Published online Oct 28, 2020. doi: 10.13105/wjma.v8.i5.411
Peer-review started: September 18, 2020
First decision: September 29, 2020
Revised: October 7, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: October 28, 2020
Processing time: 40 Days and 22.7 Hours
There are some studies investigating the relationship between antithrombotic medication and postoperative bleeding after endoscopic resection (ER) with controversial results.
To perform a meta-analysis evaluating the effects of antithrombotic therapy on postoperative bleeding after ER.
A systematic search was conducted on PubMed, Web of Science, Cochrane Library. The Newcastle-Ottawa scale was used to evaluate the quality of studies. Stata 12.0 was used for statistical analysis. The odds ratio (OR) and 95%CI were calculated and heterogeneity was quantified using Cochran’s Q test and I2.
Total 66 studies were included in the meta-analysis. Pooled data suggested that antithrombotic therapy was significantly associated with postoperative bleeding (OR = 2.302, 95%CI: 2.057-2.577, P = 0.000) after ER. The risk of postoperative bleeding after endoscopic submucosal dissection, endoscopic mucosal resection and polypectomy in the antithrombotic group was higher than the non-antithrombotic group (OR = 2.439, 95%CI: 1.916-3.105; OR = 2.688, 95%CI: 1.098-6.582; OR = 2.112, 95%CI: 1.434-3.112).
The risk of postoperative bleeding after ER correlated with the types and management of antithrombotic agents by our meta-analysis.
Core Tip: In recent years, more and more people suffering from cardiovascular disease and/or cerebrovascular disease receive antithrombotic therapy which change patients’ coagulation status and may lead to high risk of postoperative bleeding after endoscopic resection (ER). The relationship between the postoperative bleeding after ER and antithrombotic agents is still uncertain. With this reason, a systematic review and meta-analysis was carried out to identify whether the use of antithrombotic drugs increases the risk of the postoperative bleeding after ER.
- Citation: Xiang BJ, Huang YH, Jiang M, Dai C. Effects of antithrombotic agents on post-operative bleeding after endoscopic resection of gastrointestinal neoplasms and polyps: A systematic review and meta-analysis. World J Meta-Anal 2020; 8(5): 411-434
- URL: https://www.wjgnet.com/2308-3840/full/v8/i5/411.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i5.411
Endoscopic resection (ER) is deemed as an effective method for gastrointestinal neoplasia and polyp. ER is an acceptable technique to enable en bloc resection of gastric adenomas, early oesophageal, gastric and colorectal cancer and incidence and its related mortality of colorectal cancer[1-3]. This includes polypectomy, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). For example, patients with oesophageal neoplasia receiving ER can maintain the integrity of oesophageal structure and function, whereas the quality of life can be affected by oesophagectomy[4].
Although the therapeutic effect of ER has been greatly affirmed, Postoperative bleeding as a major complication is still a problem to be solved. Postoperative bleeding after ER is defined as bleeding within 30 d from a mucosal defect shown by massive melena, a decrease in blood hemoglobin level of more than 2 g/dL, or requirement of endoscopic hemostasis or transfusion[1,5,6]. A study has shown that the incidence rate of postoperative bleeding after esophageal or colorectal ESD ranged from 0.0% to 4.6%[7]. And the incidence rate of postoperative bleeding after ESD due to gastric neoplasm ranged from 1.8% to 15.6%[7]. A study that included 3788 cases of poly-pectomy by Choung found that postoperative bleeding occurred in 42 cases (1.1%)[8]. Another study with 30881 cases of polypectomy by Rutter also reported that the postoperative bleeding developed in 291 cases (0.94%)[9]. Preventive strategies such as acid secretion inhibitors and prophylactic clipping have been developed to reduce the postoperative bleeding risk after ER, but postoperative bleeding cannot be completely avoided. Some factors such as the size of polyp and a patient’s coagulation status have been reported to be associated with the risk of postoperative bleeding after ER.
In recent years, more and more people suffering from cardiovascular disease and/or cerebrovascular disease receive antithrombotic therapy which change patients’ coagulation status and may lead to high risk of postoperative bleeding after ER. The relationship between the postoperative bleeding after ER and antithrombotic agents is still uncertain. With this reason, a systematic review and meta-analysis was carried out to identify whether the use of antithrombotic drugs increases the risk of the postoperative bleeding after ER.
We carried out a systematic review and meta-analysis of the hemorrhagic data of different antithrombotic users after ER from published studies. The review and analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines[10].
We used PubMed, Web of Science and Cochrane Library to search for articles published in English from inception to February 2019. The search queries were: (1) ( ( (antithrombotic OR anticoagulant OR antiplatelet OR heparin OR warfarin OR aspirin)) AND (endoscopic submucosal dissection OR ESD)) AND (bleeding OR hemorrhage); (2) ( ( (antithrombotic OR anticoagulant OR antiplatelet OR heparin OR warfarin OR aspirin)) AND (EMR OR endoscopic mucosal resection)) AND (bleeding OR hemorrhage); (3) ( ( (antithrombotic OR anticoagulant OR antiplatelet OR heparin OR warfarin OR aspirin)) AND (endoscopic polypectomy)) AND (bleeding OR hemorrhage); and (4) ( ( (antithrombotic OR anticoagulant OR antiplatelet OR heparin OR warfarin OR aspirin)) AND (APC OR argon plasma coagulation)) AND (bleeding OR hemorrhage).
The studies that met the following inclusion criteria were included: (1) Polypectomy, EMR, ESD, polypectomy incorporated argon plasma coagulation and the hot and cold snare; (2) Randomized controlled trials, retrospective studies or cohort studies were performed to investigate the risk of postoperative bleeding after ER in patients with gastrointestinal neoplasm receiving antithrombotic medication; (3) The incidence rate of postoperative bleeding can be extracted in the antithrombotic medication group and the non-antithrombotic medication group; and (4) Anticoagulants and antiplatelet drugs were incorporated in antithrombotic agents.
The studies were excluded if: (1) The postoperative bleeding rate or antithrombotic therapy information could not be extracted; (2) Antithrombotic drugs and NSAIDS were recorded together; (3) Endoscopic treatment such as biopsy, sphincterotomy or ampullectomy was carried out; (4) Reviews, case reports, guidelines, or animal studies were screened out; (5) The articles were not written in English; and (6) The full text could not be obtained.
The Newcastle-Ottawa scale was used to evaluate the quality of the included studies. And the Newcastle-Ottawa scale includes three aspects: Selection, comparability, exposure (retrospective studies) or outcome (cohort studies)[11].
Two authors worked together to extract the basic information about the first author, publication year, country, research method (retrospective/cohort), ER method (ESD/EMR/polypectomy), number, age and gender. Moreover, the odds ratio (OR) and 95%CI of the postoperative bleeding rate were calculated in the antithrombotic group (continued/discontinued) and the non-antithrombotic group.
Statistical analysis was performed by Stata 12.0. The Cochran’s Q test and I2 (P < 0.10 was considered significant) were used to identify heterogeneity. The value I2 of 0-25% indicated insignificant heterogeneity; 26%-50%, low heterogeneity; 51%-75%, moderate heterogeneity; and greater than 75%, high heterogeneity[12]. If there was no significant heterogeneity, the OR and 95%CI were calculated in a fixed-effect model. Otherwise, a random-effect model was used. The funnel plot was used to assess publication bias.
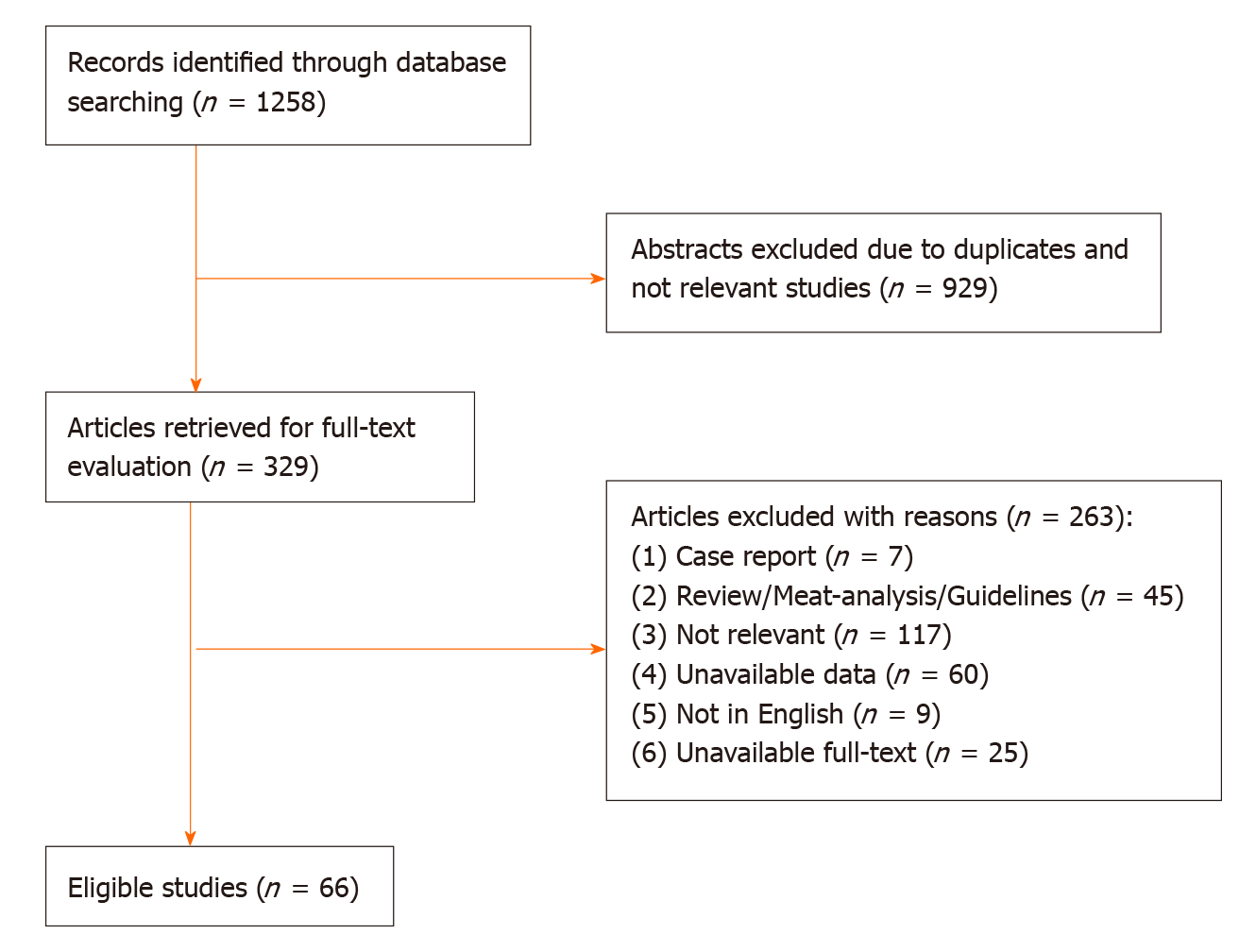
The initial literature yielded 1258 articles (454 articles from PubMed, 679 articles from Web of Science, 125 articles from Cochrane Library). After the exclusion of 929 articles due to duplicates and lack of relevance, 329 articles were retrieved for full text evaluation. 263 articles were excluded after reviewing the full text (Figure 1). Ultimately, 66 studies were included in the meta-analysis (Fifty-nine retrospective studies, seven prospective observational studies). The characteristics of included studies were described in the Table 1. The included studies were carried out from different countries (Fifty from Japan, six from Korean, five from USA, two from Italy, one from UK, one from Australia, one from Holland). The mean age was older than 60 years old in most studies.
| Ref. | Country | Research method | Location | Age (yr) | Gender male, % |
| So et al[49], 2019 | South Korea | Retrospective study | Gastric lesion | 68.8/68.5 | 954, 79.7% |
| Kishida et al[45], 2019 | Japan | Retrospective study | Colorectal lesion | 64/68 | 55, 41.66% |
| Inoue et al[65], 2019 | Japan | Prospective observational study | Gastrointestinal lesion | 67.4 ± 8.3 | 201, 58.6% |
| Harada et al[56], 2019 | Japan | Retrospective study | Gastric lesion | 72.3 ± 8.82 | 414, 69.3% |
| Arimoto et al[54], 2018 | Japan | Retrospective study | Colorectal lesion | 68.5 | 492, 58.3% |
| Azumi et al[39], 2018 | Japan | Retrospective study | Gastric lesion | 73 (41-94) | 284, 64.8% |
| Fujita et al[67], 2018 | Japan | Retrospective study | Colorectal lesion | 72.2 ± 7.4/72.9 ± 8.3 | 63, 73.8% |
| Horikawa et al[58], 2018 | Japan | Retrospective study | Gastric lesion | 78 (56-89) | 77, 77% |
| Izumikawa et al[40], 2018 | Japan | Retrospective study | Gastric lesion | - | 255, 75.25% |
| Kono et al[41], 2018 | Japan | Retrospective study | Gastric lesion | 72 (66-78) | 652, 74.77% |
| Oh et al[60], 2018 | South Korea | Retrospective study | Gastric lesion | 70 (49-85) | 173, 80.47% |
| Park et al[63], 2018 | South Korea | Prospective observationalstudy | Colorectal lesion | 55.8 ± 11.9/52.4 ± 12.3 | 2661, 68.46% |
| Sanomura et al[59], 2018 | Japan | Retrospective study | Gastric lesion | 69.8 ± 9.2 | 719, 70% |
| Seo et al[55], 2018 | South Korea | Retrospective study | Colorectal lesion | 63 (55-69.5) | 723, 60.8% |
| Sakai et al[64], 2018 | Japan | Retrospective study | Colorectal lesion n | 72.6 ± 7.2/69.1 ± 10.9 | 669, 66.63% |
| Yamashita et al[36], 2018 | Japan | Retrospective study | Colorectal lesion | 66.6 ± 10.6 | 373, 57.4% |
| Yanagisawa et al[35], 2018 | Japan | Retrospective study | Gastrointestinal lesion | - | 314, 72.02% |
| Matsumoto et al[46], 2018 | Japan | Retrospective study | Colorectal lesion | 70/65 | 551, 65.44% |
| Harada et al[61], 2017 | Japan | Prospective observational study | Gastric lesion | 76.8 ± 6.0/72.7 ± 7.9 | 40, 88.88% |
| Yano et al[33], 2017 | Japan | Retrospective study | Gastric lesion | 72 (33-94) | 1319, 74.65% |
| Ueki et al[14], 2017 | Japan | Retrospective cohort study | Gastric lesion | 71.2 ± 8.4 | 264, 72.5% |
| Yoshio et al[78], 2017 | Japan | Retrospective study | Gastric lesion | 75/76 | 90, 90.91% |
| Gotoda et al[15], 2017 | Japan | Retrospective study | Gastric lesion | 75, 68.8-81.0 | 410, 77.5% |
| Furuhata et al[17], 2017 | Japan | Retrospective study | Gastric lesion | 69 | 1377, 77.3% |
| Shibuya et al[1], 2017 | Japan | Retrospective study | Colonic lesion | - | Unclear |
| Bronsgeest et al[42], 2017 | Holland | Retrospective study | Colorectal lesion | 67.4 ± 8.3 | 201, 58.6% |
| Ishigami et al[34], 2017 | Japan | Retrospective study | Lower gastrointestinal lesion | 64.9 ± 11.1 | 526, 68% |
| Pigò et al[3], 2017 | Italy | Retrospective study | Colorectal lesion | 65.4 | 385, 63.2% |
| Kono et al[76], 2017 | Japan | Prospective observationalstudy | Upper gastrointestinal lesion | 74 ± 8.3 | 44, 89.8% |
| Lin et al[75], 2017 | United States | Retrospective study | Colorectal lesion | - | Unclear |
| Sato et al[38], 2017 | Japan | Retrospective study | Gastric lesion | 71.1 | 1786, 75.1% |
| Igarashi et al[27], 2017 | Japan | Retrospective study | Gastric lesion | 72.4 | 758, 77.7% |
| Amato et al[31], 2016 | Italy | Prospective observational study | Gastrointestinal lesion | 59 ± 12.1 | 54.3% |
| Kubo et al[32], 2016 | Japan | Retrospective study | Gastrointestinal lesion | 63.9 | 467,59.3% |
| Shindo et al[25], 2016 | Japan | Retrospective study | Gastric lesion | 71 ± 8, 32-87 | 190, 72.5% |
| Yoshida et al[52], 2016 | Japan | Retrospective study | Colorectal lesion | 68.2 ± 10.3 | Unclear |
| Ninomiya et al[53], 2015 | Japan | Retrospective study | Colorectal lesion | 67 ± 11.1 | 410, 70.4% |
| Al-Mammari et al[4], 2015 | United Kingdom | Prospective observational study | Oesophageal lesion | 71, 65-78 | 85, 72.6% |
| Odagiri et al[16], 2015 | Japan | Retrospective cohort study | Colorectal lesion | - | 4495, 59.4% |
| Namasivayam et al[5], 2014 | United States | Retrospective study | Gastrointestinal lesion | 69 | Unclear |
| Terasaki et al[21], 2014 | Japan | Retrospective study | Colorectal lesion | 66.9 ± 11.2 | 233, 64.2% |
| Tounou et al[50], 2014 | Japan | Retrospective study | Gastric lesion | 71.8, 36-92 | 257, 73.4% |
| Suzuki et al[18], 2014 | Japan | Retrospective study | Colorectal lesion | 65.5, 29-86 | 183, 57.7% |
| Matsumura et al[23], 2014 | Japan | Retrospective study | Gastric lesion | 72.1 ± 8.6 | 302, 71.1% |
| Beppu et al[74], 2014 | Japan | Retrospective study | Colorectal lesion | 59.5 ± 11.6 | 176, 84.6% |
| Inoue et al[77], 2014 | Japan | Retrospective study | Colorectal lesion | 69.2 | 95, 81.2% |
| Sanomura et al[66], 2014 | Japan | Retrospective study | Gastric lesion | 73.7 ± 8.9 | 64, 82.1% |
| Yoshio et al[47], 2013 | Japan | Retrospective study | Gastric lesion | 70 | 951, 76.1% |
| Takeuchi et al[29], 2013 | Japan | Retrospective study | Gastric lesion | 5.2 | 477, 57.2% |
| Koh et al[37], 2013 | Japan | Retrospective study | Gastric lesion | 70.3 ± 8.6 | 817, 74% |
| Mukai et al[6], 2012 | Japan | Retrospective study | Gastric lesion | 72.4 ± 8.8 | 116, 72% |
| Lim et al[51], 2012 | South Korea | Retrospective study | Gastric lesion | 62.6 | 1143, 71.8% |
| Miyahara et al[48], 2012 | Japan | Retrospective study | Gastric lesion | 71.7 ± 8.9, 36-92 | 763, 70.5% |
| Cho et al[57], 2012 | South Korea | Retrospective study | Colorectal lesion | 62.2 | 385, 74.9% |
| Toyokawa T et al[24], 2011 | Japan | Retrospective study | Gastric lesion | 26-95 | 811, 72.2% |
| Higashiyama et al[19], 2011 | Japan | Retrospective study | Gastric lesion | 69, 29-91 | 702, 76% |
| Metz et al[2], 2011 | Australia | Prospective observational study | Colonic lesion | 68, 26-93 | Unclear |
| Tokioka et al[30], 2011 | Japan | Retrospective study | Gastric lesion | 69.4 | 378, 73.4% |
| Okada K et al[22], 2011 | Japan | Retrospective study | Gastric lesion | 68.4, 33-94 | 425, 73% |
| Mannen et al[20], 2010 | Japan | Retrospective study | Gastric lesion | 71.6 ± 8.6, 36-91 | 323, 74.1% |
| Goto et al[13], 2010 | Japan | Retrospective study | Gastric lesion | 68.3 | 347, 76.4% |
| Witt et al[44], 2009 | United States | Retrospective cohort study | Colorectal lesion | 69.6 | 691, 56.4% |
| Ono et al[28], 2019 | Japan | Retrospective study | Gastric lesion | 67 | Unclear |
| Takizawa et al[26], 2008 | Japan | Retrospective study | Gastric lesion | 66 ± 10, 29-93 | 779, 80.5% |
| Sawhney et al[62], 2007 | United States | Retrospective study | Colorectal lesion | 65.1 | 169, 97.7% |
| Yousfi et al[43], 2004 | United States | Retrospective study | Gastrointestinal lesion | 70.5, 45-91 | 100, 61.7% |
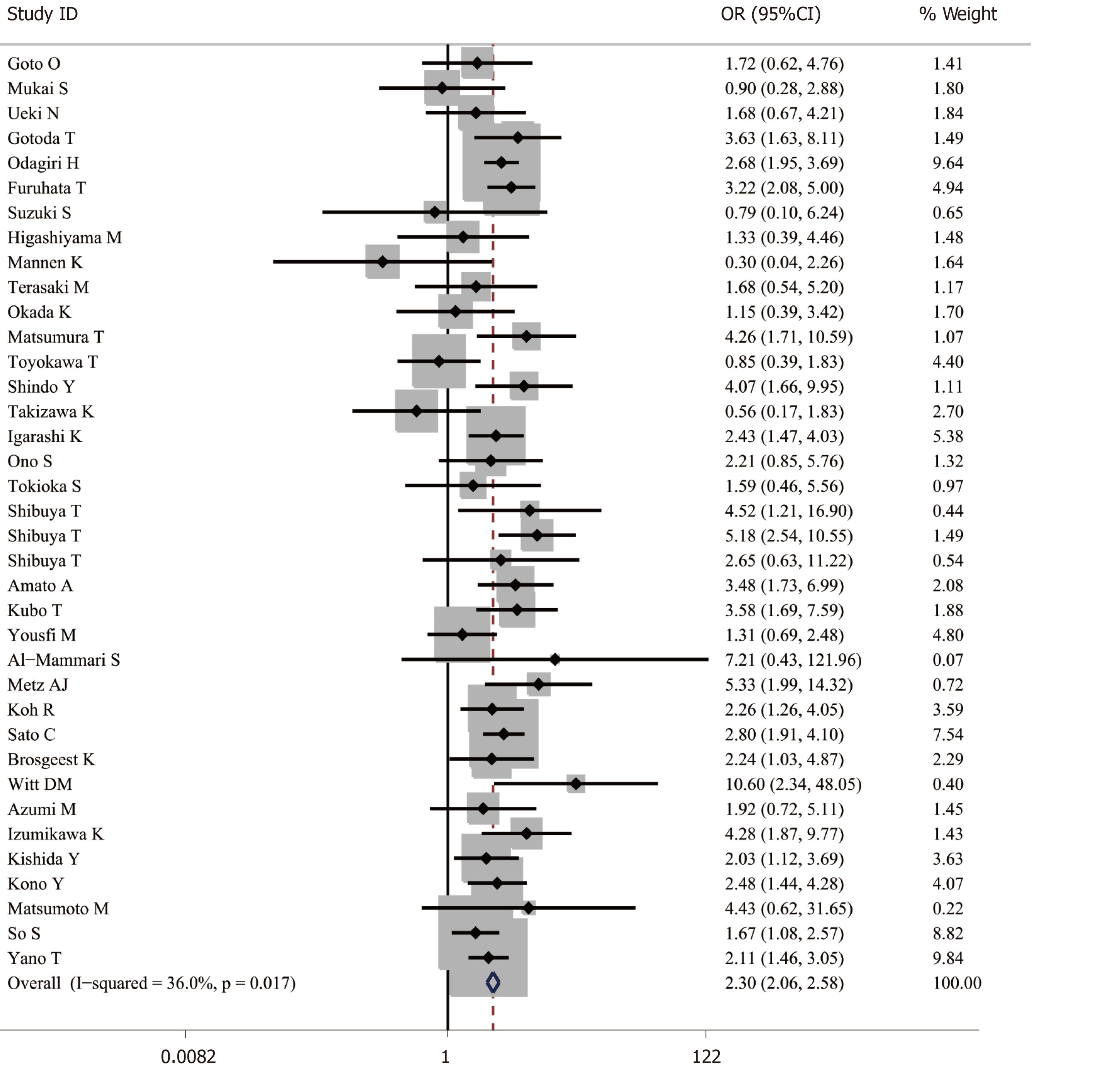
A total of 48691 cases after ER were enrolled, of which 8918 cases were receiving antithrombotic medication and 39773 cases were not taking any antithrombotic drugs[1,2,4,6,13-33]. The average postoperative bleeding rate in the antithrombotic group was 8.44%, while it was 5.28% in the non-antithrombotic group. With the random-effects model, the risk of postoperative bleeding in the antithrombotic group was higher than the non-antithrombotic group (OR = 2.421, 95%CI: 1.831-3.200, P = 0.000, I2 = 82.5%). In addition, a more homogeneous analysis (I2 = 36.0%) was carried out after six articles[3,5,29,34-36] were screened out in the sensitivity analysis and the results remained unchanged (OR = 2.302, 95%CI: 2.057-2.577, P = 0.000) (Figure 2). Besides this, the results were not changed when data from retrospective and prospective studies were separately analyzed.
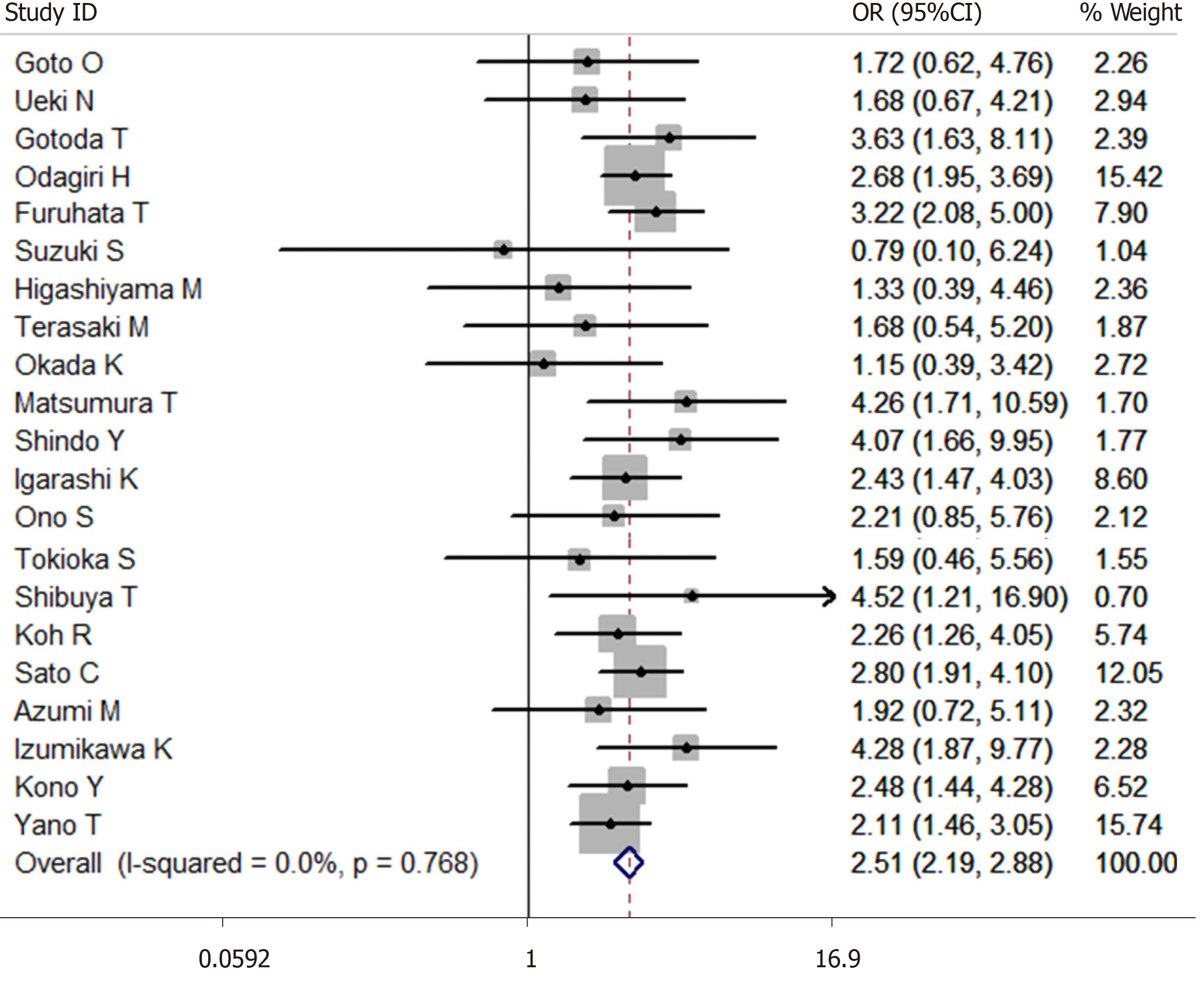
A total of 27014 cases after ESD were enrolled in this meta-analysis (3624 cases were receiving antithrombotic medication and 23390 cases were not taking antithrombotic drugs[1,6,13-30,33,37-41]). The average postoperative bleeding rate after ESD in the antithrombotic group was 13.91%, while it was 7.77% in the non-antithrombotic group. With the random-effects model, the risk of postoperative bleeding after ESD in the antithrombotic group was higher than the non-antithrombotic group (OR = 2.439, 95%CI: 1.916-3.105, P = 0.000, I2 = 63.5%). Moreover, a more homogeneous analysis (I2 = 0.0%) was carried out after six articles[6,20,24,26,29,36] were screened out in the sensitivity analysis and the results remained unchanged (OR = 2.507, 95%CI: 2.185-2.875, P = 0.000, Figure 3). The risk of postoperative bleeding after gastric ESD in the antithrombotic group was higher than the non-antithrombotic group (OR = 2.295, 95%CI: 1.757-2.998, P = 0.000, I2 = 64.1%)[6,13-15,17,19,20,22-29,33]. Meanwhile, the risk of postoperative bleeding after colorectal ESD in the antithrombotic group was higher than the non-antithrombotic group (OR = 3.305, 95%CI: 1.561-6.998, P = 0.002, I2 = 65.0%)[1,15,18,21,36].
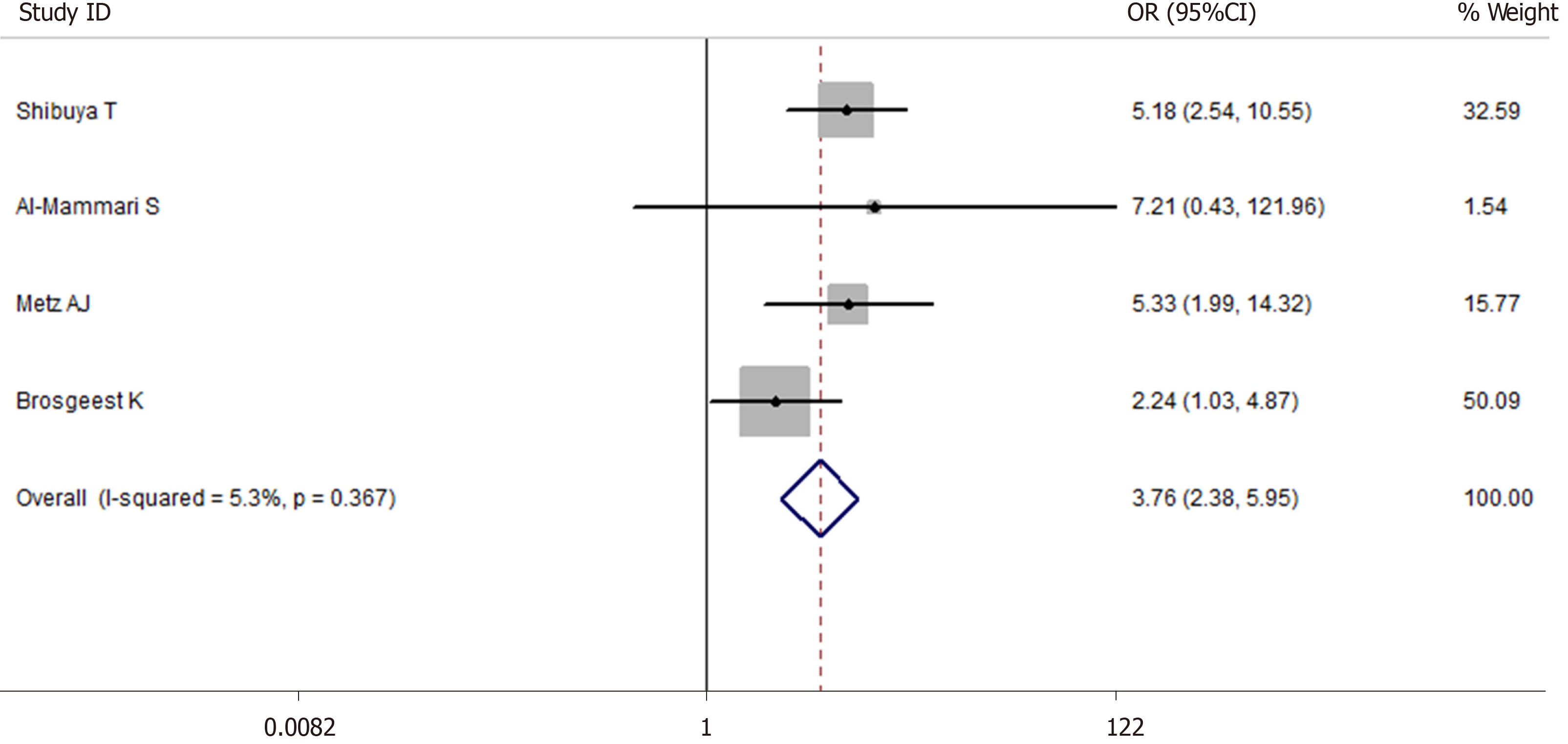
A total of 5514 cases after EMR were enrolled in this meta-analysis (1475 cases were receiving antithrombotic medication and 4039 cases
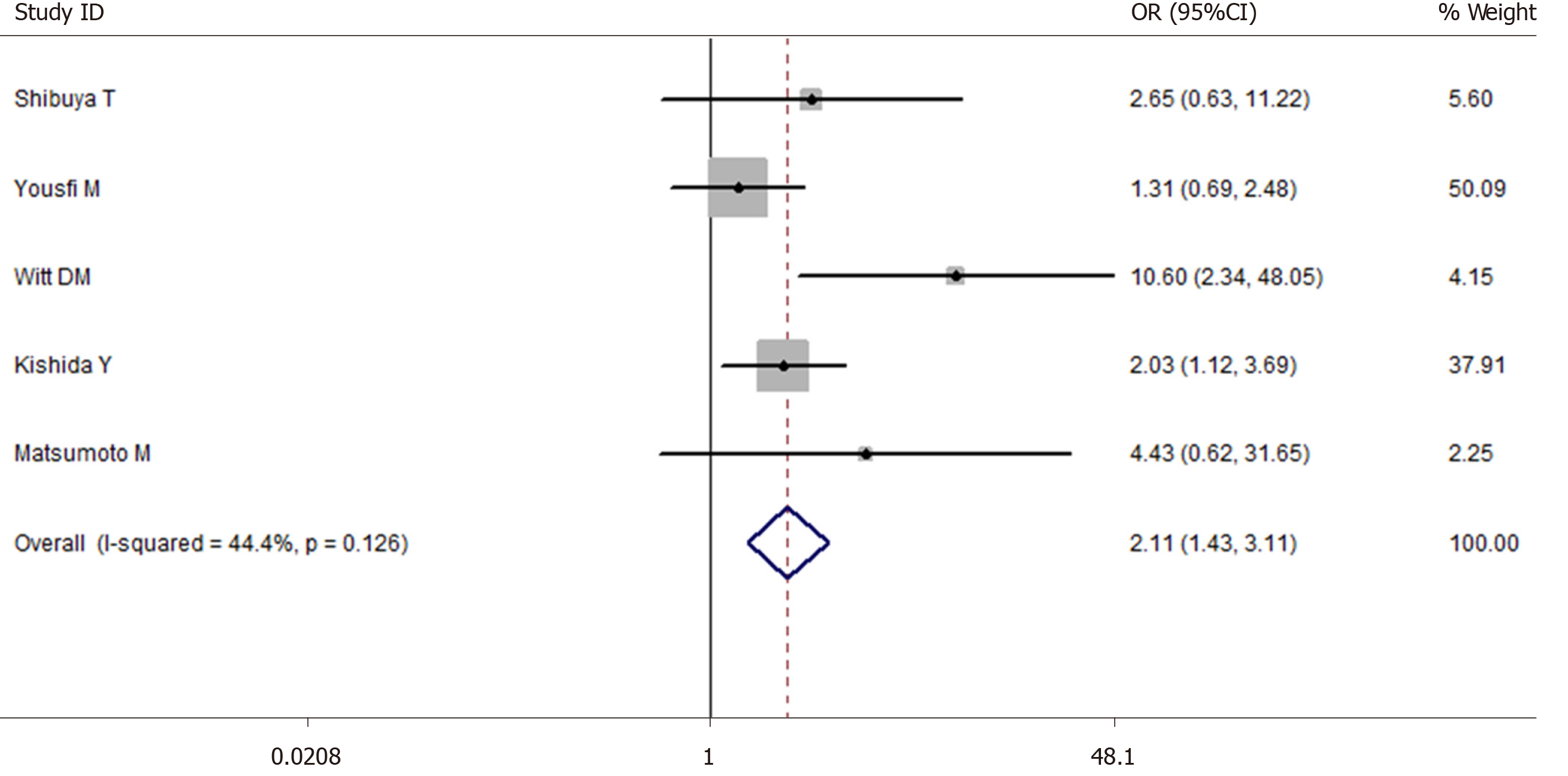
A total of 10709 cases of polypectomy were enrolled in this meta-analysis (2554 cases were receiving antithrombotic medication and 8155 cases were not taking any antithrombotic drugs[1,3,35,43-46]). The average postoperative bleeding rate in the antithrombotic group was 4.89%, while it was 1.69% in the non-antithrombotic group. With the random-effects model, there was no significant difference (OR = 2.338, 95%CI: 0.610-8.954, P = 0.215, I2 = 93.6%) in the postoperative bleeding rate between the two groups. Another more homogeneous analysis (I2 = 44.4%) was carried out after two articles[3,35] were screened out in the sensitivity analysis and the results were found to have changed (OR = 2.112, 95%CI: 1.434-3.112, P = 0.006, Figure 5). The risk of postoperative bleeding after colorectal polypectomy in the antithrombotic group was higher than the non-antithrombotic group (OR = 2.921, 95%CI: 1.821-4.687, P = 0.000, I2 = 31.9%). Table 2 shows the number of cases with or without antithrombotic agents and hemorrhagic outcome.
| Ref. | Resection method | Total | Drug | Post-bleeding | No bleeding |
| So et al[48], 2019 | ER | 1197 | Antithrombotic agent (+/-) | 40/50 | 359/748 |
| Continued antithrombotic agent (+/-) | 11/7 | 69/138 | |||
| Discontinued antithrombotic agent (+/-) | 29/43 | 330/657 | |||
| HR (+) | 5 | 9 | |||
| Kishida et al[45],2019 | Polypectomy | 6382 | Antithrombotic agent (+/-) | 15/40 | 986/5341 |
| Single APT (+) | 4 | 683 | |||
| Single anticoagulants (+) | 2 | 85 | |||
| Multiple APT (+) | 3 | 163 | |||
| Multiple antithrombotic agents (+) | 2 | 39 | |||
| Single antithrombotic agent (+) | 13 | 947 | |||
| HR (+) | 4 | 16 | |||
| Inoue et al[65], 2019 | EMR | 102 | VKA (+) | 12 | 73 |
| Discontinued VKA (+) | 0 | 4 | |||
| Continued VKA (+) | 0 | 2 | |||
| HR (+) | 15 | 98 | |||
| DOAC (+) | 3 | 14 | |||
| Discontinued DOAC (+) | 0 | 3 | |||
| Inoue et al[65], 2019 | ESD | 54 | VKA (+) | 14 | 31 |
| Discontinued VKA (+) | 1 | 2 | |||
| Continued VKA (+) | 0 | 1 | |||
| HR (+) | 13 | 31 | |||
| DOAC (+) | 2 | 7 | |||
| Discontinued DOAC (+) | 2 | 4 | |||
| Harada et al[56], 2019 | ESD | 597 | Antithrombotic agent (-) | 21 | 422 |
| Single-LDA (+) | 10 | 85 | |||
| DAPT (+) | 10 | 49 | |||
| Continued LDA (+) | 15 | 80 | |||
| Discontinued APT (+) | 5 | 54 | |||
| Arimoto et al[54], 2018 | ESD | 919 | Antithrombotic agent (-) | 26 | 757 |
| APT (+) | 5 | 131 | |||
| Discontinued APT (+) | 5 | 105 | |||
| Continued APT (+) | 0 | 26 | |||
| Azumi et al[39], 2018 | ESD | 438 | Antithrombotic agent (+/-) | 6/15 | 72/345 |
| Fujita et al[67], 2018 | EMR | 84 | Discontinued anticoagulants (+) | 1 | 42 |
| HR (+) | 4 | 37 | |||
| Horikawa et al[58], 2018 | ESD | 100 | Antithrombotic agent (-) | 1 | 49 |
| Continued LDA | 1 | 49 | |||
| Izumikawa et al[40], 2018 | ESD | 273 | Antithrombotic agent (+/-) | 15/11 | 66/207 |
| Kono et al[41], 2018 | ESD | 872 | Antithrombotic agent (+/-) | 23/38 | 159/652 |
| Single antithrombotic agent | 12 | 130 | |||
| Multiple antithrombotic agents (+) | 11 | 29 | |||
| Discontinued antithrombotic agent (+) | 8 | 120 | |||
| Discontinued | |||||
| Single APT (+) | 3 | 88 | |||
| Multiple APT (+) | 3 | 16 | |||
| Single anticoagulants (+) | 1 | 13 | |||
| Continued | |||||
| Single APT (+) | 1 | 16 | |||
| Multiple APT (+) | 4 | 2 | |||
| Single anticoagulants (+) | 7 | 13 | |||
| HR (+) | 10 | 21 | |||
| Oh et al[60], 2018 | ESD | 215 | Single APT (+) | 14 | 147 |
| Multiple APT (+) | 15 | 39 | |||
| LDA (+) | 12 | 82 | |||
| Thienopyridine (+) | 2 | 54 | |||
| Continued APT (+) | 23 | 130 | |||
| Discontinued APT (+) | 6 | 56 | |||
| Park et al[63], 2018 | Polypectomy | 3887 | APT (+) | 12 | 339 |
| Anticoagulants (+) | 0 | 15 | |||
| Sanomura et al[58], 2018 | ESD | 1243 | Antithrombotic agent (-) | 40 | 1127 |
| Anticoagulants (+) | 11 | 65 | |||
| Warfarin (+) | 5 | 32 | |||
| DOAC (+) | 4 | 14 | |||
| Seo et al[55], 2018 | ESD | 1189 | Antithrombotic agent (-) | 26 | 945 |
| APT (+) | 7 | 175 | |||
| Aspirin (+) | 2 | 139 | |||
| Warfarin (+) | 0 | 10 | |||
| DOAC (+) | 1 | 2 | |||
| Single antithrombotic agent (+) | 10 | 326 | |||
| Multiple antithrombotic agents (+) | 0 | 23 | |||
| Discontinued antithrombotic agent (+) | 7 | 206 | |||
| Continued antithrombotic agent (+) | 0 | 5 | |||
| Sakai et al[64], 2018 | Polypectomy | 1004 | Discontinued anticoagulants (+) | 12 | 0 |
| HR (+) | 8 | 70 | |||
| Warfarin (+) | 7 | 55 | |||
| DOAC (+) | 1 | 15 | |||
| Yamashita et al[36], 2018 | ESD | 650 | Antithrombotic agent (+/-) | 7/18 | 21/652 |
| Warfarin (+) | 5 | 14 | |||
| DOAC | 2 | 7 | |||
| Yanagisawa et al[35], 2018 | Polypectomy | 436 | Antithrombotic agent (+/-) | 30/2 | 188/216 |
| Discontinued anticoagulants (+) | 0 | 23 | |||
| Continued anticoagulants (+) | 10 | 83 | |||
| HR (+) | 20 | 82 | |||
| Continued warfarin (+) | 2 | 41 | |||
| Continued DOAC (+) | 8 | 42 | |||
| Warfarin (+) | 20 | 125 | |||
| DOAC (+) | 10 | 63 | |||
| Matsumoto et al[46], 2018 | Polypectomy | 1003 | Antithrombotic agent (+/-) | 2/2 | 184/815 |
| Harada et al[61], 2017 | ESD | 45 | Continued warfarin (+) | 2 | 20 |
| HR | 5 | 18 | |||
| Yano et al[33], 2017 | ESD | 144 | Antithrombotic agent (+/-) | 47/103 | 287/1330 |
| Ueki et al[14], 2017 | ESD | 364 | Antithrombotic agent (+/-) | 7/17 | 67/273 |
| Discontinued antithrombotic agent (-) | 7 | 67 | |||
| Discontinued single APT (+) | 4 | 57 | |||
| Discontinued single anticoagulants (+/-) | 2 | 4 | |||
| Aspirin (+) | 4 | 43 | |||
| Thienopyrindine (+) | 0 | 7 | |||
| Yoshio et al[78], 2017 | ESD | 97 | Warfarin (+) | 18 | 55 |
| DOAC | 5 | 19 | |||
| Gotoda et al[15], 2017 | ESD | 529 | Antithrombotic agent (+/-) | 12/14 | 96/407 |
| APT (+) | 8 | 80 | |||
| Single antithrombotic agent (+) | 6 | 80 | |||
| Multiple antithrombotic agents (+) | 7 | 17 | |||
| Single APT (+) | 3 | 69 | |||
| Multiple APT (+) | 5 | 11 | |||
| Warfarin (+) | 3 | 11 | |||
| Aspirin (+) | 2 | 33 | |||
| Thienopyridine (+) | 0 | 10 | |||
| Furuhata et al[17], 2017 | ESD | 1781 | Antithrombotic agent (+/-) | 33/68 | 220/1460 |
| Single antithrombotic agent (+) | 11 | 139 | |||
| Multiple antithrombotic agents (+) | 6 | 30 | |||
| Continued single APT (+) | 1 | 14 | |||
| HR (+) | 15 | 37 | |||
| Shibuya et al[1], 2017 | ESD | 259 | Antithrombotic agent (+/-) | 4/6 | 32/217 |
| Shibuya et al[1], 2017 | EMR | 3018 | Antithrombotic agent (+/-) | 16/15 | 510/2477 |
| Shibuya et al[1], 2017 | Polypectomy | 892 | Antithrombotic agent (+/-) | 3/5 | 163/721 |
| Bronsgeest et al[42], 2017 | EMR | Antithrombotic agent (+/-) | 13/15 | 107/277 | |
| APT (+) | 4 | 53 | |||
| Anticoagulants (+) | 4 | 43 | |||
| Ishigami et al[34], 2017 | ER | 773 | Antithrombotic agent (+/-) | 10/14 | 35/714 |
| HR (+) | 10 | 35 | |||
| Pigò et al[3], 2017 | Polypectomy | 609 | Antithrombotic agent (+/-) | 38/32 | 72/467 |
| Single APT | 14 | 57 | |||
| Multiple APT | 3 | 8 | |||
| HR (+) | 21 | 7 | |||
| Aspirin (+) | 10 | 32 | |||
| Thienopyridine | 4 | 25 | |||
| Kono et al[76], 2017 | ESD/EMR | 49 | Single antithrombotic agent (+) | 4 | 24 |
| Multiple antithrombotic agents (+) | 7 | 14 | |||
| Discontinued antithrombotic agent (+) | 5 | 20 | |||
| Continued antithrombotic agent (+) | 6 | 18 | |||
| HR (+) | 4 | 12 | |||
| Lin et al[75], 2017 | Polypectomy | 4923 | Aspirin (+) | 36 | 3897 |
| Thienopyridine (+) | 5 | 590 | |||
| Sato et al[38], 2017 | ESD | 2378 | Antithrombotic agent (+/-) | 46/76 | 401/1855 |
| APT (+) | 35 | 270 | |||
| Anticoagulants (+) | 2 | 33 | |||
| HR (+) | 6 | 33 | |||
| Aspirin (+) | 12 | 199 | |||
| Thienopyridine (+) | 0 | 19 | |||
| Warfarin (+) | 1 | 16 | |||
| DOAC (+) | 1 | 17 | |||
| Igarashi et al[27], 2017 | ESD | 976 | Antithrombotic agent (+/-) | 35/30 | 332/692 |
| Discontinued antithrombotic agent (+) | 26 | 250 | |||
| Continued antithrombotic agent (+) | 5 | 49 | |||
| HR | 4 | 33 | |||
| Multiple antithrombotic agents (+) | 9 | 70 | |||
| Single antithrombotic agent (+) | 26 | 262 | |||
| Continued aspirin (+) | 4 | 29 | |||
| Discontinue aspirin (+) | 19 | 152 | |||
| Continued thienopyridine (+) | 1 | 17 | |||
| Discontinued thienopyridine (+) | 9 | 63 | |||
| Continued anticoagulants (+) | 1 | 11 | |||
| Discontinued anticoagulants (+) | 3 | 27 | |||
| Amato et al[31], 2016 | ER | 2692 | Antithrombotic agent (+/-) | 16/16 | 595/2069 |
| APT (+) | 11 | 461 | |||
| Anticoagulants (+) | 5 | 134 | |||
| Kubo et al[32], 2016 | ER | 788 | Antithrombotic agent (+/-) | 16/13 | 194/565 |
| APT (+) | 8 | 146 | |||
| Anticoagulants (+) | 11 | 72 | |||
| HR (+) | 10 | 63 | |||
| Shindo et al[25], 2016 | ESD | 262 | Antithrombotic agent (+/-) | 10/13 | 38/201 |
| Discontinued antithrombotic agent (+) | 0 | 25 | |||
| Continued APT (+) | 2 | 8 | |||
| HR (+) | 8 | 5 | |||
| Yoshida et al[52], 2016 | ESD | 678 | Antithrombotic agent (-) | 10 | 585 |
| APT (+) | 3 | 60 | |||
| Anticoagulants (+) | 3 | 17 | |||
| Ninomiya et al[53], 2015 | ESD | 609 | Antithrombotic agent (-) | 28 | 537 |
| Discontinued APT (+) | 2 | 11 | |||
| Continued APT (+) | 5 | 26 | |||
| Al-Mammari et al[4], 2015 | EMR | 117 | Antithrombotic agent (+/-) | 1/1 | 14/101 |
| Odagiri et al[16], 2015 | ESD | 7567 | Antithrombotic agent (+/-) | 49/282 | 440/6796 |
| Namasivayam et al[5],2014 | EMR | 1712 | Antithrombotic agent (+/-) | 4/10 | 772/912 |
| APT (+) | 3 | 521 | |||
| Anticoagulants (+) | 0 | 89 | |||
| Single antithrombotic agent (+) | 1 | 617 | |||
| Multiple antithrombotic agents (+) | 3 | 111 | |||
| Thienopyridine (+/-) | 0/10 | 17/912 | |||
| Terasaki et al[21], 2014 | ESD | 363 | Antithrombotic agent (+/-) | 4/20 | 36/303 |
| Tounou et al[50], 2014 | ESD | 350 | Antithrombotic agent (-) | 16 | 245 |
| Discontinued single APT (+) | 7 | 37 | |||
| Continued single APT (+) | 2 | 12 | |||
| Dual APT (+) | 11 | 20 | |||
| Aspirin (+) | 9 | 44 | |||
| Thienopyridine (+) | 0 | 5 | |||
| Suzuki et al[18], 2014 | ESD | 317 | Antithrombotic agent (+/-) | 1/13 | 27/276 |
| HR | 0 | 6 | |||
| Matsumura et al[23], 2014 | ESD | 425 | Antithrombotic agent (+/-) | 10/10 | 77/328 |
| Discontinued antithrombotic agent (+) | 2 | 39 | |||
| Continued antithrombotic agent (+), HR (-) | 3 | 22 | |||
| HR (+) | 5 | 16 | |||
| Beppu et al[74], 2014 | ER | 208 | APT (+) | 9 | 18 |
| Anticoagulants (+) | 12 | 9 | |||
| Aspirin (+) | 6 | 11 | |||
| Thienopyridine (+) | 3 | 7 | |||
| Inoue et al[77], 2014 | Polypectomy | 117 | Discontinued antithrombotic agent (+) | 1 | 71 |
| HR (+) | 9 | 36 | |||
| Sanomura et al[66], 2014 | ESD | 78 | Continued LDA (+) | 1 | 27 |
| Discontinued LDA (+) | 3 | 63 | |||
| Yoshio et al[47], 2013 | ESD | 1250 | Antithrombotic agent (-) | 45 | 972 |
| Discontinued antithrombotic agent (-) | 12 | 197 | |||
| HR (+) | 9 | 15 | |||
| Takeuchi et al[29], 2013 | ESD | 833 | Antithrombotic agent (+/-) | 21/15 | 69/728 |
| Koh et al[37], 2013 | ESD | 1166 | Antithrombotic agent (+/-) | 17/45 | 158/946 |
| Mukai et al[6], 2012 | ESD | 161 | Antithrombotic agent (+/-) | 4/17 | 29/111 |
| Lim et al[51], 2012 | ESD | 1591 | Antithrombotic agent (-) | 68 | 1249 |
| Discontinued APT (+) | 6 | 96 | |||
| Continued APT (+) | 20 | 152 | |||
| Miyahara et al[48], 2012 | ESD | 1082 | Antithrombotic agent (-) | 68 | 883 |
| Discontinued antithrombotic agent (+) | 7 | 124 | |||
| Cho et al[57], 2012 | ESD | 514 | Antithrombotic agent (-) | 15 | 424 |
| Discontinued APT (+) | 2 | 54 | |||
| Continued APT (+) | 4 | 15 | |||
| Toyokawa et al[24], 2011 | ESD | 1123 | Antithrombotic agent (+/-) | 8/48 | 175/892 |
| Higashiyama et al[19], 2011 | ESD | 924 | Antithrombotic agent (+/-) | 123/773 | 3/25 |
| Metz et al[2], 2011 | EMR | 269 | Antithrombotic agent (+/-) | 8/11 | 30/220 |
| APT (+) | 6 | 18 | |||
| Anticoagulants (+) | 1 | 10 | |||
| HR (+) | 1 | 2 | |||
| Aspirin (+) | 5 | 12 | |||
| Thienopyridine (+) | 1 | 6 | |||
| Tokioka et al[30], 2011 | ESD | 515 | Antithrombotic agent (+/-) | 3/23 | 37/452 |
| Okada et al[22], 2011 | ESD | 582 | Antithrombotic agent (+/-) | 4/24 | 70/484 |
| Mannen et al[20], 2010 | ESD | 436 | Antithrombotic agent (+/-) | 1/38 | 32/365 |
| Goto et al[13],2010 | ESD | 454 | Antithrombotic agent (+/-) | 5/21 | 52/376 |
| Witt et al[44], 2009 | Polypectomy | 1225 | Antithrombotic agent (+/-) | 11/2 | 414/798 |
| Ono et al[28], 2019 | ESD | 444 | Antithrombotic agent (+/-) | 6/20 | 50/368 |
| Takizawa et al[26], 2008 | ESD | 968 | Antithrombotic agent (+/-) | 3/60 | 74/831 |
| Sawhney et al[62], 2007 | Polypectomy | 173 | APT (+) | 17 | 51 |
| Anticoagulants (+) | 14 | 12 | |||
| Yousfi et al[43], 2004 | Polypectomy | 162 | Antithrombotic agent (+/-) | 32/49 | 27/54 |
| APT (+) | 32 | 27 |
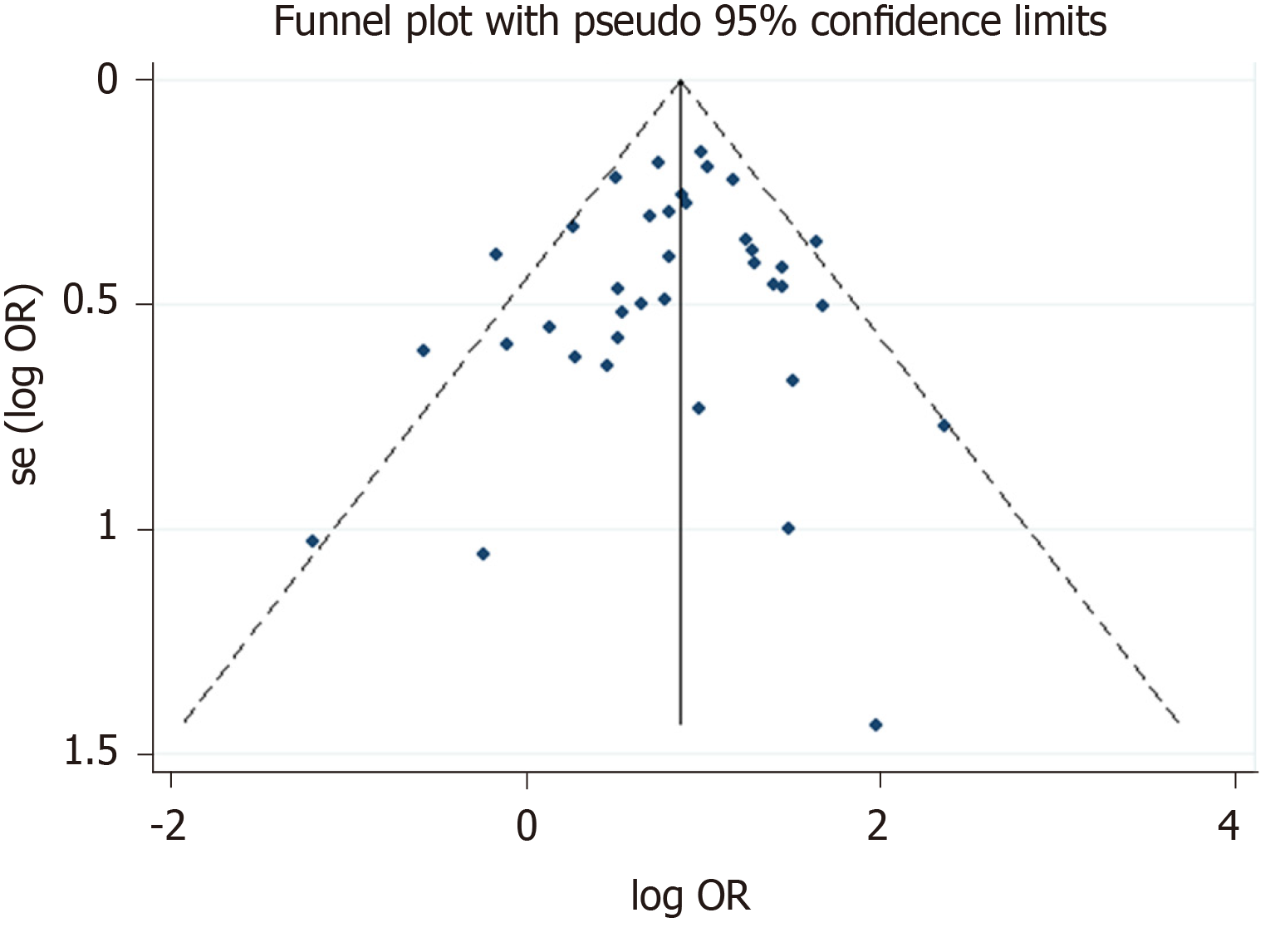
The Newcastle-Ottawa scale was used to assess the quality of the included studies in this meta-analysis. Thirteen articles had 6 stars, twenty-three articles had 7 stars, twenty-eight articles had 8 stars, and the others had 9 stars (Table 3). At the same time, the funnel plot did not show any features associated with publication bias (Figure 6).
| Ref. | Selection | Comparability | Outcome/exposure | Stars | |||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | |||
| So et al[49], 2019 | * | * | * | * | ** | * | * | * | 9 |
| Kishida et al[45], 2019 | * | * | * | * | * | * | 6 | ||
| Inoue et al[65], 2019 | * | * | * | ** | * | * | * | 8 | |
| Harada et al[56], 2019 | * | * | * | ** | * | * | * | 8 | |
| Arimoto et al[54], 2018 | * | * | * | * | * | * | * | * | 8 |
| Azumi et al[39], 2018 | * | * | * | ** | * | * | * | 8 | |
| Fujita et al[67], 2018 | * | * | * | ** | * | * | * | 8 | |
| Horikawa et al[58], 2018 | * | * | * | ** | * | * | * | 8 | |
| Izumikawa et al[40], 2018 | * | * | * | * | * | * | 6 | ||
| Kono et al[41], 2018 | * | * | * | * | * | * | * | 7 | |
| Oh et al[60], 2018 | * | * | * | * | * | * | 6 | ||
| Park et al[63], 2018 | * | * | * | ** | * | * | * | 8 | |
| Sanomura et al[59], 2018 | * | * | * | * | * | * | * | 7 | |
| Seo et al[55], 2018 | * | * | * | * | * | * | * | * | 8 |
| Sakai et al[64], 2018 | * | * | * | * | * | * | * | 7 | |
| Yamashita et al[36], 2018 | * | * | * | * | * | * | * | 7 | |
| Yanagisawa et al[35], 2018 | * | * | * | ** | * | * | * | 8 | |
| Matsumoto et al[46], 2018 | * | * | * | * | * | * | 6 | ||
| Harada et al[61], 2017 | * | * | * | * | * | * | * | 7 | |
| Yano et al[33], 2017 | * | * | * | * | * | * | * | 7 | |
| Ueki et al[14], 2017 | * | * | * | * | * | * | * | 7 | |
| Yoshio et al[78], 2017 | * | * | * | * | * | * | * | * | 8 |
| Gotoda et al[15], 2017 | * | * | * | * | * | * | 6 | ||
| Furuhata et al[17], 2017 | * | * | * | ** | * | * | * | 8 | |
| Shibuya et al[1], 2017 | * | * | * | ** | * | * | * | 8 | |
| Bronsgeest et al[42], 2017 | * | * | * | * | * | * | * | * | 8 |
| Ishigami et al[34], 2017 | * | * | * | * | * | * | * | 7 | |
| Pigò et al[3], 2017 | * | * | * | * | * | * | * | 7 | |
| Kono et al[76], 2017 | * | * | * | * | * | * | * | * | 8 |
| Lin et al[75], 2017 | * | * | * | * | * | * | * | 7 | |
| Sato et al[38], 2017 | * | * | * | ** | * | * | * | 8 | |
| Igarashi et al[27], 2017 | * | * | * | ** | * | * | 7 | ||
| Amato et al[31], 2016 | * | * | * | * | * | * | * | 7 | |
| Kubo et al[32], 2016 | * | * | * | * | * | * | * | 7 | |
| Shindo et al[25], 2016 | * | * | * | * | * | * | 6 | ||
| Yoshida et al[52], 2016 | * | * | * | * | * | * | 6 | ||
| Ninomiya et al[53], 2015 | * | * | * | * | * | * | 6 | ||
| Al-Mammari et al[4], 2015 | * | * | * | * | * | * | * | 7 | |
| Odagiri et al[16], 2015 | * | * | * | * | * | * | * | 7 | |
| Namasivayam et al[5], 2014 | * | * | * | * | * | * | 6 | ||
| Terasaki et al[21], 2014 | * | * | * | ** | * | * | 7 | ||
| Tounou et al[50], 2014 | * | * | * | * | * | * | * | 7 | |
| Suzuki et al[18], 2014 | * | * | * | * | * | * | * | 7 | |
| Matsumura et al[23], 2014 | * | * | ** | * | * | 6 | |||
| Beppu et al[74], 2014 | * | * | * | ** | * | * | * | 8 | |
| Inoue et al[77], 2014 | * | * | * | ** | * | * | * | 8 | |
| Sanomura et al[66], 2014 | * | * | * | ** | * | * | * | 8 | |
| Yoshio et al[47], 2013 | * | * | * | * | * | * | * | 7 | |
| Takeuchi et al[29], 2013 | * | * | * | ** | * | * | * | 8 | |
| Koh et al[37], 2013 | * | * | * | ** | * | * | * | 8 | |
| Mukai et al[6], 2012 | * | * | * | * | * | * | 6 | ||
| Lim et al[51], 2012 | * | * | * | ** | * | * | * | 8 | |
| Miyahara et al[48], 2012 | * | * | * | * | ** | * | * | 8 | |
| Cho et al[57], 2012 | * | * | * | ** | * | * | * | 8 | |
| Toyokawa T et al[24], 2011 | * | * | * | * | * | * | * | 7 | |
| Higashiyama et al[19], 2011 | * | * | * | * | * | * | * | 7 | |
| Metz et al[2], 2011 | * | * | * | ** | * | * | * | 8 | |
| Tokioka et al[30], 2011 | * | * | * | ** | * | * | * | 8 | |
| Okada K et al[22], 2011 | * | * | * | * | * | * | 6 | ||
| Mannen et al[20], 2010 | * | * | * | * | * | * | 6 | ||
| Goto et al[13], 2010 | * | * | * | ** | * | * | * | 8 | |
| Witt et al[44], 2009 | * | * | * | * | * | * | * | 7 | |
| Ono et al[28], 2019 | * | * | * | ** | * | * | 7 | ||
| Takizawa et al[26], 2008 | * | * | * | ** | * | * | * | 8 | |
| Sawhney et al[62], 2007 | * | * | * | ** | * | * | * | 8 | |
| Yousfi et al[43], 2004 | * | * | * | * | ** | * | * | * | 9 |
Among the ESD group, we performed several subgroup analyses to independently evaluate the effects of different types of antithrombotic agents in postoperative bleeding: (1) In gastric ESD retrospective comparison studies of single antithrombotic user (No. bleeding/total = 43/524) vs non-antithrombotic agent user (No. bleeding/total = 112/2671)[15,17,27]: The risk of postoperative bleeding in single antithrombotic agent group was significantly higher than the non-antithrombotic agent group [OR = 2.061, 95%CI: 1.405-3.024, P = 0.000 (I2 = 0.0%)]; (2) In gastric ESD retrospective comparison studies of multiple antithrombotic user (No. bleeding/total = 33/179) vs non-antithrombotic agent user (No.
Among the EMR group, we performed several subgroup analyses to evaluate the effects of different types of antithrombotic agents on postoperative bleeding: (1) APT (No. bleeding/total = 13/605) user vs non-antithrombotic user (No. bleeding/total = 36/1445)[2,5,42]: OR = 1.744, 95%CI: 0.398-7.643, P = 0.461 (I2 = 78.8%). There were two retrospective studies and one prospective study in the subgroup analysis. There were two studies about colorectal EMR and one study about gastric EMR in the subgroup analysis; (2) Anticoagulant user (No. bleeding/total = 44/567) vs non-antithrombotic user (No. bleeding/total = 218/8131)[2,5,42]: There was no significant difference in the risk of postoperative bleeding risk between the two groups [OR = 1.409, 95%CI: 0.552-3.597, P = 0.474 (I2 = 0.0%)]. There were two retrospective studies and one prospective study in the subgroup analysis. There were two studies about colorectal EMR and one study about gastric EMR in the subgroup analysis; and (3) Anticoagulant user (No. bleeding/total = 5/147) vs APT user (No. bleeding/total = 13/605)[2,5,42]: There was no significant difference in the risk of postoperative bleeding between the two groups [OR = 0.768, 95%CI: 0.261-2.261, P = 0.631 (I2 = 0.0%)]. There were two retrospective studies and one prospective study in the subgroup analysis. There were two studies about colorectal EMR and one study about gastric EMR in the subgroup analysis.
Among the polypectomy group, we also performed several subgroup analyses to evaluate the effects of different types of antithrombotic agents on postoperative bleeding: (1) APT (No. bleeding/total = 56/994) user vs non-antithrombotic user (No. bleeding/total = 121/5983)[3,43,45]: OR = 1.766, 95%CI: 1.192-2.616, P = 0.005 (I2 = 73.9%) (retrospective studies). There were two studies about colorectal polypectomy and one study about gastric polypectomy in the subgroup analysis; (2) Anticoagulant user (No. bleeding/total = 16/128) vs APT user (No. bleeding/total = 33/1106)[45,62,63]: The risk of postoperative bleeding after colorectal polypectomy in the anticoagulant agent group was significantly higher than the APT agent group [OR = 3.132, 95%CI: 1.442-6.803, P = 0.004 (I2 = 9.0%)] (retrospective studies); and (3) Warfarin user (No. bleeding/total = 32/226) vs DOAC (No. bleeding/total = 13/98)[35,36,64]: There was no significant difference in the risk of postoperative bleeding between the two groups [OR = 1.126, 95%CI: 0.557-2.275, P = 0.741 (I2 = 0.0%)] (retrospective studies). There were two studies about colorectal polypectomy and one study about gastric polypectomy in the subgroup analysis.
A subgroup analysis was planned to assess the risk of postoperative bleeding according to the difference in the size of the lesion, dosage and cessation period of antithrombotic agent, but we failed to perform the analysis because of insufficient data.
Thromboembolic event is defined as arterial thromboembolism. This includes stroke, transient ischemic attack and infarction perioperative period. These thromboembolic events in included studies were available in nineteen articles (one event in the heparin therapy group[17], five events in the antithrombotic group[5,27], three events in the HR group[35,47,64], one event in the discontinued anticoagulant therapy group[30], one event in the discontinued antithrombotic therapy group[32], two events in the withdrawal period of antiplatelet therapy group[2,51], one event in the anticoagulant therapy group[44], one event in the withdrawal period of anti-vitamin K antagonisis therapy group[65], four events in the low dose aspirin interrupted group[66]). No thromboembolic events occurred in seven studies[36,41,49,58,59,67].
Despite several practice guidelines about the cessation or continuation of antithrombotic drugs before ER made by the British Society of Gastroenterology[68], the European Society of Gastrointestinal Endoscopy[68], the American Society for Gastrointestinal Endoscopy[69] and the Japan Gastroenterological Endoscopy Society[70], the effect of antithrombotic drugs on the risk of postoperative bleeding was still controversial in some studies[4,6,13,14,16,19-22,24,26,27,31,37,48,57]. Our study found that antithrombotic agents confer a higher risk for postoperative bleeding after ESD and EMR. But the risk of postoperative bleeding after polypectomy was not significantly elevated in the patients with antithrombotic drugs from our study, which was in consistent with the results of a study by Matsumoto et al[46]. Nevertheless, there was significant heterogeneity in the analysis of antithrombotic group vs non-antithrombotic group. To explain the heterogeneity (I2 = 82.5%) of our meta-analysis, we got the following findings: (1) Different methods were used to prevent postoperative bleeding; (2) Different definitions on postoperative bleeding[2,19]; (3) Different types and doses of antithrombotic agents; and (4) Different follow-up time, ranging 24 h to 3 mo. In order to reduce the heterogeneity, we have done the subgroup analyses to assess the effect of different types of antithrombotic agents in the risk of postoperative bleeding.
Some studies found that APT did not correlate with the risk of postoperative bleeding[32,52]. At the same time, the risk of delayed postoperative bleeding after ESD was not increased in a single APT agent (continued or discontinued)[17]. In contrast, it has been demonstrated that APT (especially dual APT) increases the risk of postoperative bleeding[50]. A retrospective study by Singh et al[71] showed that clopidogrel alone was not an independent risk factor for postoperative bleeding, but a randomized trial by Chan et al[72] showed that continued clopidogrel use results in a higher risk of postoperative bleeding compared to the discontinued clopidigrel use group. Our study found that continued single APT agent use did not increase the risk of postoperative bleeding, but multiple APT agents increased the risk of postoperative bleeding after ER.
Some studies found that low dose aspirin and continued use of aspirin didn’t induce a higher risk of postoperative bleeding after polypectomy and gastric ESD[23,43,50]. However, Ninomiya et al[53] found that continued use of aspirin increased the risk of postoperative bleeding after colorectal ESD. A study by Metz et al[2] demonstrated that the use of aspirin within 7 d of the operation was an independent risk factor for postoperative bleeding after colonic EMR. In a meta-analysis by Shalman et al[73], the risk of immediate bleeding in patients with aspirin was not increased, but the risk of delayed bleeding in patients with aspirin or thienopyridine derivatives was increased. Our study found that the use
Several guidelines about gastroenterological endoscopy recommend that anticoagulant agent should be discontinued with HR[68-70]. APT plus HR (meaning that anticoagulants were substituted by heparin before polypectomy) were not correlated with postoperative bleeding, but anticoagulant or anticoagulant plus HR were risk factors for postoperative bleeding[32]. Besides, HR alone was related to postoperative bleeding in univariate analysis but was not in multivariate analysis[32]. And our study has reached the same conclusion. Cessation of antithrombotic therapy could result in thromboembolic events such as cerebral infarction and hemorrhagic shock. But the risk of the thromboembolic events in the included studies is relatively low.
There were several drawbacks in this meta-analysis. First of all, the results of our meta-analysis were derived from retrospective studies. Retrospective studies may underestimate the risk of postoperative bleeding. Further prospective studies are needed to confirm our results. Secondly, the surveillance periods of included studies were not exactly the same. Finally, different types and doses of antithrombotic agents were used in the included studies, which may lead to bias.
In conclusion, the risk of postoperative bleeding after ER (polypectomy, EMR and ESD) correlated with the types and management of the antithrombotic agents according to our meta-analysis. Interrupting or switching antithrombotic therapy might result in the increased risk of serious thromboembolic events. Therefore, it is important to comprehensively assess the risk of postoperative bleeding and thromboembolic events in the patients with antithrombotic drugs after ER.
Endoscopic resection (ER) is deemed as an effective method for gastrointestinal neoplasia, polyp, gastric adenomas, early oesophageal, gastric and colorectal cancer. More and more people suffering from cardiovascular disease and/or cerebrovascular disease receive antithrombotic therapy which change patients’ coagulation status and may lead to high risk of postoperative bleeding after ER. The relationship between the postoperative bleeding after ER and antithrombotic agents is still uncertain.
This study explored the relationship between the postoperative bleeding after ER and antithrombotic agents.
The aim of this study is to identify whether the use of antithrombotic drugs increases the risk of the postoperative bleeding after ER by a systematic review and meta-analysis.
A systematic search was conducted on PubMed, Web of Science, Cochrane library. The Newcastle-Ottawa scale was used to evaluate the quality of studies. Stata 12.0 was used for statistical analysis. The odds ratio and 95%CI were calculated and heterogeneity was quantified using Cochran’s Q test and I2.
Total 66 studies were included in the meta-analysis. Pooled data suggested that antithrombotic therapy was significantly associated with postoperative bleeding after ER. The risk of postoperative bleeding after endoscopic submucosal dissection, endoscopic mucosal resection and polypectomy in the antithrombotic group was higher than the non-antithrombotic group.
The risk of postoperative bleeding after ER correlated with the types and management of antithrombotic agents by our meta-analysis.
Our results can guide the use of antithrombotic drugs before ER and evaluate the risk of postoperative bleeding.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Hosoe N, Komeda Y, Rege S S-Editor: Wang JL L-Editor: A P-Editor: Ma YJ
| 1. | Shibuya T, Nomura O, Kodani T, Murakami T, Fukushima H, Tajima Y, Matsumoto K, Ritsuno H, Ueyama H, Inami Y, Ishikawa D, Matsumoto K, Sakamoto N, Osada T, Nagahara A, Ogihara T, Watanabe S. Continuation of antithrombotic therapy may be associated with a high incidence of colonic post-polypectomy bleeding. Dig Endosc. 2017;29:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Metz AJ, Bourke MJ, Moss A, Williams SJ, Swan MP, Byth K. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Pigò F, Bertani H, Manno M, Mirante VG, Caruso A, Mangiafico S, Manta R, Rebecchi AM, Conigliaro RL. Colonic Postpolypectomy Bleeding Is Related to Polyp Size and Heparin Use. Clin Endosc. 2017;50:287-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Al-Mammari S, Owen R, Findlay J, Koutsoumpas A, Gillies R, Marshall R, Bailey AA, Maynard N, Sgromo B, Braden B. Endoscopic mucosal resection of early oesophageal neoplasia in patients requiring anticoagulation: is it safe? Surg Endosc. 2016;30:2390-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Namasivayam V, Prasad GA, Lutzke LS, Dunagan KT, Borkenhagen LS, Okoro NI, Tomizawa Y, Buttar NS, Michel WL, Wang KK. The risk of endoscopic mucosal resection in the setting of clopidogrel use. ISRN Gastroenterol. 2014;2014:494157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Mukai S, Cho S, Kotachi T, Shimizu A, Matuura G, Nonaka M, Hamada T, Hirata K, Nakanishi T. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012;2012:875323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol. 2016;22:5927-5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Choung BS, Kim SH, Ahn DS, Kwon DH, Koh KH, Sohn JY, Park WS, Kim IH, Lee SO, Lee ST, Kim SW. Incidence and risk factors of delayed postpolypectomy bleeding: a retrospective cohort study. J Clin Gastroenterol. 2014;48:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21613] [Cited by in RCA: 18159] [Article Influence: 1134.9] [Reference Citation Analysis (0)] |
| 11. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12631] [Article Influence: 842.1] [Reference Citation Analysis (0)] |
| 12. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46468] [Article Influence: 2112.2] [Reference Citation Analysis (3)] |
| 13. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Ueki N, Futagami S, Akimoto T, Maruki Y, Yamawaki H, Kodaka Y, Nagoya H, Shindo T, Kusunoki M, Kawagoe T, Gudis K, Miyake K, Iwakiri K. Effect of Antithrombotic Therapy and Long Endoscopic Submucosal Dissection Procedure Time on Early and Delayed Postoperative Bleeding. Digestion. 2017;96:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Gotoda T, Hori K, Iwamuro M, Kono Y, Miura K, Kanzaki H, Kawano S, Kawahara Y, Okada H. Evaluation of the bleeding risk with various antithrombotic therapies after gastric endoscopic submucosal dissection. Endosc Int Open. 2017;5:E653-E662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Odagiri H, Yasunaga H, Matsui H, Fushimi K, Iizuka T, Kaise M. Hospital volume and the occurrence of bleeding and perforation after colorectal endoscopic submucosal dissection: analysis of a national administrative database in Japan. Dis Colon Rectum. 2015;58:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Furuhata T, Kaise M, Hoteya S, Iizuka T, Yamada A, Nomura K, Kuribayashi Y, Kikuchi D, Matsui A, Ogawa O, Yamashta S, Mitani T. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer. 2017;20:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Suzuki S, Chino A, Kishihara T, Uragami N, Tamegai Y, Suganuma T, Fujisaki J, Matsuura M, Itoi T, Gotoda T, Igarashi M, Moriyasu F. Risk factors for bleeding after endoscopic submucosal dissection of colorectal neoplasms. World J Gastroenterol. 2014;20:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Higashiyama M, Oka S, Tanaka S, Sanomura Y, Imagawa H, Shishido T, Yoshida S, Chayama K. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc. 2011;23:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, Iwakiri R, Fujimoto K. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Terasaki M, Tanaka S, Shigita K, Asayama N, Nishiyama S, Hayashi N, Nakadoi K, Oka S, Chayama K. Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis. 2014;29:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, Nakajima A, Hoshino E, Igarashi M. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Shindo Y, Matsumoto S, Miyatani H, Yoshida Y, Mashima H. Risk factors for postoperative bleeding after gastric endoscopic submucosal dissection in patients under antithrombotics. World J Gastrointest Endosc. 2016;8:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Igarashi K, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H, Ono H. Should antithrombotic therapy be stopped in patients undergoing gastric endoscopic submucosal dissection? Surg Endosc. 2017;31:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Ono S, Ishikawa M, Matsuda K, Tsuda M, Yamamoto K, Shimizu Y, Sakamoto N. Clinical impact of the perioperative management of oral anticoagulants in bleeding after colonic endoscopic mucosal resection. BMC Gastroenterol. 2019;19:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Tokioka S, Umegaki E, Murano M, Takeuchi N, Takeuchi T, Kawakami K, Yoda Y, Kojima Y, Higuchi K. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol. 2012;27 Suppl 3:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Amato A, Radaelli F, Dinelli M, Crosta C, Cengia G, Beretta P, Devani M, Lochis D, Manes G, Fini L, Paggi S, Passoni GR, Repici A; SIED Lombardy group. Early and delayed complications of polypectomy in a community setting: The SPoC prospective multicentre trial. Dig Liver Dis. 2016;48:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Kubo T, Yamashita K, Onodera K, Iida T, Arimura Y, Nojima M, Nakase H. Heparin bridge therapy and post-polypectomy bleeding. World J Gastroenterol. 2016;22:10009-10014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Yano T, Tanabe S, Ishido K, Suzuki M, Kawanishi N, Yamane S, Watanabe A, Wada T, Azuma M, Katada C, Koizumi W. Different clinical characteristics associated with acute bleeding and delayed bleeding after endoscopic submucosal dissection in patients with early gastric cancer. Surg Endosc. 2017;31:4542-4550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Ishigami H, Arai M, Matsumura T, Maruoka D, Minemura S, Okimoto K, Kasamatsu S, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Heparin-bridging therapy is associated with a high risk of post-polypectomy bleeding regardless of polyp size. Dig Endosc. 2017;29:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Yanagisawa N, Nagata N, Watanabe K, Iida T, Hamada M, Kobayashi S, Shimbo T, Akiyama J, Uemura N. Post-polypectomy bleeding and thromboembolism risks associated with warfarin vs direct oral anticoagulants. World J Gastroenterol. 2018;24:1540-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 36. | Yamashita K, Oka S, Tanaka S, Boda K, Hirano D, Sumimoto K, Mizumoto T, Ninomiya Y, Tamaru Y, Shigita K, Hayashi N, Sanomura Y, Chayama K. Use of anticoagulants increases risk of bleeding after colorectal endoscopic submucosal dissection. Endosc Int Open. 2018;6:E857-E864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Sato C, Hirasawa K, Koh R, Ikeda R, Fukuchi T, Kobayashi R, Kaneko H, Makazu M, Maeda S. Postoperative bleeding in patients on antithrombotic therapy after gastric endoscopic submucosal dissection. World J Gastroenterol. 2017;23:5557-5566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Azumi M, Takeuchi M, Koseki Y, Kumagai M, Kobayashi Y, Takatsuna M, Yoshioka A, Yoshikawa S, Miura T, Terai S. The search, coagulation, and clipping (SCC) method prevents delayed bleeding after gastric endoscopic submucosal dissection. Gastric Cancer. 2019;22:567-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Izumikawa K, Iwamuro M, Inaba T, Ishikawa S, Kuwaki K, Sakakihara I, Yamamoto K, Takahashi S, Tanaka S, Wato M, Okada H. Bleeding in patients who underwent scheduled second-look endoscopy 5 days after endoscopic submucosal dissection for gastric lesions. BMC Gastroenterol. 2018;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kono Y, Obayashi Y, Baba Y, Sakae H, Gotoda T, Miura K, Kanzaki H, Iwamuro M, Kawano S, Kawahara Y, Tanaka T, Okada H. Postoperative bleeding risk after gastric endoscopic submucosal dissection during antithrombotic drug therapy. J Gastroenterol Hepatol. 2018;33:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Bronsgeest K, Huisman JF, Langers A, Boonstra JJ, Schenk BE, de Vos Tot Nederveen Cappel WH, Vasen HFA, Hardwick JCH. Safety of endoscopic mucosal resection (EMR) of large non-pedunculated colorectal adenomas in the elderly. Int J Colorectal Dis. 2017;32:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Yousfi M, Gostout CJ, Baron TH, Hernandez JL, Keate R, Fleischer DE, Sorbi D. Postpolypectomy lower gastrointestinal bleeding: potential role of aspirin. Am J Gastroenterol. 2004;99:1785-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Witt DM, Delate T, McCool KH, Dowd MB, Clark NP, Crowther MA, Garcia DA, Ageno W, Dentali F, Hylek EM, Rector WG; WARPED Consortium. Incidence and predictors of bleeding or thrombosis after polypectomy in patients receiving and not receiving anticoagulation therapy. J Thromb Haemost. 2009;7:1982-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Kishida Y, Hotta K, Imai K, Ito S, Yoshida M, Kawata N, Tanaka M, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Ono H. Risk Analysis of Colorectal Post-Polypectomy Bleeding Due to Antithrombotic Agent. Digestion. 2019;99:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Matsumoto M, Yoshii S, Shigesawa T, Dazai M, Onodera M, Kato M, Sakamoto N. Safety of Cold Polypectomy for Colorectal Polyps in Patients on Antithrombotic Medication. Digestion. 2018;97:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Yoshio T, Nishida T, Kawai N, Yuguchi K, Yamada T, Yabuta T, Komori M, Yamaguchi S, Kitamura S, Iijima H, Tsutsui S, Michida T, Mita E, Tsujii M, Takehara T. Gastric ESD under Heparin Replacement at High-Risk Patients of Thromboembolism Is Technically Feasible but Has a High Risk of Delayed Bleeding: Osaka University ESD Study Group. Gastroenterol Res Pract. 2013;2013:365830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Miyahara K, Iwakiri R, Shimoda R, Sakata Y, Fujise T, Shiraishi R, Yamaguchi K, Watanabe A, Yamaguchi D, Higuchi T, Tominaga N, Ogata S, Tsuruoka N, Noda T, Hidaka H, Mannen K, Endo H, Yamanouchi K, Yamazato T, Sakata H, Fujimoto K. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion. 2012;86:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | So S, Ahn JY, Kim N, Na HK, Jung KW, Lee JH, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. Comparison of the effects of antithrombotic therapy on delayed bleeding after gastric endoscopic resection: a propensity score-matched case-control study. Gastrointest Endosc 2019; 89: 277-285. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Tounou S, Morita Y, Hosono T. Continuous aspirin use does not increase post-endoscopic dissection bleeding risk for gastric neoplasms in patients on antiplatelet therapy. Endosc Int Open. 2015;3:E31-E38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS, Jung HC, Song IS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Yoshida N, Naito Y, Murakami T, Hirose R, Ogiso K, Siah KT, Inada Y, Yagi N, Itoh Y. High incidence of postoperative hemorrhage in colorectal endoscopic submucosal dissection during anticoagulant therapy. Int J Colorectal Dis. 2016;31:1487-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Ninomiya Y, Oka S, Tanaka S, Nishiyama S, Tamaru Y, Asayama N, Shigita K, Hayashi N, Chayama K. Risk of bleeding after endoscopic submucosal dissection for colorectal tumors in patients with continued use of low-dose aspirin. J Gastroenterol. 2015;50:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Arimoto J, Higurashi T, Chiba H, Misawa N, Yoshihara T, Kato T, Kanoshima K, Fuyuki A, Ohkubo H, Goto S, Ishikawa Y, Tachikawa J, Ashikari K, Nonaka T, Taguri M, Kuriyama H, Atsukawa K, Nakajima A. Continued Use of a Single Antiplatelet Agent Does Not Increase the Risk of Delayed Bleeding After Colorectal Endoscopic Submucosal Dissection. Dig Dis Sci. 2018;63:218-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Seo M, Song EM, Cho JW, Lee YJ, Lee BI, Kim JS, Jeon SW, Jang HJ, Yang DH, Ye BD, Byeon JS. A risk-scoring model for the prediction of delayed bleeding after colorectal endoscopic submucosal dissection. Gastrointest Endosc 2019; 89: 990-998. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Harada H, Suehiro S, Murakami D, Nakahara R, Nagasaka T, Ujihara T, Sagami R, Katsuyama Y, Hayasaka K, Amano Y. Feasibility of gastric endoscopic submucosal dissection with continuous low-dose aspirin for patients receiving dual antiplatelet therapy. World J Gastroenterol. 2019;25:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Horikawa Y, Mizutamari H, Mimori N, Kato Y, Sawaguchi M, Fushimi S, Sato S, Okubo S. Effect of Continued Administration of Low-dose Aspirin for Intraoperative Bleeding Control in Gastric Endoscopic Submucosal Dissection. Digestion. 2019;100:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Sanomura Y, Oka S, Tanaka S, Yorita N, Kuroki K, Kurihara M, Mizumoto T, Yoshifuku Y, Chayama K. Taking Warfarin with Heparin Replacement and Direct Oral Anticoagulant Is a Risk Factor for Bleeding after Endoscopic Submucosal Dissection for Early Gastric Cancer. Digestion. 2018;97:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Oh S, Kim SG, Kim J, Choi JM, Lim JH, Yang HJ, Park JY, Han SJ, Kim JL, Chung H, Jung HC. Continuous Use of Thienopyridine May Be as Safe as Low-Dose Aspirin in Endoscopic Resection of Gastric Tumors. Gut Liver. 2018;12:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Harada H, Suehiro S, Murakami D, Shimizu T, Nakahara R, Katsuyama Y, Miyama Y, Tounou S, Hayasaka K. Continuous use of low-dose warfarin for gastric endoscopic submucosal dissection: a prospective study. Endosc Int Open. 2017;5:E348-E353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Sawhney MS, Salfiti N, Nelson DB, Lederle FA, Bond JH. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 63. | Park SK, Seo JY, Lee MG, Yang HJ, Jung YS, Choi KY, Kim H, Kim HO, Jung KU, Chun HK, Park DI. Prospective analysis of delayed colorectal post-polypectomy bleeding. Surg Endosc. 2018;32:3282-3289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Sakai T, Nagami Y, Shiba M, Hayashi K, Kinoshita Y, Maruyama H, Kato K, Minamino H, Ominami M, Fukunaga S, Otani K, Hosomi S, Tanaka F, Taira K, Kamata N, Yamagami H, Tanigawa T, Watanabe T, Fujiwara Y. Heparin-bridging therapy is associated with post-colorectal polypectomy bleeding in patients whose oral anticoagulation therapy is interrupted. Scand J Gastroenterol. 2018;53:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Inoue T, Iijima H, Yamada T, Okuyama Y, Takahashi K, Nishida T, Ishihara R, Akasaka T, Kobayashi I, Kuroshima T, Yasunaga Y, Yamamoto K, Nakahara M, Doi Y, Nakajima S, Mukai A, Masuda E, Yoshii S, Hayashi Y, Minamiguchi H, Sakata Y, Yamamoto K, Tsujii M, Takehara T. A prospective multicenter observational study evaluating the risk of periendoscopic events in patients using anticoagulants: the Osaka GIANT Study. Endosc Int Open. 2019;7:E104-E114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Sanomura Y, Oka S, Tanaka S, Numata N, Higashiyama M, Kanao H, Yoshida S, Ueno Y, Chayama K. Continued use of low-dose aspirin does not increase the risk of bleeding during or after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2014;17:489-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Fujita M, Murao T, Osawa M, Hirai S, Fukushima S, Yo S, Nakato R, Ishii M, Matsumoto H, Tamaki T, Sakakibara T, Shiotani A. Colonic endoscopic mucosal resection in patients taking anticoagulants: Is heparin bridging therapy necessary? J Dig Dis. 2018;19:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, Dumonceau JM. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 69. | ASGE Standards of Practice Committee, Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 70. | Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K, Mine T, Yoshino J, Kinoshita Y, Ichinose M, Matsui T; Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 71. | Singh M, Mehta N, Murthy UK, Kaul V, Arif A, Newman N. Postpolypectomy bleeding in patients undergoing colonoscopy on uninterrupted clopidogrel therapy. Gastrointest Endosc. 2010;71:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Chan FKL, Kyaw MH, Hsiang JC, Suen BY, Kee KM, Tse YK, Ching JYL, Cheong PK, Ng D, Lam K, Lo A, Lee V, Ng SC. Risk of Postpolypectomy Bleeding With Uninterrupted Clopidogrel Therapy in an Industry-Independent, Double-Blind, Randomized Trial. Gastroenterology 2019; 156: 918-925. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Shalman D, Gerson LB. Systematic review with meta-analysis: the risk of gastrointestinal haemorrhage post-polypectomy in patients receiving anti-platelet, anti-coagulant and/or thienopyridine medications. Aliment Pharmacol Ther. 2015;42:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Beppu K, Osada T, Sakamoto N, Shibuya T, Matsumoto K, Nagahara A, Terai T, Ogihara T, Watanabe S. Optimal timing for resuming antithrombotic agents and risk factors for delayed bleeding after endoscopic resection of colorectal tumors. Gastroenterol Res Pract. 2014;2014:825179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Lin D, Soetikno RM, McQuaid K, Pham C, Doan G, Mou S, Shergill AK, Somsouk M, Rouse RV, Kaltenbach T. Risk factors for postpolypectomy bleeding in patients receiving anticoagulation or antiplatelet medications. Gastrointest Endosc. 2018;87:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Kono Y, Matsubara M, Toyokawa T, Takenaka R, Suzuki S, Nasu J, Yoshioka M, Nakagawa M, Mizuno M, Sakae H, Abe M, Gotoda T, Miura K, Kanzaki H, Iwamuro M, Hori K, Tsuzuki T, Kita M, Kawano S, Kawahara Y, Okada H. Multicenter Prospective Study on the Safety of Upper Gastrointestinal Endoscopic Procedures in Antithrombotic Drug Users. Dig Dis Sci. 2017;62:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Inoue T, Nishida T, Maekawa A, Tsujii Y, Akasaka T, Kato M, Hayashi Y, Yamamoto S, Kondo J, Yamada T, Shinzaki S, Iijima H, Tsujii M, Takehara T. Clinical features of post-polypectomy bleeding associated with heparin bridge therapy. Dig Endosc. 2014;26:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Yoshio T, Tomida H, Iwasaki R, Horiuchi Y, Omae M, Ishiyama A, Hirasawa T, Yamamoto Y, Tsuchida T, Fujisaki J, Yamada T, Mita E, Ninomiya T, Michitaka K, Igarashi M. Effect of direct oral anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection. Dig Endosc. 2017;29:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |