact of biological therapy on body composition of patients with Chron's disease. Rev Assoc Med Bras (1992). 2017;63:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Yadav DP, Kedia S, Madhusudhan KS, Bopanna S, Goyal S, Jain S, Vikram NK, Sharma R, Makharia GK, Ahuja V. Body Composition in Crohn's Disease and Ulcerative Colitis: Correlation with Disease Severity and Duration. Can J Gastroenterol Hepatol. 2017;2017:1215035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 434] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Martinsen OG, Grimnes S. Bioimpedance and bioelectricity basics. 2nd ed. New York: Academic Press, 2008. Hardcover ISBN: 9780123740045; eBook ISBN: 9780080568805. |
| 46. | Paiva SI, Borges LR, Halpern-Silveira D, Assunção MC, Barros AJ, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer. 2010;19:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Belarmino G, Gonzalez MC, Torrinhas RS, Sala P, Andraus W, D'Albuquerque LA, Pereira RM, Caparbo VF, Ravacci GR, Damiani L, Heymsfield SB, Waitzberg DL. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 683] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 49. | Barbosa-Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. 2005;8:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 50. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C; Composition of the ESPEN Working Group. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1772] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 51. | Avesani CM, Draibe SA, Kamimura MA, Cendoroglo M, Pedrosa A, Castro ML, Cuppari L. Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant. 2004;19:2289-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Barbosa-Silva MC, Barros AJ, Post CL, Waitzberg DL, Heymsfield SB. Can bioelectrical impedance analysis identify malnutrition in preoperative nutrition assessment? Nutrition. 2003;19:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int. 1994;46:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 486] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 55. | Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, Pierson RN. Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991;53:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, M W J Schols A, Pichard C; ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1445] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 57. | Back IR, Marcon SS, Gaino NM, Vulcano DSB, Dorna MS, Sassaki LY. Body composition in patients with Crohn's disease and ulcerative colitis. Arq Gastroenterol. 2017;54:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Urbano APS, Sassaki LY, Dorna MS, Presti PT, Carvalhaes MABL, Martini LA, Ferreira ALA. Associations among body composition, inflammatory profile and disease extent in ulcerative colitis patients. Rev Assoc Med Bras (1992). 2018;64:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1042] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 60. | Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn's disease: what is the actual risk? Gut. 2011;60:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 62. | Burke JP, Velupillai Y, O'Connell PR, Coffey JC. National trends in intestinal resection for Crohn's disease in the post-biologic era. Int J Colorectal Dis. 2013;28:1401-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Adamina M, Gerasimidis K, Sigall-Boneh R, Zmora O, de Buck van Overstraeten A, Campmans-Kuijpers M, Ellul P, Katsanos K, Kotze PG, Noor N, Schäfli-Thurnherr J, Vavricka S, Wall C, Wierdsma N, Yassin N, Lomer M. Perioperative Dietary Therapy in Inflammatory Bowel Disease. J Crohns Colitis. 2020;14:431-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 64. | Brazilian Study Group of Inflammatory Bowel Diseases. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. 2010;47:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, Doherty G, El-Hussuna A, Ellul P, Fiorino G, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gisbert JP, Gomollon F, González Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Kucharzik T, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Stassen L, Torres J, Uzzan M, Vavricka S, Verstockt B, Zmora O. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 66. | Yamamoto T, Shimoyama T, Umegae S, Kotze PG. Impact of Preoperative Nutritional Status on the Incidence Rate of Surgical Complications in Patients With Inflammatory Bowel Disease With Vs Without Preoperative Biologic Therapy: A Case-Control Study. Clin Transl Gastroenterol. 2019;10:e00050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, Thiele RH, Everett S, Grocott M, Gan TJ, Shaw AD, Thacker JKM, Miller TE, Hedrick TL, McEvoy MD, Mythen MG, Bergamaschi R, Gupta R, Holubar SD, Senagore AJ, Abola RE, Bennett-Guerrero E, Kent ML, Feldman LS, Fiore JF Jr; Perioperative Quality Initiative (POQI) 2 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy Within a Surgical Enhanced Recovery Pathway. Anesth Analg. 2018;126:1883-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 68. | Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 429] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 69. | Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm Bowel Dis. 2019;25:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 70. | Bryant RV, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, Lim A, Bartholomeusz FD, Andrews JM. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 71. | Scaldaferri F, Pizzoferrato M, Lopetuso LR, Musca T, Ingravalle F, Sicignano LL, Mentella M, Miggiano G, Mele MC, Gaetani E, Graziani C, Petito V, Cammarota G, Marzetti E, Martone A, Landi F, Gasbarrini A. Nutrition and IBD: Malnutrition and/or Sarcopenia? Gastroenterol Res Pract. 2017;2017:8646495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 72. | Erős A, Soós A, Hegyi P, Szakács Z, Benke M, Szűcs Á, Hartmann P, Erőss B, Sarlós P. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: a meta-analysis. Surg Today. 2020;50:1138-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Fiorindi C, Cuffaro F, Piemonte G, Cricchio M, Addasi R, Dragoni G, Scaringi S, Nannoni A, Ficari F, Giudici F. Effect of long-lasting nutritional prehabilitation on postoperative outcome in elective surgery for IBD. Clin Nutr. 2021;40:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Stobäus N, Pirlich M, Valentini L, Schulzke JD, Norman K. Determinants of bioelectrical phase angle in disease. Br J Nutr. 2012;107:1217-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Piccoli A. Bioelectric impedance measurement for fluid status assessment. Contrib Nephrol. 2010;164:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Piccoli A, Pastori G. BIVA software. Department of Medical and Surgical Sciences, University of Padova, Padova, Italy, 2002. |
| 77. | Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 2002;18:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Lukaski HC, Vega Diaz N, Talluri A, Nescolarde L. Classification of Hydration in Clinical Conditions: Indirect and Direct Approaches Using Bioimpedance. Nutrients. 2019;11:809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 79. | Fernandes SA, Leonhardt LR, da Silva DM, Alves FD, Marroni CA. Bioelectrical impedance vector analysis evaluates cellularity and hydration in cirrhotic patients. World J Hepatol. 2020;12:1276-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |