Published online Dec 28, 2021. doi: 10.13105/wjma.v9.i6.522
Peer-review started: July 16, 2021
First decision: August 9, 2021
Revised: September 9, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: December 28, 2021
Processing time: 164 Days and 10.6 Hours
Viral hepatitis in the entirety of its clinical spectrum is vast and most discussion are often restricted to hepatotropic viral infections, including hepatitis virus (A to E). With the advent of more advanced diagnostic techniques, it has now become possible to diagnose patients with non-hepatotropic viral infection in patients with hepatitis. Majority of these viruses belong to the Herpes family, with characteristic feature of latency. With the increase in the rate of liver transplantation globally, especially for the indication of acute hepatitis, it becomes even more relevant to identify non hepatotropic viral infection as the primary hepatic insult. Immunosuppression post-transplant is an established cause of reactivation of a number of viral infections that could then indirectly cause hepatic injury. Antiviral agents may be utilized for treatment of most of these infections, although data supporting their role is derived primarily from case reports. There are no current guidelines to manage patients suspected to have viral hepatitis secondary to non-hepatotropic viral infection, a gap that needs to be addressed. In this review article, the authors analyze the common non hepatotropic viral infections contributing to viral hepatitis, with emphasis on recent advances on diagnosis, management and role of liver transplantation.
Core Tip: With growing numbers of patients receiving solid organ transplantation including liver transplant, and subsequent immunosuppression there is an increasing incidence of non-hepatotropic viruses causing hepatitis. Several gaps exist in the diagnosis and management of such patients. Through this review article we attempt to outline the important non hepatotropic viruses causing liver injury. We also address the challenges in diagnosis, current and future prospects in treatment as well as prevention of these infections.
- Citation: Gupta M, Manek G, Dombrowski K, Maiwall R. Newer developments in viral hepatitis: Looking beyond hepatotropic viruses. World J Meta-Anal 2021; 9(6): 522-542
- URL: https://www.wjgnet.com/2308-3840/full/v9/i6/522.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i6.522
Viral hepatitis in the entirety of its clinical spectrum is vast and most discussion are often restricted to hepatotropic viral infections, including hepatitis virus (A to E). With the advent of more advanced diagnostic techniques, it has now become possible to diagnose patients with non-hepatotropic viral infection in patients with hepatitis. Non hepatotropic viruses do not infect the liver as the primary organ. These infections can present as hepatitis as a part of systemic infection. In a study performed in India, 10.5% of the patients with acute hepatitis and acute on chronic hepatitis were found to be secondary to non-hepatotropic viral infection[1]. Majority of these viruses belong to the Herpes family, with characteristic feature of latency. With the increase in the rate of liver transplantation globally, especially for the indication of acute hepatitis, it becomes even more relevant to identify non hepatotropic viral infection as the primary hepatic insult. Immunosuppression post-transplant is an established cause of reactivation of a number of viral infections that could then indirectly cause hepatic injury. Antiviral agents may be utilized for treatment of most of these infections, although data supporting their role is derived primarily from case reports. There are no current guidelines to manage patients suspected to have viral hepatitis secondary to non-hepatotropic viral infection, a gap that needs to be addressed. In this review article, the authors analyze the common non hepatotropic viral infections contributing to viral hepatitis, with emphasis on recent advances on diagnosis, management and role of liver transplantation.
The most important and common cause of viral hepatitis is infection with hepatotropic virus, including hepatitis A-E. However, a small percentage of individuals exhibit signs and symptoms of hepatitis without testing positive for any of the hepatotropic viruses. In such patients, the differential diagnosis should be expanded to include other non-hepatotropic virus, listed in Table 1.
| Herpesvirus | HSV1, HSV2, HHV6, HHV7, HHV8, EBV, CMV, VZV |
| Adenovirus | |
| Enterovirus | Coxsackie B virus, Echovirus |
| Paramyxovirus | Measles |
| Togavirus | Rubella |
| Parvovirus | Parvovirus B19 |
| Coronavirus | COVID-19 |
Cytomegalovirus (CMV) or human herpes virus-5 (HHV-5) belongs to the herpesvirus family. It is an enveloped, double-stranded DNA virus that remains latent in the body in two-thirds of the patients after primary infection. The capacity of the virus to remain latent in the host cells leads to risk of endogenous reactivation in a susceptible host, in addition to the risk of exogenous transmission. It is one of the most common viruses causing chronic infections as reflected in seroprevalence rates (ranging from 40%-100%) in adults and increases with age[2]. Demographic variability exists; women, non-white population and people belonging to the lower socioeconomic strata exhibit a higher prevalence[3-5]. CMV infection and disease are defined as distinct entities. CMV infection is any evidence of replication of the virus regardless of symptoms whereas CMV disease is infection along with symptoms that are explained by the virus[6]. CMV hepatitis is exceedingly rare in immunocompetent individuals and is more prevalent in immunocompromised patients, particularly post liver transplant (LT)[7]. Based on data available from population-based studies, 1%-4% of adults with acute hepatitis are due to CMV in developed countries[1].
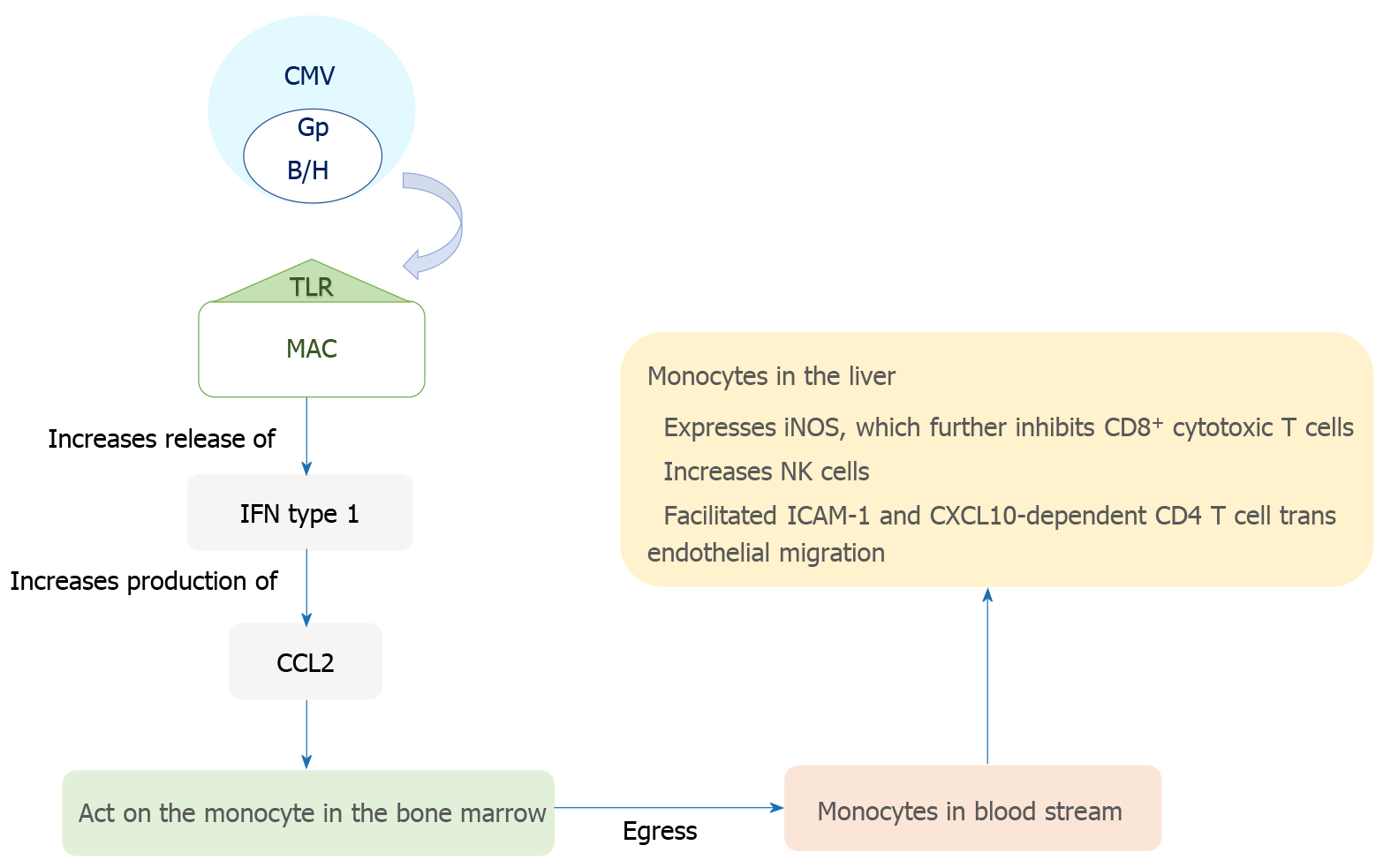
The transmission of CMV is through multiple routes including sexual exposure and close contact with bodily fluids such as saliva and breast milk[8]. The virus initially infects mucosal epithelial cells, and has broad cellular tropism allowing it to interact with a myriad of cell surfaces. Systemic dissemination occurs hematogenously with polymorphonuclear leukocytes, macrophages in the gastrointestinal and pulmonary tissues, and infected monocytes, all playing a role[9-12]. CMV has a predilection for hematopoietic, connective tissue and parenchymal cells; it specifically infects hepatocytes and macrophages in the hepatic tissue[11,13]. The virus then has complex interactions with the immune system leading to the repression of the primary infection, which is often followed by the stage of latent infection[14]. It plays a role in modulating both the humoral and adaptive immune responses in humans[15]. The sinusoidal endothelial cells of the liver, instead of being a barrier, provide an ideal environment for viral dispersion through the organ and act as sites for latency and reactivation[16,17]. The sinusoidal cells also facilitate immune activation in the liver by modulating T cell recruitment and activation via trans endothelial migration of CXCL10 and ICAM-1 dependent CD4+ T cells[18]. Notably, the sinusoidal cells play a role in viral latency, reactivation and dissemination within the liver but have a limited capacity for viral replication. Hepatocytes, on the other hand, play a major role in viral reproduction but have a limited role in latency. The pathogenesis of CMV disease is summarized in Figure 1.
Various factors lead to the reactivation of the virus such as allogeneic tran
CMV causes indirect cytotoxicity in the liver via cytotoxic T cell activation and alterations in vasculature, subsequently causing necrosis[24-26]. Additionally, it also has a direct cytotoxic effect on hepatocytes as evidenced in a study by Sinzger et al[11] that demonstrated lysis of CMV infected hepatocytes. Thus, in contrast to other herpetic infections such as Epstein-Barr virus (EBV), CMV affects the liver both indirectly via continuous immune activation and cytokine release as well as with direct cytotoxicity.
The clinical features vary according to the patient's immune status. Both stages, acute and chronic stages, are seen with the viral infection. In immunocompetent patients, a mononucleosis-like syndrome is seen with splenomegaly and hepatic dysfunction. Only case reports exist describing uncommonly seen CMV hepatitis in immunocompetent hosts[11,13]. Immunocompromised patients, especially LT patients, have a high incidence of CMV tissue invasive disease including hepatitis, esophagitis, gastritis, enteritis and/or colitis[21,23,27]. The risk of CMV hepatitis occurs with the highest frequency in the combination of seropositive donor/ seronegative recipient patients (incidence estimate of 44%-65%), followed by the combination of seropositive donor/seropositive recipient patients or seronegative donor/seropositive recipient (8%-18%), and with the least frequency in the com
A study by Toghill et al[29] studied 70 patients with cirrhosis due to a variety of causes including alcoholic cirrhosis, primary biliary cirrhosis, secondary biliary cirrhosis, hemochromatosis, congenital hepatic fibrosis and cryptogenic cirrhosis. The authors did not find evidence of CMV disease as the cause for the liver cirrhosis and the antibody titers in these patients were similar to that of the general population. CMV disease was, however, found to be an important cause of chronic rejection in post-LT patients and associated with increased mortality in patients with cirrhosis[30-32]. Liver involvement in CMV is varied and can manifest as mild hepatitis, necrotizing hepatitis, granulomatous hepatitis or even portal vein thrombosis.
The diagnosis of CMV is starts with serological testing to detect CMV IgM and IgG antibodies, antigenic testing of CMV pp65 that detects CMV antigens in leukocytes, polymerase chain reaction (PCR), culture and biopsy. Serological testing can provide risk assessment prior to LT but its role is limited in the diagnosis of CMV in immunocompromised patients due to the inability of these patients to mount an immune response[20,33,34]. In immunocompetent patients, serological tests may be falsely positive due to cross reactivity with other herpetic viruses, persistence of antibody levels after primary infection, reactivation or presence of rheumatoid factor[32,35].
Serological tests may provide quick diagnosis in immunocompetent patients after other etiologies of hepatitis have been ruled out. The pp65 antigen assay has a sensitivity of 64% and a specificity of 81% but since it detects antigens in leukocytes, it may not be reliable in patients with leukopenia[35,36]. The utility of viral culture is limited due to the long turnaround time, with one study demonstrating sensitivity of only 52% with cell culture[37]. The use of shell vial assay has the advantage of faster turnaround time (12 h), similar specificity to traditional culture and higher sensitivity[38]. PCR has a high sensitivity and specificity, ranging from 61%-92% and 75%-99%, respectively[39,40]. It can provide both quantitative and qualitative measurements from body fluids or tissue samples and is particularly useful in immunocompromised patients to determine the need for preemptive therapy and monitoring disease response[35,41]. While liver biopsy is not mandatory for diagnosis, it may be required when the diagnosis is uncertain. It is also required in LT patients to distinguish between acute graft rejection and CMV infection since CMV is a risk factor for rejection[8,28]. CMV hepatitis has characteristic histology with cytoplasmic and intranuclear inclusion bodies, nonspecific hepatocellular necrosis, mononuclear cell infiltrate and micro abscesses[42,43]. The degree of inflammation on the biopsy depends on the immune status of the patient. To increase the sensitivity, immunohistochemistry and/or DNA hybridization can be added to the liver biopsy[6,44].
Agents acting on CMV DNA polymerase including ganciclovir, valganciclovir, foscarnet and cidofovir are recommended for treatment of CMV hepatitis in immunocompromised individuals[6,28]. Immunocompetent patients usually have self-limited infectious mononucleosis (IM) like syndrome which does not require treatment. In immunocompetent patients with severe disease, limited data suggests using the above mentioned anti-viral agents[45-47]. There also have been reports of acute liver failure (ALF) from CMV hepatitis requiring LT[47-49].
About 18%-29% patients receiving LT are affected by CMV disease and it remains one of the most common infectious complications following solid organ transplant (SOT)[28,50]. Infection in the LT recipient can either be a primary infection, re-infection or reactivation of the latent virus. CMV disease in LT patients leads to other co-morbidities such as acute or chronic rejection, graft loss, post-transplant lymphoproliferative disorders (PTLD), increased infections, vascular thrombosis and increased mortality[28,51]. Increased rates of bacterial infection, invasive fungal infection such as Nocardia and viral co-infection such as EBV, HHV6, HHV7 and HCV has been described in literature[28,52-54].
Two basic approaches have been proposed to prevent CMV disease post-liver transplantation: Prophylactic and pre-emptive. The prophylactic approach refers to treatment which is immediately started post-transplant and continued for three to six months while the pre-emptive therapy refers to close monitoring for evidence of CMV replication with prompt initiation of antiviral therapy upon detection[23]. Both approaches have been shown to have comparable efficacy [0.34, 95% confidence interval (CI): 0.24-0.48 with prophylactic approach vs 0.30, 95%CI: 0.15-0.60 with preventative approach] in a meta-analysis. Notably, the population used in this meta-analysis was treated with ganciclovir as opposed to preferred alternative, valganciclovir[55].
For high-risk recipients (seropositive donor/seronegative recipient), prophylactic therapy is preferred with acyclovir, valacyclovir, intravenous ganciclovir and valganciclovir, if available for use[6,22]. Valganciclovir has demonstrated better efficacy, lower incidence at 6 mo and 12 mo follow up and better safety profile in multiple studies[21-23]. Preemptive therapy requires resource intensive monitoring which may not be achievable in all clinical settings. It can still be employed for high-risk LT patients (seropositive donor/seronegative recipient) and intermediate risk LT patients (seropositive donor/seropositive recipient, seronegative donor/seropositive recipient). Intermediate risk LT patients can also be managed with prophylactic therapy[13]. Low-risk LT patients (seronegative donor/seronegative recipient) do not require routine prophylaxis. Table 2 outlines the strategies for CMV prevention in LT patients based on risk stratification.
| Risk status | Donor/Recipient CMV serological status | Prevention strategy |
| High risk | Donor positive/recipient negative | Prophylactic therapy for 3-6 mo |
| Or | ||
| Pre-emptive therapy requiring close monitoring | ||
| Intermediate risk | Donor positive/recipient positive | Prophylactic therapy for 3 mo |
| Or | ||
| Pre-emptive therapy requiring close monitoring | ||
| Intermediate risk | Donor negative/recipient positive | Prophylactic therapy for 3 mo |
| Or | ||
| Pre-emptive therapy requiring close monitoring | ||
| Low risk | Donor negative/recipient negative | No routing prophylaxis |
Another high-risk patient population for CMV disease are patients undergoing hematopoietic stem cell transplant patients (HSCT). This field is rapidly evolving with ongoing research on multiple strategies for management of disease and risk mitigation. The concept of adoptive transfer of T-cells with protective effects against CMV is currently being studied[56-58]. Letromovir, a viral terminase complex inhibitor, has been approved for prophylactic CMV treatment for HSCT transplant patients and acts against both viral replication as well as latent infection[59]. Maribavir, an inhibitor of the viral kinase UL97, is also being evaluated in patients undergoing HSCT and has shown better safety profile with regards to hematologic side effects as well as nephrotoxic effects when compared to ganciclovir and valganciclovir[60]. A phase III trial comparing maribavir and placebo did not show any difference in patients with HSCT[61]. However, the trial used low-dose maribavir and repeating the trial with higher doses may reveal different, perhaps, positive results[62]. Maribavir is also being evaluated in an ongoing phase III clinical trial as a treatment for CMV disease in transplant recipients with resistance to ganciclovir, cidofovir and foscarnet (NCT02931539). The therapies used in HSCT patients may have a future in patients undergoing liver transplantation, given the overlap in immune status. Therapies against CMV latency can have significant clinical benefits. As indicated by in vitro studies, vincristine has the potential to be a therapeutic agent with the ability to kill latent infected cells; however, its use is limited by the extensive adverse effect profile[63]. A protein named F49A-fusion toxin protein (FTP) which kills infected cells has been developed, which may be a possible future therapeutic agent to target latent disease[13,64]. Apart from this, studies have also suggested using immunotherapeutic strategies which force the virus to be partially reactive only to be detected and demolished by the host immune system[14,65].
Several vaccine candidates have been developed including live attenuated viral vaccines, and subunit vaccines against CMV phosphoprotein 65 and glycoprotein[13,66]. Till date, the most efficacious results are from a subunit recombinant vaccine against CMV glycoprotein with MF59 adjuvant indicating 50% efficacy in young mothers as well as in recipient negative/donor positive transplant patients[67].
The most common presentation of primary EBV is IM which manifests as fever, cervical lymphadenopathy, tonsillitis and splenomegaly. In 90% of these cases, abnormal liver function tests are noted with hepatomegaly observed in about 14% cases[68]. However, a much smaller percentage of the population, estimated to be 0.85%-1% in population-based studies, are diagnosed with EBV hepatitis[69,70]. According to the available literature, the incidence of ALF secondary to EBV is estimated to be 0.21%[71]. In a recently published Russian study, EBV DNA was detected in 58.1% of the patients with viral hepatitis and correlation indicated worse outcomes in hepatitis C patients, coinfected with EBV[72]. The median age for EBV hepatitis in a British population-based study was noted to be 40 years and 41% of the individuals were above the age of 60 years[70]. Subsequently, another population-based study indicated the median age of patients to be 17 years, overlapping the age group most commonly affected by IM[69]. The scarcity of data and the difficulty in determining causation of EBV in patients with viral hepatitis or hepatitis of unknown etiology stems largely from lack of a diagnostic criteria. This forms the basis for the need to develop better diagnostic tools to identify these patients and initiate early treatment.
EBV or herpes human virus 4 belongs to the family of herpesvirus and has predilection for epithelial cells of the oropharynx and B lymphocytes. Once the virus infects B lymphocytes, it causes polyclonal expansion of T lymphocytes (specifically cytotoxic CD8 T cells). As EBV does not directly infect hepatocyte, vascular or biliary epithelium, the primary mechanism of damage is mediated indirectly through cellular immune responses. In majority immunocompetent patients (approximately 90%), hepatic involvement is subacute, mild, anicteric and self-limiting. In rarer cases, despite immunocompetence, the involvement can be acutely severe, recurrent or chronic[69,70]. In immunocompromised individuals, severe hepatitis with icterus is more commonly seen[72].
Another important concern in immunocompromised individuals following transplantation is the development of PTLD. EBV has been recognized as the cause for development of PTLD in 70% cases and occurs due to unregulated replication of EBV infected B cells in an environment of T cell immunosuppression. Depending on the source of EBV infected B cells that generate the clone pathognomic of PTLD in these patients, the disorder can be classified as host-derived PTLD and donor-derived PTLD. In patients receiving hematopoietic stem cell transplant, PTLD is often systemic and secondary to activation of latent EBV infection in the host[73,74]. Following LT, one study showed latent EBV infection in the donor as a likely cause[75]. The clinical manifestations range from constitutional symptoms to extra nodal lymphadenopathy and organ dysfunction (including allograft dysfunction)[76].
Liver involvement as a result of EBV infection can also be a manifestation of hemophagocytic lymphohistiocytosis (HLH)[77-79]. This rare life-threatening clinical entity occurs as a result of excessive immune system activation, primarily of lymphocytes and macrophages, that results in severe cytopenia, coagulopathy and splenomegaly in addition to hepatitis[80].
Liver enzymes, aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) are elevated up to 5-fold in majority of patients with subacute hepatitis presentation[81]. In the rare case that acute severe hepatitis develops, transaminase levels can exceed 5 times the upper normal limit. Serum bilirubin levels are elevated in only 5%-10% of the patients[68]. Cholestatic pattern of injury [elevated alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT)], compared to other viral etiologies, is seen in some patients with EBV[70]. As part of initial blood work, lymphocytosis with atypical lymphocytes is characteristically seen[82]. In the subgroup with HLH, additional laboratory abnormalities of note are bicytopenia (92% patients), hyperferritinemia (> 500 mcg/L in 94% patients) and hypofibrinogenemia (90% patients)[83]. Heterophile antibodies, although nonspecific, is rapid and has reasonable sensitivity ranging from 85%-100%, depending on assay used. The Paul Bunnell test (against sheep erythrocytes), the Monospot test (against horse erythrocytes) and the enzyme linked immunosorbent assay against other substrates such as ox or goat erythrocytes are some examples of widely available confirmatory tests for EBV infection[84]. In individuals with negative heterophile test but high clinical suspicion, further testing with specific antibody assays against EBV can be used. The immunogenic components of EBV used as basis for antibody testing are viral capsid antigen (VCA) and EBV nuclear antigen (EBNA). Given that 90%-95% of the general adult population in the United States is seropositive for anti-VCA IgG, it is difficult to use it as a diagnostic test in clinical practice[81]. The presence of anti-VCA IgM antibodies in the serum is considered to be a more reliable marker of active EBV infection and lasts for 4-6 wk after infection. IgG antibodies against EBNA, on the other hand, are established 6-12 wk after infection and are a marker for latency or convalescence. Thus, the combination of presence of anti-VCA IgM antibodies and with the absence of anti-EBNA-1 IgG antibodies is key to diagnosis of active EBV infection[85].Additionally, autoantibodies such as anti-nuclear antibodies, anti-smooth muscle antibodies may be seen in EBV infection due to cross reactivity of EBV proteins with cellular antigens. As a result, in immunocompromised individuals, autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus could hypothetically be triggered, further confounding the etiology of hepatitis[86].
In patients diagnosed with IM, the presence of elevated transaminase is sufficient to diagnose EBV hepatitis. However, the diagnosis of isolated EBV hepatitis in the absence of IM is trickier. Liver biopsy is indicated in these patients to establish etiology. The interpretation of the biopsy is challenging as a small percentage of EBV infected lymphocytes may be present in the liver in seropositive individuals without hepatitis. The diagnosis, thus, requires serum testing to establish the context for interpretation of the histopathological features in liver biopsy. Typically, portal and intra-sinusoidal lymphocytic infiltration (B and T cells) with few apoptotic cells is seen in EBV hepatitis. The most common lymphocytic population visualized in EBV hepatitis was CD3 positive cytotoxic T cells[71,87]. The diagnosis is further confirmed by either EBV-DNA PCR or EBER-RISH (EBV encoded RNA in situ hybridization), both methods demonstrating comparable sensitivity[88].
In an analysis published in 2015, ALF secondary to EBV was shown to have a high case fatality rate. While the study population was treated with antivirals (acyclovir, famciclovir and ganciclovir) and high dose steroids, the efficacy of either treatment option (alone or in combination) is not clearly established[89]. Antivirals such as ganciclovir have shown efficacy in both immunocompromised and immunocompetent individuals[90]. Oral valganciclovir has also been used in immunocompetent individuals, although with uncertain benefit[91]. While steroids have been used in acute hepatitis to limit inflammatory response, steroid use has been proposed to be associated with EBV reactivation, most likely at the time of withdrawal of high dose steroids. This mechanism is possibly the rebound increase of suppressed cytotoxic T cells that attack infected B cell infiltrate in latent EBV[92].
The definitive treatment for management of ALF currently remains LT. Orthotopic liver transplantation has been described in cases of fulminant hepatitis secondary to EBV infection[89,93]. Subsequent treatments with antivirals such as acyclovir has been suggested in a few case studies of patients developing fulminant hepatitis requiring orthotopic liver transplantation. The rationale behind it is similar to the concern outlined with steroid use in management of ongoing hepatitis. While immunosuppression is required post-transplant, reinfection of graft liver remains a possibility, warranting antiviral therapy[94].
Another important concern related to transplantation, both liver and HSCT, is the risk of development of PTLD. This risk can be lowered by cautious use of immunosuppression post-transplant (in terms of dosage, duration and choice of drug regiment), careful donor-recipient matching (in term of avoiding serodiscordant match) and antiviral prophylaxis. While there is no clear data supporting efficacy of antiviral prophylaxis for EBV in adults, oral acyclovir and intravenous ganciclovir have been used in patients receiving liver allograft. The milder spectrum of PTLD is seen to resolve with the cessation of tacrolimus[95]. In patients who cannot tolerate tapering or changing of immune suppression regiment or persist to have PTLD despite cessation of immune suppression, treatment with anti CD20 agent rituximab has shown success. Single agent treatment with rituximab has shown 40%-50% remission. Other therapeutic options for these patients include surgical removal of affected organ (if localized PTLD) or chemotherapy[96-101].
Herpes simplex virus (HSV) 1 and 2 affect the majority of adults in the western world with prevalence being 80% and 30% respectively. Like other members of the Herpesviridae family, the virus exhibits latency in the human body persisting in the neurons. Majority of patients who suffer from HSV hepatitis are immunocompromised such as organ transplant recipients, patients on immunosuppressive medications, patients with acquired immuno-deficiency syndrome, neonates and pregnant women in their second and third trimesters[42,102,103]. A study on HSV hepatitis with 137 patients revealed 24% patients were immunocompetent, 23% were pregnant and 53% patients were taking immunosuppressant medications either for organ transplantation or for other reasons[42,104]. HSV hepatitis can also affect immunocompetent patients[105]. Interestingly there have been case reports suggesting reactivation of latent HSV by inhaled anesthetic agents such as enflurane, isoflurane, desflurane and nitric oxide[105,106].
A multitude of theories exist regarding HSV pathogenesis in causing hepatitis. As herpes is known to be a neurovirulent virus, studies have shown hepatovirulent strains of HSV that can cause fulminant hepatitis. Another theory suggests an acute infection superimposed on a latent HSV reactivation as causing liver failure. With regards to viral dissemination to the liver, one hypothesis suggests that the virus spreads to the liver from the herpetic lesions in the setting of impaired immunity and delayed type hypersensitivity reaction. While another suggests that during initial infection, a large inoculum of the virus may overwhelm the innate host defenses leading to dissemination to the visceral organs including the liver[107,108].
HSV hepatitis occurs during the primary infection and rarely as a reinfection in immunocompromised individuals. It presents with non-specific features such as fever, abdominal pain in the right upper quadrant, nausea/vomiting with jaundice rarely present. The characteristic herpetic skin rash is present in only about 18% to 50% of patients. Patients also present with leukopenia, thrombocytopenia, markedly elevated liver enzymes, and mild bilirubin increase[103]. Cases of fulminant hepatitis present with aminotransferase levels 50 to 100 times the upper limit of normal.
Patients may also develop acute kidney injury, disseminated intravascular coa
A thorough physical examination of the skin and pelvis should be conducted in patients with suspicion for HSV infection to detect characteristic herpetic lesions. HSV serology (IgM and IgG antibodies) have limited utility due to false negative and false positive results. PCR of HSV DNA utilizing blood samples is rapid, with a better yield than serology and even viral cultures[112,113]. A liver biopsy is imperative in the diagnosis of HSV hepatitis with typical biopsy findings of intranuclear inclusions (Cowdry Type A) occurring in the foci of coagulative or sometimes extensive hemorrhagic necrosis which are irregular in distribution[103]. There is a characteristic scarcity of inflammatory cells in the portal veins or the parenchyma under light microscopy[107]. Due to risk of increased bleeding with the percutaneous approach in patients with ALF, a trans jugular approach is preferred with consideration of administering factor VII recombinant to reduce the risk[113-115]. Computed tomo
The disease is curable and carries a high mortality, hence treatment must be initiated as soon as possible. While no standardized guidelines or prospective studies exist, literature exists that has shown reduction in mortality and the need for LT from 88% to 51% in patients receiving treatment[104]. The most important aspect is that in patients with high suspicion of HSV hepatitis, empiric acyclovir should be considered until it is ruled out via PCR and/or biopsy. Cidofovir and foscarnet can be used in cases of acyclovir resistance which are quite uncommon about 0.27% in immunocompetent patients and 7% in immunocompromised patients[119,120]. Expert consensus recommends treatment from 2 wk to up to 4 wk[104]. Very limited data exists on the use of therapeutic plasmapheresis which theoretically works by removing infectious particles, reducing viral load and buying time for the immune system to mount a stronger response[121].
An urgent LT is indicated in patients not responding to antiviral therapy as above as a final treatment option. Although disseminated HSV is not a contraindication for transplant, thorough evaluation is necessary since sepsis, and multi organ failure is usually present in these cases which can make it difficult to initiate an immunosuppressive regimen post-transplant. Patients who do receive transplant have a higher risk of HSV recurrence , and require life-long acyclovir which contributes towards acyclovir resistance[119,122,123].
In patients who have received LT, HSV tends to occur in the early post-operative period from 20 ± 12 d and is associated with increased mortality[124]. The early recurrence of HSV in LT patients may be due to acquisition of the virus from the donor or due to immunosuppression. A very high index of suspicion is to be maintained since acute cellular rejection or biliary complications are the commonest issues in the early post-operative period. Early diagnosis improves survival and patients should empirically be started on acyclovir as soon as the suspicion arises[125,126]. In patients with LT who are not receiving CMV prophylaxis which also has activity against HSV, prophylactic treatment is associated with low incidence on clinical disease[127].
The first attempt at the HSV vaccine was in 1964 by Kern and Schiff[128]. Since a live attenuated vaccine was developed for varicella zoster virus, a member of the alpha- herpesvirus, there was a possibility to develop a vaccine against HSV-2 as well[129]. Currently no effective vaccine exists for HSV-2, however, Heprevac- a truncated glycoprotein D2 (gD2) vaccine did show efficacy for prevention of genital HSV-1 disease (58%) and HSV-1 infection (32%) in a clinical trial[130].
Various types of vaccines including whole killed virus, attenuated virus, subunit vaccines (glycoprotein) as well as DNA based vaccines have been attempted to come up with a preventative/therapeutic vaccine against HSV-2[131]. A promising can
The coronavirus disease 2019 (COVID-19) pandemic has ravaged the world affecting over 3 million people worldwide, as of July 12, 2021. Case studies from China, where the pandemic emerged, indicated that 2%-11% patients affected by COVID-19 had prior liver comorbidities and abnormal levels of liver enzymes (ALT and AST) were seen in 14%-53% cases[133]. Prothrombin time abnormalities signifying synthetic function of liver were also seen in COVID-19 patients with gastrointestinal symptoms[134]. Another large study of 1099 patients across 552 hospitals in China demonstrated that patients with severe COVID-19 infection had abnormal liver enzyme levels as compared to those with less severe disease[135]. Li et al[136] conducted a study among COVID-19 patients and found that patients with elevated C-reactive protein levels greater than 20 mg/L and lymphopenia with counts less that 1.1 × 109 per liter were related to ALT elevation thus highlighting the fact that COVID-19 disease severity correlates with liver dysfunction[136].
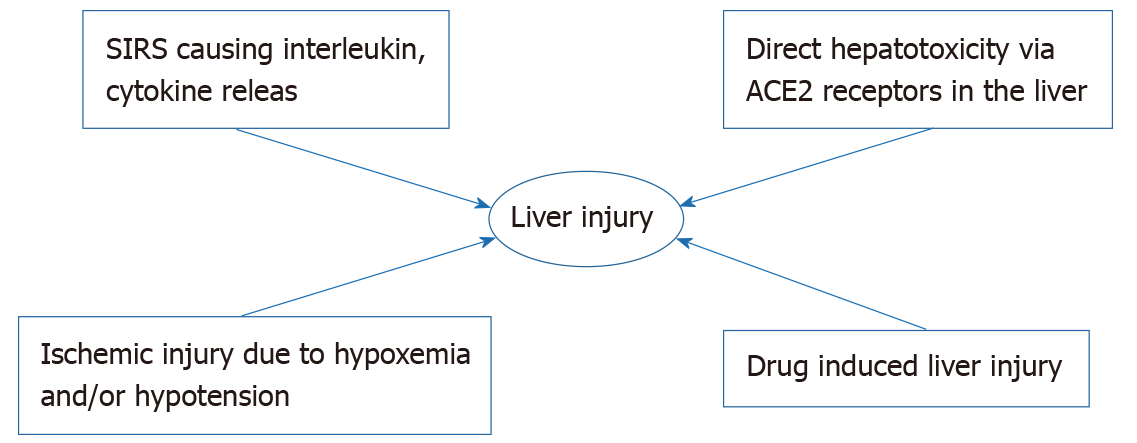
The pathogenesis of COVID-19 induced liver injury continues to evolve as we learn more about the virus. The virus is known to cause immune dysregulation causing systemic inflammatory response syndrome which causes release of inflammatory mediators including interleukins causing a cytokine storm causing hepatocellular injury with the intrahepatic cytotoxic T cells as well as Kupffer cells playing a role[137,138]. The virus probably also has direct cytotoxic effect but the ACE2 receptors, which the virus has an affinity for, is expressed in the bile duct cells more than the hepatocytes[139]. It would thus be expected that patients would have elevated ALP levels but patients with COVID-19 hepatitis usually have elevated AST and ALT levels. The virus predominantly affects the lung and in severe disease causes refractory hypoxemia as well as hypotension leading to ischemic liver injury which adds up to another mechanism of liver induced injury caused by the virus. Another important consideration in the pathogenesis of liver dysfunction in patients with COVID-19 is the myriad of drugs that have been tried and are currently being used for treatment that cause hepatic injury via hepatocellular damage and cholestasis[140,141]. Reactivation of hepatitis B is associated with use of biological agents such as tocilizumab which has been used and studied in the treatment of COVID-19[142]. Thus a multitude of factors including inflammatory mediated damage, direct cytotoxicity, hypoxemia/hypoten
The pattern of liver injury seen in COVID-19 is typically elevated AST and ALT levels with a predominance of AST elevation[143]. Serum bilirubin levels can also be mildly increased but it’s relation to disease severity is unclear in contrast the levels of aminotransferases that correlate with disease severity[144]. Hypoalbuminemia can also be seen along with increased levels of GGT in severe cases, but the levels of ALP are usually normal in mild or severe cases[139,145]. A case report of a patient initially presenting with hepatitis that was later diagnosed as COVID-19 infection, has also been reported[146]. Patients with pre-existing liver disease may be more susceptible to suffer from liver damage from COVID-19 according to the meta-analysis done by Mantovani et al[138]. Patients with non-alcoholic fatty liver disease (NAFLD) were demonstrated to have higher risk of disease progression, longer viral shedding time and higher likelihood of abnormal liver function tests[147]. Table 3 describes relevant studies in the context of COVID-19 and liver disease[148-157].
| Ref. | Patients | Type | Study highlight with regards to liver disease |
| Xie et al[148] | 79 | Retrospective study | Liver injury maybe related to systemic inflammation and liver function should be monitored in patients with severe pulmonary lesions on imaging |
| Zhang et al[149] | 115 | Retrospective study | Liver enzymes as well as INR significantly elevated in patients with severe COVID-19; Albumin low in severe cases |
| Huang et al[145] | 41 | Prospective case series | Two percent patients had chronic liver disease; 37% patients had elevated AST which was more pronounced in ICU patients |
| Fan et al[141] | 148 | Retrospective case series | In patients with abnormal liver function, more received treatment with lopinavir/ritonavir as compared to those with normal liver function |
| Wang et al[150] | 138 | Retrospective study | Of 2.9% patients had chronic liver disease, AST elevation > ALT and seen more in ICU patients |
| Xu et al[151] | 62 | Retrospective study | Of 12% patients had underlying liver disease; 16% patients had elevated AST |
| Shi et al[152] | 81 | Retrospective study | AST more elevated in patients with increasing pulmonary lesions on imaging; 9% patients had hepatitis or cirrhosis on imaging |
| Zhang et al[153] | 82 | Retrospective study; Jul 2020 | Of 2.4% patients had underlying liver disease; 1.2% patients died due to liver disease; 30.6%, 61.1% and 30.6% had elevated levels of ALT, AST and Total bilirubin respectively |
| Guan et al[135] | 1099 | Retrospective study | There are 2.1% patients had hepatitis B; AST, ALT and Total bilirubin were elevated in 22.2%, 21.3% and 10.5% patients respectively |
| Ji et al[147] | 202 | Retrospective study | Liver injury frequent but mild in nature with mostly hepatocellular pattern; Patients with NAFLD and BMI had higher risk for persistent liver injury. Patients with NAFLD had higher risk for severe COVID-19 and longer viral shedding. |
| Mao et al[154] | 6686 | Systematic Review and Meta-analysis | Pooled prevalence of liver comorbidities was 3%. Pooled prevalence of liver injury was 19%; Patients with severe COVID-19 had higher risk for abnormal liver enzymes. |
| Singh et al[155] | 2780 | Multicenter research network study | Patients with cirrhosis and pre-existing liver disease are at increased risk for hospitalization and death |
| Bloom et al[143] | 60 | Prospective cohort study | Predominant AST elevation commonly seen in COVID-19 and correlates with disease severity |
| Wang et al[156] | 105 | Retrospective study | Elevated liver enzymes more likely in patients with severe COVID-19 |
| Cai et al[157] | 417 | Cross sectional study | Of 76.3% patients had abnormal liver enzymes and 21.5% had liver injury during hospitalization; Patients who received lopinavir/ritonavir had higher odds of liver injury. Patients with abnormal liver tests had higher chance of severe COVID-19 |
The risk of COVID-19 infection and its severity remain unclear in patients with LT, although a preliminary analysis of the SOT recipient registry from the University of Washington reported that the risk of contracting COVID-19 in SOT recipients is comparable to the general population[158]. With regards to mortality in LT patients, older patients with LT seem to have a higher mortality[159]. International voluntary registries that collect information on COVID-19 patients with underlying liver disease and LT described 81% patients hospitalized with 30% requiring intensive care unit care and 19% expired[160]. A systematic review described a case fatality rate of 37.5% among LT recipients[161].
Organ procurement has decreased due to the limitations of the pandemic whereas telemedicine is increasingly utilized in evaluation LT recipients[162,163]. All the major societies recommend that patients with high MELD scores, risk for decompensation or HCC progression only be considered for LT[162,164,165]. AASLD recommends that patients with COVID-19 do not receive LT but the procedure can be undertaken 21 d after symptom resolution and negative test in recipient. With regards to immunosuppression in the post-transplant period, all the major societies recommend against reducing it as there has been no data to suggest immunosuppression as a risk factor for severe COVID-19[162,164,165]. AASLD however recommends lowering antimetabolite medication dosages while maintaining the same doses of calcineurin inhibitors (CNI) in LT patients with COVID-19, based on similar principles for managing an active infection in LT patients. Managing immunosuppressive therapy is challenging and should be done cautiously in patients with LT who had COVID-19 due to interactions between corticosteroids and CNI, and the liver toxicity associated with remdesevir and tocilizumab[166]. AASLD recommends vaccination preferable 3 mo after liver transplant once the doses of immunosuppressant medications have been reduced[167]. Table 4 highlights important studies in the context of COVID-19 and liver transplant[168-175].
| Ref. | Patients | Type | Study highlight with regards to liver transplant |
| Coll et al[168] | 110 | Retrospective | Higher incidence of COVID (two-fold) in solid organ transplant patients. Eighty-five percent patients had adjustment in their immunosuppression |
| Becchetti et al[169] | 57 | Multicenter Prospective | Of 12% overall fatality rate and 17% in-hospital fatality rate. Patients with history of cancer had poorer outcomes |
| Colmonero et al[170] | 111 | Prospective | LT patients with increased risk of contracting COVID-19 but lower mortality when compared with matched general population. Dose reduction/withdrawal in mycophenolate helped prevent severe COVID-19 but complete discontinuation of immunosuppressants discouraged. |
| Webb et al[171] | 151 | Multicenter Prospective | Need for invasive mechanical ventilation and ICU admission more in LT group when compared with a control cohort – 20% vs 5 % and 28% vs 8% respectively. LT not independently associated with death, but presence of comorbidities and increased age were |
| Belli et al[172] | 240 | Multicenter retrospective | Of 84% patients required hospitalization, 25% of hospitalized patients died. Use of Tacrolimus associated with increased survival probability |
| Bhoori et al[159] | 111 | Retrospective | Three patients died of COVID-19 and all of them were male, > 65 years with multiple comorbidities and minimal immunosuppression |
| Rabiee et al[173] | 112 | Prospective | Hospital and ICU mortality rates lower rates in matched patients with chronic liver disease without LT |
| Mansoor et al[174] | 126 | Retrospective | Higher risk of hospitalization in LT patients. No difference in mortality and need for ICU in LT patients vs non- LT patients |
| Tejedor-Tejada et al[175] | 16 | Retrospective | Post COVID-19 syndrome present with mild symptoms but no loss of liver graft or graft dysfunction noted |
The topic of non-hepatotropic viral infection is very broad and covers a number of infections that do not have liver as the primary site of infection. Majority of the known infections belong to the family of herpes virus infections and often require reactivation, as seen in immunocompromised individuals. Since the development of systemic disease with these infections depends on immune dysregulation, full blown disease is rarely seen in immunocompetent patients. Moreover, the infection is more severe in immunocompromised individuals, especially post-transplant (including liver transplant). These patients can also suffer from allograft rejection, in addition to hepatitis of varying degree of severity. The diagnosis, despite the presence of new testing modalities, is often based on exclusion of hepatotropic infection and liver biopsy findings inconsistent with other etiologies of hepatitis. There is a lack of guidelines regarding management of each viral infection. Antivirals are often the first line, with or without steroid use. Patients with poor prognosis are worked up for liver transplant and studies have indicated continued use of antivirals following transplant to cover for latent infection. Lastly, there is growing literation on the involvement of liver in coronavirus 2019 pandemic and warrants it to be included in the differential diagnosis of hepatitis, once hepatotropic infection is ruled out.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao HC S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Gupta E, Ballani N, Kumar M, Sarin SK. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol. 2015;34:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bull World Health Organ. 1973;49:103-106. [PubMed] |
| 3. | Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 477] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 1015] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 5. | Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 685] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 6. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 826] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 7. | Da Cunha T, Wu GY. Cytomegalovirus Hepatitis in Immunocompetent and Immunocompromised Hosts. J Clin Transl Hepatol. 2021;9:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Fakhreddine AY, Frenette CT, Konijeti GG. A Practical Review of Cytomegalovirus in Gastroenterology and Hepatology. Gastroenterol Res Pract. 2019;2019:6156581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Hassan-Walker AF, Mattes FM, Griffiths PD, Emery VC. Quantity of cytomegalovirus DNA in different leukocyte populations during active infection in vivo and the presence of gB and UL18 transcripts. J Med Virol. 2001;64:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sinzger C, Plachter B, Grefte A, The TH, Jahn G. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis. 1996;173:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Jean Beltran PM, Cristea IM. The life cycle and pathogenesis of human cytomegalovirus infection: lessons from proteomics. Expert Rev Proteomics. 2014;11:697-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Bunchorntavakul C, Reddy KR. Epstein-Barr Virus and Cytomegalovirus Infections of the Liver. Gastroenterol Clin North Am. 2020;49:331-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Wills MR, Poole E, Lau B, Krishna B, Sinclair JH. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol Immunol. 2015;12:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Terrazzini N, Kern F. Cell-mediated immunity to human CMV infection: a brief overview. F1000Prime Rep. 2014;6:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Sacher T, Podlech J, Mohr CA, Jordan S, Ruzsics Z, Reddehase MJ, Koszinowski UH. The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe. 2008;3:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kühnapfel B, Reddehase MJ, Grzimek NK. Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol. 2009;83:8869-8884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Bruns T, Zimmermann HW, Pachnio A, Li KK, Trivedi PJ, Reynolds G, Hubscher S, Stamataki Z, Badenhorst PW, Weston CJ, Moss PA, Adams DH. CMV infection of human sinusoidal endothelium regulates hepatic T cell recruitment and activation. J Hepatol. 2015;63:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:315-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Kanj SS, Sharara AI, Clavien PA, Hamilton JD. Cytomegalovirus infection following liver transplantation: review of the literature. Clin Infect Dis. 1996;22:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Paya CV, Hermans PE, Wiesner RH, Ludwig J, Smith TF, Rakela J, Krom RA. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989;160:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Bruminhent J, Razonable RR. Management of cytomegalovirus infection and disease in liver transplant recipients. World J Hepatol. 2014;6:370-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 23. | Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, Jenkins G, Larson T, Hellinger WC, Spivey JR, Paya CV. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Infect Dis. 2001;184:1461-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Griffiths PD, Grundy JE. The status of CMV as a human pathogen. Epidemiol Infect. 1988;100:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Grundy JE, Shanley JD, Griffiths PD. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987;2:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Kyriazis AP, Mitra SK. Multiple cytomegalovirus-related intestinal perforations in patients with acquired immunodeficiency syndrome. Report of two cases and review of the literature. Arch Pathol Lab Med. 1992;116:495-499. [PubMed] |
| 27. | Seehofer D, Rayes N, Tullius SG, Schmidt CA, Neumann UP, Radke C, Settmacher U, Müller AR, Steinmüller T, Neuhaus P. CMV hepatitis after liver transplantation: incidence, clinical course, and long-term follow-up. Liver Transpl. 2002;8:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Yadav SK, Saigal S, Choudhary NS, Saha S, Kumar N, Soin AS. Cytomegalovirus Infection in Liver Transplant Recipients: Current Approach to Diagnosis and Management. J Clin Exp Hepatol. 2017;7:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Toghill PJ, Williams R, Stern H. Cytomegalovirus infection in chronic liver disease. Gastroenterology. 1969;56:936-937. [PubMed] |
| 30. | Arnold JC, Portmann BC, O'Grady JG, Naoumov NV, Alexander GJ, Williams R. Cytomegalovirus infection persists in the liver graft in the vanishing bile duct syndrome. Hepatology. 1992;16:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Faivre M, Cottet V, Bour JB, Richou C, Valmary-Degano S, Thiefin G, Andreoletti L, Geist C, Schvoerer E, Malvé B, Habersetzer F, Fafi-Kremer S, Binquet C, Jouve JL, Bronowicki JP, Doffoel M, Hillon P, Herbein G, Monnet E, Di Martino V; CirCE Study Group. Impact of Cytomegalovirus Infection on the Outcome of Patients With Cirrhosis: A Preliminary Study. J Clin Gastroenterol. 2019;53:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Lautenschlager I, Höckerstedt K, Jalanko H, Loginov R, Salmela K, Taskinen E, Ahonen J. Persistent cytomegalovirus in liver allografts with chronic rejection. Hepatology. 1997;25:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Gorensek MJ, Carey WD, Vogt D, Goormastic M. A multivariate analysis of risk factors for cytomegalovirus infection in liver-transplant recipients. Gastroenterology. 1990;98:1326-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Humar A, Mazzulli T, Moussa G, Razonable RR, Paya CV, Pescovitz MD, Covington E, Alecock E; Valganciclovir Solid Organ Transplant Study Group. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R- transplant recipients. Am J Transplant. 2005;5:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Ross SA, Novak Z, Pati S, Boppana SB. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets. 2011;11:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 36. | Greanya ED, Partovi N, Yoshida EM, Shapiro RJ, Levy RD, Sherlock CH, Stephens GM. The role of the cytomegalovirus antigenemia assay in the detection and prevention of cytomegalovirus syndrome and disease in solid organ transplant recipients: A review of the British Columbia experience. Can J Infect Dis Med Microbiol. 2005;16:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Piiparinen H, Höckerstedt K, Grönhagen-Riska C, Lautenschlager I. Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J Clin Virol. 2004;30:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Gleaves CA, Smith TF, Shuster EA, Pearson GR. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1985;21:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 239] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Mhiri L, Kaabi B, Houimel M, Arrouji Z, Slim A. Comparison of pp65 antigenemia, quantitative PCR and DNA hybrid capture for detection of cytomegalovirus in transplant recipients and AIDS patients. J Virol Methods. 2007;143:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sanghavi SK, Abu-Elmagd K, Keightley MC, St George K, Lewandowski K, Boes SS, Bullotta A, Dare R, Lassak M, Husain S, Kwak EJ, Paterson DL, Rinaldo CR. Relationship of cytomegalovirus load assessed by real-time PCR to pp65 antigenemia in organ transplant recipients. J Clin Virol. 2008;42:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Seehofer D, Meisel H, Rayes N, Stein A, Langrehr JM, Settmacher U, Neuhaus P. Prospective evaluation of the clinical utility of different methods for the detection of human cytomegalovirus disease after liver transplantation. Am J Transplant. 2004;4:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Noor A, Panwala A, Forouhar F, Wu GY. Hepatitis caused by herpes viruses: A review. J Dig Dis. 2018;19:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Kunno A, Abe M, Yamada M, Murakami K. Clinical and histological features of cytomegalovirus hepatitis in previously healthy adults. Liver. 1997;17:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | McDonald GB, Sarmiento JI, Rees-Lui G, Myerson D. Cytomegalovirus hepatitis after bone marrow transplantation: An autopsy study with clinical, histologic and laboratory correlates. J Viral Hepat. 2019;26:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Fernández-Ruiz M, Muñoz-Codoceo C, López-Medrano F, Faré-García R, Carbonell-Porras A, Garfia-Castillo C, Muñoz-Gómez R, Aguado-García JM. Cytomegalovirus myopericarditis and hepatitis in an immunocompetent adult: successful treatment with oral valganciclovir. Intern Med. 2008;47:1963-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Serna-Higuera C, González-García M, Milicua JM, Muñoz V. Acute cholestatic hepatitis by cytomegalovirus in an immunocompetent patient resolved with ganciclovir. J Clin Gastroenterol. 1999;29:276-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Leonardsson H, Hreinsson JP, Löve A, Björnsson ES. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Yu YD, Park GC, Park PJ, Choi YI, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, Moon DB, Ha TY, Lee SG. Cytomegalovirus infection-associated fulminant hepatitis in an immunocompetent adult requiring emergency living-donor liver transplantation: report of a case. Surg Today. 2013;43:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Shusterman NH, Frauenhoffer C, Kinsey MD. Fatal massive hepatic necrosis in cytomegalovirus mononucleosis. Ann Intern Med. 1978;88:810-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Singh N. Optimal prevention of late-onset cytomegalovirus (CMV) disease and other sequelae of CMV infection in organ transplant recipients. Clin Infect Dis. 2008;47:296-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol. 2014;20:10658-10667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Madalosso C, de Souza NF Jr, Ilstrup DM, Wiesner RH, Krom RA. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation. 1998;66:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Humar A, Washburn K, Freeman R, Paya CV, Mouas H, Alecock E, Razonable RR; PV16000 Study Group. An assessment of interactions between hepatitis C virus and herpesvirus reactivation in liver transplant recipients using molecular surveillance. Liver Transpl. 2007;13:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Humar A, Kumar D, Raboud J, Caliendo AM, Moussa G, Levy G, Mazzulli T. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant. 2002;2:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 947] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 57. | Perales MA. Editorial commentary: Adoptive therapy of cytomegalovirus-specific T lymphocytes: is less more? Clin Infect Dis. 2011;52:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Lindemann M, Eiz-Vesper B, Steckel NK, Tischer S, Fiedler M, Heinold A, Klisanin V, Maecker-Kolhoff B, Blasczyk R, Horn PA, Beelen DW, Koldehoff M. Adoptive transfer of cellular immunity against cytomegalovirus by virus-specific lymphocytes from a third-party family donor. Bone Marrow Transplant. 2018;53:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Ligat G, Cazal R, Hantz S, Alain S. The human cytomegalovirus terminase complex as an antiviral target: a close-up view. FEMS Microbiol Rev. 2018;42:137-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 60. | Frange P, Leruez-Ville M. Maribavir, brincidofovir and letermovir: Efficacy and safety of new antiviral drugs for treating cytomegalovirus infections. Med Mal Infect. 2018;48:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M; Maribavir 1263-300 Clinical Study Group. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 62. | Marty FM, Boeckh M. Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed? Curr Opin Virol. 2011;1:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Weekes MP, Tan SY, Poole E, Talbot S, Antrobus R, Smith DL, Montag C, Gygi SP, Sinclair JH, Lehner PJ. Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection. Science. 2013;340:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Humby MS, O'Connor CM. Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells. J Virol. 2015;90:2959-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A. 2005;102:4140-4145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 66. | Anderholm KM, Bierle CJ, Schleiss MR. Cytomegalovirus Vaccines: Current Status and Future Prospects. Drugs. 2016;76:1625-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Jenei B, Pócsik E, Lázár G, Medgyesi GA. Comparison of hypotensive response to aggregated IgG or to bacterial LPS in rats. Inflamm Res. 1997;46:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Björnsson HK, Olafsson S, Bergmann OM, Björnsson ES. A prospective study on the causes of notably raised alanine aminotransferase (ALT). Scand J Gastroenterol. 2016;51:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Vine LJ, Shepherd K, Hunter JG, Madden R, Thornton C, Ellis V, Bendall RP, Dalton HR. Characteristics of Epstein-Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther. 2012;36:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Drebber U, Kasper HU, Krupacz J, Haferkamp K, Kern MA, Steffen HM, Quasdorff M, Zur Hausen A, Odenthal M, Dienes HP. The role of Epstein-Barr virus in acute and chronic hepatitis. J Hepatol. 2006;44:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Schechter S, Lamps L. Epstein-Barr Virus Hepatitis: A Review of Clinicopathologic Features and Differential Diagnosis. Arch Pathol Lab Med. 2018;142:1191-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, Osterhaus AD, Gratama JW, Löwenberg B, Verdonck LF, Cornelissen JJ. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 74. | Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, Wagner JE. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 75. | Randhawa PS, Jaffe R, Demetris AJ, Nalesnik M, Starzl TE, Chen YY, Weiss LM. Expression of Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver specimens from transplant recipients with post-transplantation lymphoproliferative disease. N Engl J Med. 1992;327:1710-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 127] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Nalesnik MA, Jaffe R, Starzl TE, Demetris AJ, Porter K, Burnham JA, Makowka L, Ho M, Locker J. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173-192. [PubMed] |
| 77. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 958] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 78. | McClain K, Gehrz R, Grierson H, Purtilo D, Filipovich A. Virus-associated histiocytic proliferations in children. Frequent association with Epstein-Barr virus and congenital or acquired immunodeficiencies. Am J Pediatr Hematol Oncol. 1988;10:196-205. [PubMed] |
| 79. | Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 80. | Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 81. | Kofteridis DP, Koulentaki M, Valachis A, Christofaki M, Mazokopakis E, Papazoglou G, Samonis G. Epstein Barr virus hepatitis. Eur J Intern Med. 2011;22:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Brigden ML, Au S, Thompson S, Brigden S, Doyle P, Tsaparas Y. Infectious mononucleosis in an outpatient population: diagnostic utility of 2 automated hematology analyzers and the sensitivity and specificity of Hoagland's criteria in heterophile-positive patients. Arch Pathol Lab Med. 1999;123:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, Burbelo PD, Turk SP, Fulton R, Wayne AS, Little RF, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop MR, Wilson W, Straus SE. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117:5835-5849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 84. | Linderholm M, Boman J, Juto P, Linde A. Comparative evaluation of nine kits for rapid diagnosis of infectious mononucleosis and Epstein-Barr virus-specific serology. J Clin Microbiol. 1994;32:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J Virol. 2012;1:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (4)] |
| 86. | Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Chiba T, Goto S, Yokosuka O, Imazeki F, Tanaka M, Fukai K, Takahashi Y, Tsujimura H, Saisho H. Fatal chronic active Epstein-Barr virus infection mimicking autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2004;16:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Suh N, Liapis H, Misdraji J, Brunt EM, Wang HL. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol. 2007;31:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Mellinger JL, Rossaro L, Naugler WE, Nadig SN, Appelman H, Lee WM, Fontana RJ. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | Adams LA, Deboer B, Jeffrey G, Marley R, Garas G. Ganciclovir and the treatment of Epstein-Barr virus hepatitis. J Gastroenterol Hepatol. 2006;21:1758-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Pisapia R, Mariano A, Rianda A, Testa A, Oliva A, Vincenzi L. Severe EBV hepatitis treated with valganciclovir. Infection. 2013;41:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Cacopardo B, Nunnari G, Mughini MT, Tosto S, Benanti F, Nigro L. Fatal hepatitis during Epstein-Barr virus reactivation. Eur Rev Med Pharmacol Sci. 2003;7:107-109. [PubMed] |
| 93. | Feranchak AP, Tyson RW, Narkewicz MR, Karrer FM, Sokol RJ. Fulminant Epstein-Barr viral hepatitis: orthotopic liver transplantation and review of the literature. Liver Transpl Surg. 1998;4:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Zhang W, Chen B, Chen Y, Chamberland R, Fider-Whyte A, Craig J, Varma C, Befeler AS, Bisceglie AM, Horton P, Lai JP. Epstein-Barr Virus-Associated Acute Liver Failure Present in a 67-Year-Old Immunocompetent Female. Gastroenterology Res. 2016;9:74-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | McDiarmid SV, Jordan S, Kim GS, Toyoda M, Goss JA, Vargas JH, Martín MG, Bahar R, Maxfield AL, Ament ME, Busuttil RW. Prevention and preemptive therapy of postransplant lymphoproliferative disease in pediatric liver recipients. Transplantation. 1998;66:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | Yang J, Tao Q, Flinn IW, Murray PG, Post LE, Ma H, Piantadosi S, Caligiuri MA, Ambinder RF. Characterization of Epstein-Barr virus-infected B cells in patients with posttransplantation lymphoproliferative disease: disappearance after rituximab therapy does not predict clinical response. Blood. 2000;96:4055-4063. [PubMed] |
| 97. | Garnier JL, Stevenson G, Blanc-Brunat N, Touraine JL, Milpied N, Leblond V, Blay JY. Treatment of post-transplant lymphomas with anti-B-cell monoclonal antibodies. Recent Results Cancer Res. 2002;159:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA, Tsai DE. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 99. | Blaes AH, Peterson BA, Bartlett N, Dunn DL, Morrison VA. Rituximab therapy is effective for posttransplant lymphoproliferative disorders after solid organ transplantation: results of a phase II trial. Cancer. 2005;104:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Jain AB, Marcos A, Pokharna R, Shapiro R, Fontes PA, Marsh W, Mohanka R, Fung JJ. Rituximab (chimeric anti-CD20 antibody) for posttransplant lymphoproliferative disorder after solid organ transplantation in adults: long-term experience from a single center. Transplantation. 2005;80:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.6.1. Cancer risk after renal transplantation. Post-transplant lymphoproliferative disease (PTLD): prevention and treatment. Nephrol Dial Transplant. 2002;17 Suppl 4:31-33, 35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Wertheim RA, Brooks BJ, Rodriguez FH, Lesesne HR, Jennette JC. Fatal herpetic hepatitis in pregnancy. Obstet Gynecol. 1983;63:38s-42s. |
| 103. | Gallegos-Orozco JF, Rakela-Brödner J. Hepatitis viruses: not always what it seems to be. Rev Med Chil. 2010;138:1302-1311. [PubMed] |
| 104. | Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 105. | Katz J, Magee J, Baker B, Eger EI 2nd. Hepatic necrosis associated with herpesvirus after anesthesia with desflurane and nitrous oxide. Anesth Analg. 1994;78:1173-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 106. | Fisher NA, Iwata RT, Eger EI 2nd, Smuckler EA. Hepatic necrosis associated with herpes virus after isoflurane anesthesia. Anesth Analg. 1985;64:1131-1133. [PubMed] |
| 107. | Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Miyazaki Y, Akizuki S, Sakaoka H, Yamamoto S, Terao H. Disseminated infection of herpes simplex virus with fulminant hepatitis in a healthy adult. A case report. APMIS. 1991;99:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Peters DJ, Greene WH, Ruggiero F, McGarrity TJ. Herpes simplex-induced fulminant hepatitis in adults: a call for empiric therapy. Dig Dis Sci. 2000;45:2399-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Rimawi BH, Meserve J, Rimawi RH, Min Z, Gnann JW Jr. Disseminated Herpes Simplex Virus with Fulminant Hepatitis. Case Reports Hepatol. 2015;2015:463825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Riediger C, Sauer P, Matevossian E, Müller MW, Büchler P, Friess H. Herpes simplex virus sepsis and acute liver failure. Clin Transplant. 2009;23 Suppl 21:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 113. | Levitsky J, Duddempudi AT, Lakeman FD, Whitley RJ, Luby JP, Lee WM, Fontana RJ, Blei AT, Ison MG; US Acute Liver Failure Study Group. Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transpl. 2008;14:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Tsochatzis E, Papatheodoridis GV, Elefsiniotis I, Thanelas S, Theodossiades G, Moulakakis A, Archimandritis AJ. Prophylactic and therapeutic use of recombinant activated factor VII in patients with cirrhosis and coagulation impairment. Dig Liver Dis. 2007;39:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | McAfee JH, Keeffe EB, Lee RG, Rösch J. Transjugular liver biopsy. Hepatology. 1992;15:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Tripuraneni V, Patel K, Brennan TV, Ho LM. Fulminant herpes simplex viral hepatitis: ultrasound and CT imaging appearance and a review of the imaging literature. Clin Imaging. 2014;38:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Murakami T, Baron RL, Peterson MS. Liver necrosis and regeneration after fulminant hepatitis: pathologic correlation with CT and MR findings. Radiology. 1996;198:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Mortelé KJ, Barish MA, Yucel KE. Fulminant herpes hepatitis in an immunocompetent pregnant woman: CT imaging features. Abdom Imaging. 2004;29:682-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Stránská R, Schuurman R, Nienhuis E, Goedegebuure IW, Polman M, Weel JF, Wertheim-Van Dillen PM, Berkhout RJ, van Loon AM. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol. 2005;32:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Chaudhary D, Ahmed S, Liu N, Marsano-Obando L. Acute Liver Failure from Herpes Simplex Virus in an Immunocompetent Patient Due to Direct Inoculation of the Peritoneum. ACG Case Rep J. 2017;4:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Holt EW, Guy J, Gordon SM, Hofmann JC, Garcia-Kennedy R, Steady SL, Bzowej NH, Frederick RT. Acute liver failure caused by herpes simplex virus in a pregnant patient: is there a potential role for therapeutic plasma exchange? J Clin Apher. 2013;28:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Shanley CJ, Braun DK, Brown K, Turcotte JG, Greenson JK, Beals TF, Tiballi RN, Campbell DA Jr. Fulminant hepatic failure secondary to herpes simplex virus hepatitis. Successful outcome after orthotopic liver transplantation. Transplantation. 1995;59:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Devictor D, Desplanques L, Debray D, Ozier Y, Dubousset AM, Valayer J, Houssin D, Bernard O, Huault G. Emergency liver transplantation for fulminant liver failure in infants and children. Hepatology. 1992;16:1156-1162. [PubMed] |
| 124. | Côté-Daigneault J, Carrier FM, Toledano K, Wartelle-Bladu C, Willems B. Herpes simplex hepatitis after liver transplantation: case report and literature review. Transpl Infect Dis. 2014;16:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Basse G, Mengelle C, Kamar N, Ribes D, Selves J, Cointault O, Suc B, Rostaing L. Disseminated herpes simplex type-2 (HSV-2) infection after solid-organ transplantation. Infection. 2008;36:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Navaneethan U, Lancaster E, Venkatesh PG, Wang J, Neff GW. Herpes simplex virus hepatitis - it's high time we consider empiric treatment. J Gastrointestin Liver Dis. 2011;20:93-96. [PubMed] |
| 127. | Lee DH, Zuckerman RA; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 128. | Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Dropulic LK, Cohen JI. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev Vaccines. 2012;11:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 130. | Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, Morse SA, St Louis ME, Weiss JB, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald GA, Novotny J, Weisfuse I, Goldberg M, O'Donnell JA, Knaup R. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis. 1998;178:1795-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Kardani K, Basimi P, Fekri M, Bolhassani A. Antiviral therapy for the sexually transmitted viruses: recent updates on vaccine development. Expert Rev Clin Pharmacol. 2020;13:1001-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Bernstein DI, Flechtner JB, McNeil LK, Heineman T, Oliphant T, Tasker S, Wald A, Hetherington S; Genocea study group. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine. 2019;37:3443-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 133. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 134. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1203] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 135. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18850] [Article Influence: 3770.0] [Reference Citation Analysis (7)] |
| 136. | Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, Liu J, Chen Y, Li J. Risk factors related to hepatic injury in patients with corona virus disease 2019. 2020 Preprint. Available from: medRxiv. [DOI] [Full Text] |
| 137. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1207] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 138. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 139. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 140. | Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 141. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 142. | Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 143. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 144. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12964] [Article Influence: 2592.8] [Reference Citation Analysis (1)] |
| 145. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30071] [Article Influence: 6014.2] [Reference Citation Analysis (3)] |
| 146. | Wander P, Epstein M, Bernstein D. COVID-19 Presenting as Acute Hepatitis. Am J Gastroenterol. 2020;115:941-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 147. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 148. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 149. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 150. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14751] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 151. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 152. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2309] [Article Influence: 461.8] [Reference Citation Analysis (0)] |
| 153. | Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 154. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 750] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 155. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 156. | Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, Cao Y, Ding R, Wang JJ, Cheng C, Xie W. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 157. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 158. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 492] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 159. | Belli LS, Duvoux C, Karam V, Adam R, Cuervas-Mons V, Pasulo L, Loinaz C, Invernizzi F, Patrono D, Bhoori S, Ciccarelli O, Morelli MC, Castells L, Lopez-Lopez V, Conti S, Fondevila C, Polak W. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 160. | SECURE-Liver Registry. Reporting COVID-19 Liver disease to AASLD. [cited 10 May 2021]. Available from: https://covidcirrhosis.web.unc.edu. |
| 161. | Nacif LS, Zanini LY, Waisberg DR, Pinheiro RS, Galvão F, Andraus W, D'Albuquerque LC. COVID-19 in solid organ transplantation patients: A systematic review. Clinics (Sao Paulo). 2020;75:e1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 162. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 163. | Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95-e96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 164. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 165. | Lau G, Sharma M; APASL Covid-19 Task Force. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 166. | Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474-e484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 687] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 167. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 168. | Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, Bada-Bosch T, Oppenheimer F, Moreso F, López-Oliva MO, Melilli E, Rodríguez-Ferrero ML, Bravo C, Burgos E, Facundo C, Lorenzo I, Yañez Í, Galeano C, Roca A, Cabello M, Gómez-Bueno M, García-Cosío M, Graus J, Lladó L, de Pablo A, Loinaz C, Aguado B, Hernández D, Domínguez-Gil B; Spanish Group for the Study of COVID-19 in Transplant Recipients. COVID-19 in transplant recipients: The Spanish experience. Am J Transplant. 2021;21:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 169. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 170. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 171. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 172. | Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C; ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 173. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 174. | Mansoor E, Perez A, Abou-Saleh M, Sclair SN, Cohen S, Cooper GS, Mills A, Schlick K, Khan A. Clinical Characteristics, Hospitalization, and Mortality Rates of Coronavirus Disease 2019 Among Liver Transplant Patients in the United States: A Multicenter Research Network Study. Gastroenterology. 2021;160:459-462.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 175. | Tejedor-Tejada J, Fuentes-Valenzuela E, Alonso-Martin C, Almohalla-Alvarez C, Garcia-Pajares F. COVID-19 and short and medium-term outcomes in liver transplant patients: A spanish single-center case series. J Clin Exp Hepatol. . [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |