INTRODUCTION
Fecal microbiota transplantation (FMT) refers to the transplantation of healthy human intestinal flora into the intestinal tract of patients to treat diseases by reconstructing the normal composition of intestinal microbes[1]. The earliest record of treating diseases with human feces was written in the book “Zhou Hou Bei Ji Fang” by Ge Hong in the fourth century AD, which was used to treat severe food poisoning[2]. In modern medicine, FMT is a treatment option that can quickly reconstruct the normal composition of intestinal microbes[3]. It is recommended by clinical guidelines for treatment of recurrent Clostridium difficile (C. difficile) infection (CDI)[4-6] and acute graft-versus-host reaction (GVHD)[7]. In addition, it has been found that FMT has potential therapeutic value for inflammatory bowel disease (IBD), irritable bowel syndrome, autism, metabolic syndrome, antibiotic-associated diarrhea, hepatic encephalopathy, and other related diseases[1,8,9], even systemic diseases associated with intestinal microbiota, such as obesity[10]. Although FMT has been shown to have potential in treating various diseases, its therapeutic mechanism remains unclear[11]. Like other treatments, FMT needs to be scientifically evaluated for its advantages and disadvantages, and its safety needs to be enhanced[12]. The degree of FMT standardization is also low due to different donors for this technique[11] and separate guidelines in different regions[9]. Therefore, in this review, we discuss the use of germ-free animal systems to understand the mechanism of FMT efficacy and screen the functional strains of FMT, to discover the functional bacteria that play a role in FMT, and develop a safe, controllable, standard, and effective combination of functional bacteria.
CAUSAL RELATIONSHIP AND MECHANISM OF FMT EFFICACY NEEDS TO BE CLARIFIED
CDI is an important risk factor for exacerbation and death in hospitalized patients, with recurrent infection occurring in up to 30% of patients receiving conventional treatment, suggesting that the mechanism of antibiotic treatment for CDI is the direct killing of C. difficile, but it may not be eradicated. FMT is a new approach to solve this complex problem. In the treatment of recurrent CDI, the effective rate of FMT is nearly 90%[13]. Different from the direct killing effect of antibiotics, the recovery of intestinal flora diversity is considered as the main mechanism of FMT in treating CDI[14]. FMT has been proved to be effective in treating an increasing number of diseases, and its clinical application continues to grow. For example, the efficacy of FMT in the treatment of IBD has also been confirmed. By including 75 patients with ulcerative colitis (UC), Costello et al[15] demonstrated that FMT was significant in relieving UC, with nine of 38 patients (24%) in the FMT group having remission and two of 37 patients (5%) in the placebo group having remission at the end of treatment.
FMT has great clinical potential, and it is a type of donor-specific treatment, with heterogeneous efficacy for different pathological conditions. At present, the whole spectrum of bacteria and randomness of donors are still used for clinical treatment[16], and there is no universal super feces[17]; it is therefore necessary to clarify the mechanism of FMT to better exert its effects in diseases other than CDI. According to existing research reports, FMT works through direct or indirect approaches. Direct approaches include the introduction of bacteriophages, bacteriocins, and metabolites (short-chain fatty acids and bile acids), and nutritional competition between normal flora and C. difficile. Indirect approaches include participation in the metabolism of short-chain fatty acids and bile acids, thus participating in the regulation of immune and mechanical barriers to exert efficacy[9,18]. It has been found that human FMT compensates for the microbial imbalance in the feces of colitis rats by restoring diversity and increasing the relative abundance of health-related microbes[19]. FMT also regulates the microbial composition of the colon, which leads to recovery of colon length, and significantly reduces the severity of epithelial injury and disease[19]. Natividad et al[20] found that ecobiotherapy rich in Firmicutes can reduce the susceptibility to colitis in a humanized gnotobiotic mouse model, and downregulate colonic inflammation and TH17 pathways in mice; it is clear that the mechanism of FMT against colitis may be the enriched Firmicutes[20].
SAFETY OF FMT NEEDS TO BE EVALUATED
Currently, FMT has been applied to clinical treatment of various diseases. However, as an emerging clinical treatment, the short- and long-term safety issues of FMT have become the focus of attention, requiring further clinical and laboratory data to confirm. China and the United States have established the China Microflora Platform (www.cmts.com)[21] and the United States FMT National registry system (FMT National Registry System)[22,23], respectively, for the follow-up and registration of all patients receiving FMT for up to 10 years to assess its long-term safety. In the systematic review reported by Zhang Faming’s research group from 2000 to 2020, FMT-related adverse events were divided into transplantation-related and microecologically related adverse events for the first time, and mucosal barrier injury-associated microbiota-related adverse events were defined. The total incidence of FMT-related adverse events was 19%, with diarrhea, abdominal discomfort, pain, and spasm being the most common, and the incidence of FMT-related serious adverse events was 1.4%. The incidence of microecologically related serious adverse events was 0.99%, and all serious adverse events occurred in patients with impaired intestinal mucosal barrier function[24].
FMT is associated with a risk of infection and needs to be taken seriously. As with all treatments, the advantages and disadvantages need to be assessed[12]. For example, FMT has been used as a potential treatment option for Crohn’s disease (CD), but there is still a lack of evidence for safety based on the large number of CD samples undergoing FMT. The study by Wang et al[25] showed that 184 FMT frequency tests were performed in 139 patients receiving FMT, and 13.6% of mild adverse events including fever, abdominal pain, flatulence, hematochezia, vomiting, abdominal distension, and increased frequency of herpes zoster occurred within 1 mo after FMT. In two separate clinical trials, after FMT, two patients developed extended-spectrum β-lactamase (ESBL)-producing Escherichia coli bacteremia; both of which were related to the same fecal donor, and one of the patients died. Therefore, donor screening should be enhanced to limit microbial transmission that could lead to adverse infectious events to determine the benefits and risks of FMT in different patient populations[26]. A multicenter retrospective study by Nicholson et al confirmed that the treatment of CDI in pediatric patients was safe and effective, but 4.7% of patients still had serious adverse events during a follow-up period of 3 mo[27]. Ianiro et al[28] studied the safety of FMT for recurrent CDI in 290 patients; 109 were treated with FMT and 181 with antibiotics. After 90-d treatment, 40 patients in the antibiotic group had bloodstream infection (BSI). The analysis of matched patients showed that compared with antibiotic treatment, the risk of BSI in the FMT group decreased by 23%, but there were still five cases of BSI in the FMT group. Bilinski et al[29] published a case report in which the patient developed severe diarrhea and norovirus infection after FMT, accompanied by grade III nausea, weight loss, and rising blood eosinophilia. DeFilipp et al[30] hypothesized that FMT may restore intestinal microbiome diversity after allogeneic hematopoietic cell transplantation (allo-HCT). In this open-label single-group pilot study, 13 patients received FMT capsules. Some patients developed grade 3-4 acute gastrointestinal tract GVHD after FMT, and one of these patients developed Klebsiella pneumoniae bacteremia and sepsis, followed by multiple organ failure. There was one case of C. difficile colitis observed after FMT and six patients developed moderate-severe chronic GVHD[30]. Take into account this, the FDA issued a warning to researchers that fecal screening in FMT studies should be expanded to include specific resistant strains[31] and opportunistic pathogens should also be considered. In addition, when improving the safety of FMT to reduce the risk of systemic infection (such as sepsis caused by FMT spreading drug-resistant pathogens), besides screening for pathogens in the donor fecal bacteria, the composition and structure of the flora should also be considered, such as flora diversity, key bacterial species (such as short-chain fatty acid-producing bacteria), and the ratio of obligate and facultative anaerobic bacteria, which can improve the safety of FMT[31]. At present, there is insufficient evidence to prove that FMT is used to treat diseases other than CDI, and long-term safety still needs to be evaluated[32].
At present, FMT has not been approved as a new drug, which is primarily due to the lack of preclinical safety studies. Experimental animal research can clarify the relationship between safety and bacterial solution treatment methods, dosage, and other factors, and provide scientific data in support of the safety of FMT in clinical application. Since 2014, Zhang Faming’s team has begun to use the automatic fecal isolation system (genFMTer) to ultrafilter, purify, and centrifugally wash the fecal microbiota[33,34], and select different routes for transplantation into patients, which is defined as washed microbiota transplantation (WMT)[4,35]. Preclinical studies have confirmed the safety of WMT after automatic machine isolation in experimental animals, and its clinical application can significantly reduce FMT-related adverse events without affecting the efficacy of patients[4,25,36].
STANDARDIZATION AND ACCEPTABILITY OF FMT SHOULD BE IMPROVED
The material of FMT is variable, which is caused by biological variation and sample handling. Unlike drugs, FMT products are more personalized due to different donors[11]. Human feces (microorganisms and chemical components) vary greatly from person to person. Even among healthy individuals, the diversity and abundance of microorganisms in each individual vary widely, and there is a strong niche specificity within and between individuals, and many diseases are linked to specific microorganisms and chemicals in feces[37]. Therefore, during the process of FMT, different donors and improper preparation of samples may lead to differences or variations in these microorganisms, affecting the therapeutic effect and even causing adverse events. In addition, the choice of treatment for FMT varies from region to region, resulting in low standardization of FMT[9].
However, there is no clear and universal screening standard for fecal donors. The reported screening methods are strict, and usually < 20% of volunteers meet the screening standards of blood, feces, and urine[38]. Therefore, it is obviously necessary to develop FMT substitutes for patients with microecological disorders, including bacteria, fungi, protozoa, viruses, cytokines, and metabolites, and it is necessary to determine which of them are beneficial factors, which will establish a link between the disease and beneficial microorganisms of therapeutic value[11]. In addition, the development of targeted probiotic microorganisms or combinations can be considered, and alternatives including bacteriophages should also be considered[12], which may be an important research direction.
Recent investigations of doctors, medical students, donors, and patients have shown that FMT is less acceptable compared with traditional treatment methods, especially fecal bacterial suspension prepared by the rough method[4,39,40]. Zhang et al[4] found that the bacteria prepared by standardized formula have greater acceptance with the same efficacy, while improving the activity of the flora, which meets the standards of modern medical aesthetics. Therefore, it is necessary to improve the standardized preparation technology of fecal suspension to improve the acceptability of FMT, and the screening of effective bacteria or metabolites should be considered to improve the standardization and acceptability. In 2019, China’s first national consensus opinion on the methodology for WMT was confirmed and published, aiming to regulate the processes such as donor screening, fecal microbiota preparation, storage and transportation, selection of transplantation route, and safety monitoring and management of FMT, so as to improve the safety of FMT and ensure its clinical efficacy[35].
Therefore, screening of the functional strain is prepared under the condition of clarifying the mechanism of action of the FMT effective strain. At the same time, FMT operation procedures are improved to reach a consensus, which makes the FMT strain preparation easier to control the production, safer, more acceptable, and more standardized.
PRECLINICAL SAFETY, EFFECTIVENESS EVALUATION, AND EFFECTIVE MICROBIAL SCREENING OF FMT BASED ON GERM-FREE ANIMAL SYSTEMS
Germ-free animals refer to animals without microorganisms living in or on them. These animals have become an irreplaceable research tool to study the relationship between a single strain or multiple strains and the host, and also an indispensable model for studying functional microorganisms in humans and animals[41,42]. Germ-free animal disease models can be used to evaluate the effectiveness of FMT, to study the causal relationship between microbiota transplantation and therapeutic effect, and clarify the mechanism of action between FMT and disease in the absence of host microbial interference. In addition, germ-free animals can be evaluated for FMT safety and provide preclinical scientific data for FMT by evaluating cardiac, liver, and renal toxicity, immune disturbance, impact on behavior, and the risk of local and translocation infection. The use of germ-free immunodeficient animals can correspond to patients with low clinical immune function, and the humanized flora can be transplanted to germ-free immunodeficient animals for FMT safety test, to enhance the sensitivity of FMT activity and ensure the preclinical safety of FMT to treat immunodeficient individuals. For example, two immunocompromised patients developed ESBL-producing E. coli infection after FMT and one of them died[26]. However, safety assessment with germ-free immunodeficient animals in advance may avoid this adverse event.
Papanicolas et al[31] reviewed that the protection of symbiotic bacteria and the reduction of pathogenic bacteria in feces could improve the safety of FMT, and the change in fecal flora might reduce the risk of sepsis. Technology based on germ-free animals and multiomics provides the possibility to modify the fecal flora. Based on the clear causal relationship between FMT and germ-free animal models of human diseases, a function-oriented system for screening and evaluating transplanted flora could be established. By using metagenomics and cultureomics, effective target bacteria of FMT are obtained, and the formula flora with a clear function can be formed, so that the functional bacteria of FMT can be visible, accessible, and available.
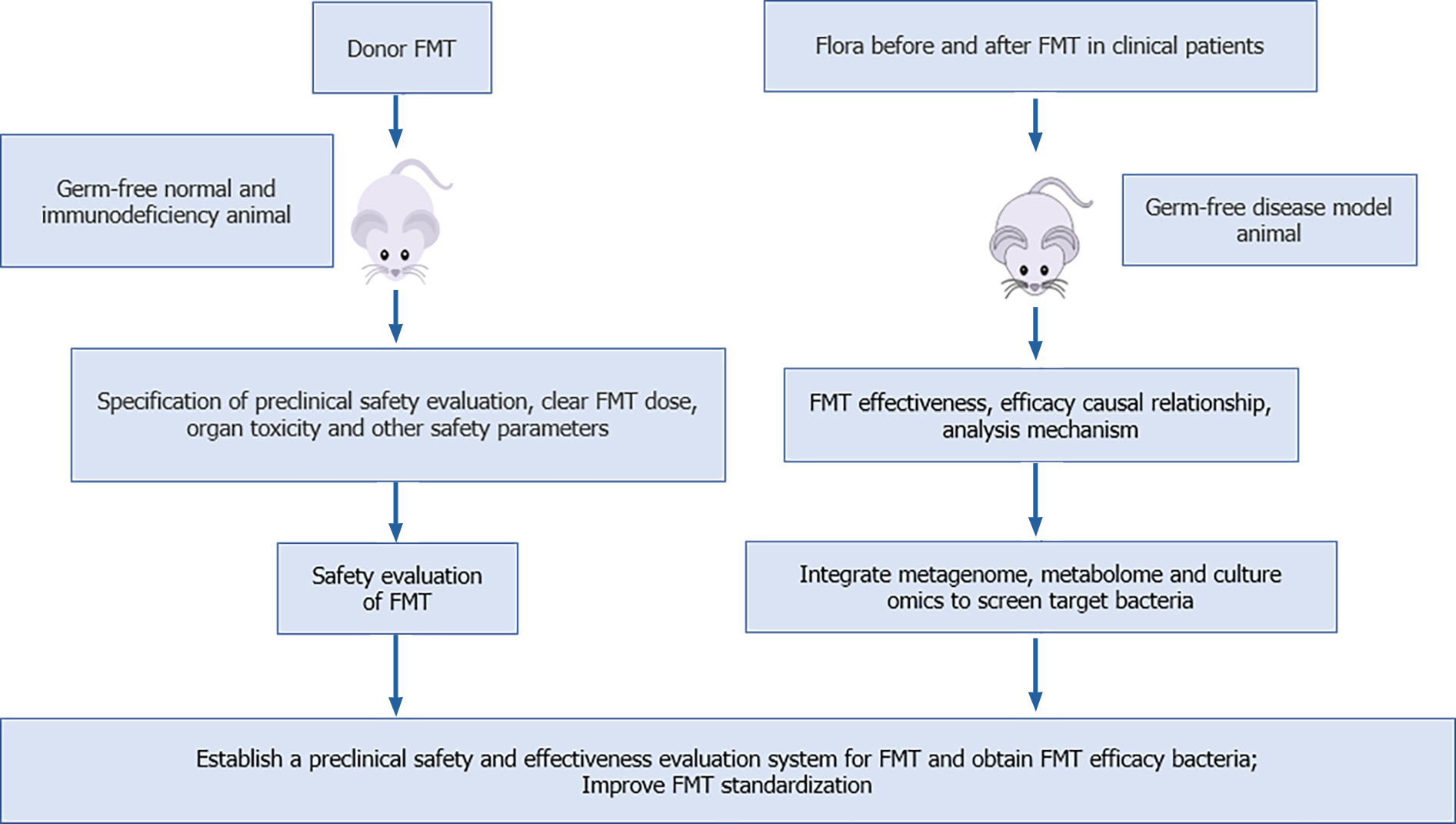
The use of germ-free animals to standardize the preclinical safety evaluation of FMT, establish an effective evaluation system that can clarify the causal relationship and mechanism of the efficacy of FMT, and combine multiomics technology to screen the effective bacteria can improve the safety, standardization, controllability, and acceptance of FMT. Therefore, we propose a safety and effectiveness research system of FMT based on germ-free animals, and the screening of FMT efficient bacteria (Figure 1): (1) The causal relationship between therapeutic effect and flora transplantation is validated. For patients who have been effectively treated with FMT, the flora before and after FMT was transplanted into the corresponding germ-free disease model mice to verify the causal mechanism of FMT efficacy. We can use the standard germ-free disease model for FMT effectiveness evaluation, to ensure the scientific validity and versatility of FMT effectiveness data, clarify the causal relationship between flora transplantation and therapeutic effect, and clarify the mechanism of FMT; (2) The humanized flora model is constructed based on germ-free animals to evaluate the safety of FMT. The preclinical safety evaluation of FMT is conducted in strict accordance with the international classics and standard preclinical safety evaluation specifications of new drugs, medical devices, and medical technology. In addition, germ-free immunodeficient animals were used to evaluate the safety of FMT; cardiac, liver, and renal toxicity of FMT; interference with the immune system; behavioral effects of FMT; and the risk of local and translocation infections. Germ-free immunodeficient animals are also used to enhance the sensitivity of FMT, and better identify the route and dose of FMT, and other indicators to ensure its safety; and (3) At present, there are insufficient studies to obtain the effective flora of FMT. Therefore, after clarifying the causal relationship of FMT in step one, we can combine with metagenomics and cultureomics techniques to establish a function-oriented system for screening, obtaining, and evaluating flora, and finally, the effective target bacteria of FMT can be obtained and functional formula flora can be formed. The efficacy of the formulated bacteria can be verified again by using germ-free animal models of human diseases, so as to achieve standardization of FMT, improve the acceptance level, and reduce infection risk.
Figure 1 Preclinical safety, effectiveness evaluation, and effective bacterial screening of fecal microbiota transplantation based on germ-free animals.
An FMT safety evaluation system is established based on germ-free normal and immunodeficient animals. The causal mechanism for the efficacy of fecal microbiota transplantation (FMT) is verified based on germ-free disease animal models, to clarify the effectiveness of FMT, and multiomics technology is used to screen the effective bacteria of FMT, and to improve the standardization of FMT. FMT: Fecal microbiota transplantation.
CONCLUSION
Gut microbiota plays a major role in the development and treatment of many diseases, especially gastrointestinal disorders. FMT is a therapeutic option that can rapidly reconstruct the normal composition of intestinal microorganisms. FMT has potential to treat various diseases, such as CDI, IBD, constipation, and cancer. However, the therapeutic mechanism of FMT is unclear, its safety needs to be improved, and it is not standardized due to a variety of reasons. Germ-free animals, as the most appropriate models for studying the interaction between microorganisms and hosts, are applicable research tools for studying the mechanism of action of FMT, screening the functional bacteria of FMT, evaluating the safety of FMT, and improving standardization of FMT. Therefore, evaluation of the safety and effectiveness of FMT animal experiments based on germ-free animals will be set up. By using a function-oriented in vivo experimental system, combined with clinical analysis and culturomics technology, the functional strains of FMT for microbial diseases can be obtained and verified. And the standard, safe, effective, controllable, and product-oriented flora of FMT can be developed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Microbiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Leardini D, Link A S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu M