Published online Jun 28, 2020. doi: 10.13105/wjma.v8.i3.220
Peer-review started: March 2, 2020
First decision: April 24, 2020
Revised: May 20, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: June 28, 2020
Processing time: 127 Days and 11.5 Hours
Thrombocytopenia is a multifactorial disorder that is common in patients with chronic liver disease (CLD), leading to challenging perioperative planning. As thrombocytopenia in CLD is associated with thrombopoietin (TPO) deficiency, the use of TPO-receptor agonists (TPO-RAs) to increase platelet counts is a promising approach. This has led to the development of various TPO-RAs, including romiplostim, eltrombopag, avatrombopag, and lusutrombopag. Of these, only avatrombopag and lusutrombopag are approved by the United States Food and Drug Administration for the perioperative treatment of thrombocytopenia in patients with CLD. Platelet transfusion is commonly used for the clinical management of thrombocytopenia in patients with CLD undergoing invasive procedures. However, the limitations and possible risks of transfusion, including short duration of efficacy, development of antiplatelet antibodies, risk of infections and such complications as transfusion-related acute lung injury or circulatory overload, and possibility of refractoriness, limit its use. Moreover, there is no consensus among guidelines as to the platelet count at which transfusions are indicated. Results from studies using TPO-RAs perioperatively in patients with thrombocytopenia and CLD are promising and provide an alternative to platelet transfusions in the pre- and post-operative setting. These TPO-RAs are the subject of this review, with focus on their use in the perioperative setting in patients with thrombocytopenia, associated supporting clinical trials, efficacy and safety data, and their use with respect to platelet transfusions.
Core tip: Thrombocytopenia in patients with chronic liver disease complicates perioperative planning. Platelet transfusions are typically used as periprocedural treatment in these patients, but their use is complicated due to risk factors such as the development of infections and refractoriness. This has led to the development of thrombopoietin-receptor agonists, such as avatrombopag and lusutrombopag, that can increase platelet counts in patients with compromised thrombopoietin production and chronic liver disease. These thrombopoietin-receptor agonists can provide physicians with a safe and effective alternative to platelet transfusions and their use in clinical practice is the focus of this review.
- Citation: Qureshi K, Bonder A. Thrombopoietin-receptor agonists in perioperative treatment of patients with chronic liver disease. World J Meta-Anal 2020; 8(3): 220-232
- URL: https://www.wjgnet.com/2308-3840/full/v8/i3/220.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i3.220
Thrombopoietin (TPO) is a hormone produced by the liver that regulates platelet production by binding to and activating TPO receptors that are present on the surface of megakaryocytes. This induces a series of signaling cascades, leading to increased platelet production[1]. As a result, TPO levels typically decrease as liver disease progresses. This impaired TPO production, along with other factors, including platelet sequestration due to hypersplenism[2], bone marrow suppression, altered TPO metabolism, and development of anti-platelet antibodies, can contribute to thrombocytopenia[3].
Recombinant TPOs, as a treatment option, were developed to counter the lowered TPO levels by stimulating the TPO receptor. These pharmacologic agents mimic the action of TPO by binding to and activating different receptors on the megakaryocytes. The first generation of TPO-receptor agonists (TPO-RAs) included recombinant human thrombopoietin (known as rhTPO) and pegylated-human recombinant megakaryocyte growth and development factor (known as Peg-rHuMGDF). These resulted in the development of neutralizing autoantibodies that cross-reacted with endogenous TPO and were therefore discontinued. The next-generation TPO-RAs developed did not have homology to endogenous TPO and did not, therefore, produce an antigenic effect. These included romiplostim (NPLATE®) and eltrombopag (Promacta®/Revolade®), and most recently, avatrombopag (Doptelet®) and lusutrombopag (Mulpleta®)[4-7]. Of these, avatrombopag and lusutrombopag are approved by the United States Food and Drug Administration for the treatment of thrombocytopenia in patients with chronic liver disease (CLD) who are scheduled to undergo a procedure. Hetrombopag is another TPO-RA that is currently undergoing Phase 2 trials.
Patients with thrombocytopenia can have a negative perioperative outcome and platelet transfusions can be used to manage platelet counts during this period. Bleeding risk associated with low platelet counts has been demonstrated in several studies; however, the literature is equivocal, as some studies do not support an association. The optimal platelet count prior to performance of surgery varies, depending on the procedure and on patient variables, and while various guidelines advocate different platelet count thresholds for different invasive procedures, there are inadequate data to support these recommendations. For instance, the British Society of Hematology proposes thresholds of 50, 80 and 100 × 109/L according to the type of invasive procedure[8].
This review will provide an overview of the currently available TPO-RAs, with a focus on their use in the perioperative setting in patients with thrombocytopenia and CLD, associated supporting clinical trials, including the efficacy and safety data, and their use with respect to platelet transfusions.
Romiplostim is a recombinant fusion protein (peptibody)[4] containing two identical single-chain subunits, each consisting of a human immunoglobulin IgG1 Fc domain covalently linked to a peptide, itself containing two TPO receptor binding domains. It has no sequence homology to endogenous TPO and is produced using recombinant DNA technology in Escherichia coli. Romiplostim increases platelet production in a dose-dependent manner by the binding and activation of the TPO receptor, c-Mpl. As it is given intravenously, romiplostim avoids first-pass metabolism and is cleared by its binding to c-Mpl receptors and via the renal pathway. A single subcutaneous dose of 1 mcg/kg to 10 mcg/kg in patients with immune thrombocytopenia (ITP) results in a peak platelet count 1.3-14.9 times greater than the baseline platelet count over a 2-wk to 3-wk study period[4].
Romiplostim is a subcutaneously administered drug indicated in the treatment of thrombocytopenia in patients with chronic ITP who have shown an insufficient response to corticosteroids, immunoglobulins, or splenectomy. Several studies have explored the efficacy of romiplostim for the management of perioperative thrombocytopenia in patients with CLD (Table 1). However, the small sample size in these studies is a major limitation. A 3-mo, single-center, single-arm, open-label study by Moussa et al[3] evaluated the efficacy of preoperative romiplostim treatment in 35 male patients with chronic hepatitis C, liver cirrhosis, and thrombocytopenia secondary to hepatitis C virus infection over 90 d. Romiplostim administered at 2 μg/kg once a week for up to 1 mo before the scheduled surgeries resulted in an increase in platelet counts (≥ 70 × 109/L) in 33 of the 35 patients, making them eligible for the procedure. Additionally, no serious adverse events (AEs) were observed during the treatment period (up to day 30) and none of the patients experienced postoperative bleeding or a thrombotic event within 60 d of the operation[3]. The authors concluded that preoperative treatment with romiplostim could be a viable and cost-effective alternative for patients who are unresponsive to standard therapy.
| Ref. | Dosing | Efficacy results | AEs |
| Romiplostim | |||
| Basu et al[38], 2012 | 65 patients with CLD and thrombocytopenia randomized 1:1:1 to 500 μg romiplostim: 75 mg eltrombopag: 7 units of platelet transfusion | Improved platelet count > 180 × 109/L in all groups | Nausea, vomiting, dry mouth, headache, insomnia, irritability, local skin rash, shortness of breath, myalgia, arthralgia, erythema |
| Moussa et al[3], 2013 | 35 male patients with thrombocytopenia and CLD secondary to hepatitis C infection, dosed 2 μg/kg romiplostim weekly | Improved platelet count ≥ 70 × 109/L | No serious AEs reported |
| Marshall et al[39], 2015 | 18 patients with various etiologies of thrombocytopenia, including CLD, undergoing wide range of procedures | Improved platelet counts in all patients; all patients could receive surgery without delay or cancellation | No venous thromboembolic events |
| Al-Samkari et al[9], 2018 | 48 patients with various etiologies of thrombocytopenia, including CLD, undergoing 51 procedures, dosed 3 μg/kg romiplostim weekly (range 1-10 μg/kg/wk) | Improved platelet counts achieved in all patients after 1, 2 or 3 doses | Bleeding and thromboembolic events within acceptable limits |
| Eltrombopag | |||
| ELEVATE (Eltrombopag); Afdhal et al[11], 2012 | 292 patients with CLD administered | Platelet transfusion not required in 72% of patients in the eltrombopag group and 19% of patients in the placebo group | Early study termination due to six portal vein thrombotic events in the eltrombopag group; headache, pyrexia, abdominal pain, diarrhea, nausea, etc. |
| 75 mg/d or placebo for 14 d | |||
| Avatrombopag | |||
| ADAPT-1; Terrault et al[14], 2018 | 231 patients with CLD and thrombocytopenia divided into low (<40 × 109/L) and high (40 to < 50 × 109/L) baseline platelet count cohorts | Platelet transfusion, or rescue procedure for bleeding not required in 65.6% of avatrombopag-treated patients and 22.9% of placebo-treated patients in the low platelet count cohort and 88.1% of avatrombopag-treated patients and 38.2% of placebo-treated patients in the high platelet count cohort | Mild to moderate in severity in all treatment groups; most common TEAEs: abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache; no thromboembolic TEAEs |
| Both cohorts were randomized 2:1 to receive avatrombopag or placebo; the low baseline cohort was treated with 60 mg and the high baseline cohort was treated with 40 mg avatrombopag, or placebo | |||
| ADAPT-2; Terrault et al[14], 2018 | 204 patients with CLD and thrombocytopenia; same dosing as ADAPT-1 | Platelet transfusion, or rescue procedure for bleeding not required in 68.6% of avatrombopag-treated patients and 34.9% of placebo-treated patients in the low platelet count cohort and 87.9% of avatrombopag-treated patients and 33.3% of placebo-treated patients in the high platelet count cohort | Same as ADAPT-1; three thromboembolic TEAEs reported |
| Lusutrombopag | |||
| L-PLUS-1; Hidaka et al[18], 2018 | 97 patients with thrombocytopenia and CLD randomized to receive 3 mg of lusutrombopag or placebo once daily for up to 7 d | Platelet transfusion, or rescue procedure for bleeding not required in 79.2% of lusutrombopag-treated patients and 12.5% of placebo-treated patients | Most common TEAEs: Nausea, pyrexia, headache, pain, and portal vein thrombosis; most common SAE with lusutrombopag was portal vein thrombosis |
| L-PLUS-2; Peck-Radosavljevic et al[17], 2019 | 215 patients; same dosing as L-PLUS-1 | Platelet transfusion, or rescue procedure for bleeding not required in 64.8% of lusutrombopag-treated patients and 29% of placebo-treated patients | Most TEAEs were mild or moderate in severity; four asymptomatic thrombotic events, two each in the lusutrombopag and placebo groups, respectively; none attributed to lusutrombopag |
A recent single-center retrospective review of 47 patients treated with romiplostim perioperatively showed that the median platelet counts improved from 47 × 109/L at the time of romiplostim initiation to 164 × 109/L at the time of surgery. Romiplostim at a dose of 3 μg/kg per week for 2 wk increased the platelet count to over 100 × 109/L in 79% of patients. Additionally, bleeding and thromboembolic events were within acceptable limits in these patients[9]. The large cohort of patients, the fact that patients in this study had a wide variety of etiologies of thrombocytopenia, and that they underwent major surgical procedures, such as open cardiac surgery (unlike previous studies where procedures were minor in nature), makes this study stand out from the rest. However, the facts that this was a retrospective study without randomization, that no uniform platelet count threshold was set prior to proceeding with the medical procedure, that romiplostim doses were not standardized, and that bone marrow evaluation was not performed limits the conclusions that can be drawn[9]. Nevertheless, this study provides evidence for the use of romiplostim perioperatively in patients with chronic thrombocytopenia; although, this remains an off-label use for romiplostim.
Romiplostim treatment was also found to cause partial portal vein thrombosis (PVT), as reported for a 50-year-old woman with Child class B liver cirrhosis and hepatitis C-associated ITP; upon discontinuation of treatment complete recanalization of the portal vein was noted[10]. Based on this case study, caution must be exercised in the use of romiplostim in patients with advanced liver cirrhosis.
Eltrombopag is a small-molecule non-peptide TPO-RA that interacts with the transmembrane domain of the TPO receptor on megakaryocyte precursors and megakaryocytes, leading to increased platelet production[5]. Eltrombopag is metabolized via cleavage, oxidation, and conjugation with glucuronic acid, glutathione, or cysteine. The cytochrome P450 enzymes involved in the oxidation pathway include CTP1A2 and CYP2C8, whereas UGT1A1 and UFT1A3 are involved in the glucuronidation pathway[5]. Eltrombopag increases platelet production after 7 d of repeated dosing, and platelet counts decrease to baseline 1 wk to 2 wk after discontinuing treatment[1]. Eltrombopag is approved for the treatment of thrombocytopenia in adults and children with chronic ITP who have an insufficient response to corticosteroids, immunoglobulins, or splenectomy, in patients with chronic hepatitis C virus infection to allow initiation and maintenance of interferon-based therapy, and in patients with severe aplastic anemia who have an insufficient response to immunosuppressive therapy[5].
The Eltrombopag Evaluated for Its Ability to Overcome Thrombocytopenia and Enable Procedures (referred to as “ELEVATE”) study was a double-blind, randomized, placebo-controlled, Phase 3 trial conducted in 288 patients with CLD and platelet counts below 50 × 109/L to study the ability of eltrombopag to increase platelet counts and to reduce the need for platelet transfusions in patients undergoing an elective invasive procedure[11]. Patients received either 75 mg eltrombopag once daily or placebo. The primary efficacy endpoint was the proportion of patients who did not require a platelet transfusion before, during, and up to 7 d after the elective invasive procedure, and the secondary endpoint was the proportion of patients with bleeding before, during, and up to 7 d after the procedure. A majority of patients in the eltrombopag and placebo groups (62% and 56%, respectively) underwent elective invasive procedures that were in the lowest bleeding risk category. At the end of 2 wk, 59% of patients treated with eltrombopag and 5% of those receiving placebo had an increased platelet count, and primary endpoint was achieved in 72% and 19% of patients, respectively. With the exception of thrombotic events being higher in the eltrombopag group (4% vs 1% in the placebo group), the incidence and severity of AEs were similar in both groups. Serious AEs occurred in 13% and 12% of patients in the eltrombopag and placebo groups, respectively. A total of 8 patients, comprising 6 in the eltrombopag group and 2 in the placebo group, experienced 10 thrombotic events, with 9 of those events involving the portal venous system. It must be noted that none of the 8 total patients with a thrombotic event had received both eltrombopag and a platelet/blood product transfusion, and of the 26 patients who received both eltrombopag and a transfusion, none had a thrombotic event. Additionally, 5 out of the total 8 patients who had a thrombotic event were found to have cancer. However, a separate post hoc analysis identified an association between higher platelet counts (> 200 × 109/L) and an increased risk of thrombotic events[11], and the study was terminated early due to the increased incidence of PVT in the eltrombopag arm. Key efficacy and safety data from the study are outlined in Table 1.
In the absence of better identification of risk factors for the development of thrombotic events, dose optimization, and the risk of PVT in patients with thrombocytopenia and CLD undergoing an elective procedure, eltrombopag must be used with caution and is not recommended as an alternative to platelet transfusions in this group of patients; this is an off-label use of eltrombopag.
Avatrombopag is an oral, small-molecule non-peptide TPO-RA that, like eltrombopag, binds to the TPO receptor to bring out a number of cellular reactions that give rise to megakaryocytic proliferation and differentiation and increased platelet production. Similar to eltrombopag and lusutrombopag[7], it does not compete with TPO for binding to the TPO receptor, and may thus have an additive effect with TPO on platelet production[6,12]. Cytochrome P450 enzymes CYP2C9 and 3A are responsible for metabolizing avatrombopag to corresponding 4-hydroxylated products, with the former enzyme playing the predominant role[13]. It is the first TPO-RA to be approved for use perioperatively to treat thrombocytopenia in adult patients with CLD scheduled to undergo a procedure[6]. Avatrombopag is an orally administered drug that is taken with food for 5 d consecutively prior to an elective procedure. Avatrombopag results in a dose- and exposure-dependent increase in platelet counts in adults within 3-5 d of treatment, with a peak effect occurring after 10-13 d and counts remaining elevated over 50 × 109/L for at least 7 d post-procedure. Baseline levels are achieved after 35 d[6].
There have been two randomized, double-blind, placebo-controlled Phase 3 trials[14] - ADAPT-1 and ADAPT-2 - with identical designs that have studied the effectiveness and safety of avatrombopag in reducing the need for platelet transfusion and rescue from bleeding in patients with CLD scheduled for a procedure. Patients were screened to exclude several criteria, including presence of arterial or venous thrombosis and a portal vein blood flow below 10 cm/s. Included patients were divided into low and high baseline platelet count cohorts and received 60 mg/d and 40 mg/d of avatrombopag, respectively. The primary endpoint of these studies was the proportion of patients who did not require a platelet transfusion or rescue procedure for bleeding after randomization and up to 7 d after a scheduled procedure. Secondary efficacy endpoints included the proportion of patients achieving the target platelet count of ≥ 50 × 109/L on the day of the procedure and the mean change in platelet count from baseline to procedure day. Patients treated with avatrombopag demonstrated increased platelet counts that were approximately 30-50 × 109/L higher on average compared with patients treated with placebo before minor invasive procedures, with a net doubling of counts on average. There were no meaningful differences in AEs of special interest among the avatrombopag-treated and placebo-treated patients, including thromboembolism, and the rates of occurrence of AEs across both the studies were comparable in the avatrombopag- and placebo-treated groups[14]. A treatment-emergent AE (TEAE) of partial PVT was observed in 1 avatrombopag-treated patient in the high baseline platelet count cohort in the ADAPT-2 trial at 13 d after the last avatrombopag dose and was assessed as nonserious and possibly related to the drug. Thromboembolic events were also observed in 2 placebo-treated patients in the ADAPT-2 trial, one being an acute myocardial infarction and the other a disseminated intravascular coagulation/ pulmonary embolus. Thromboembolic TEAEs were not noted in the ADAPT-1 trial. One non-TEAE of PVT was observed in the avatrombopag group (60 mg) at 31 d after the final dose of avatrombopag and was assessed as serious but not related. Table 1 outlines the key efficacy and safety results from these trials.
Results from a pharmacokinetic modeling study have shown that repeated dosing of avatrombopag as early as 12 d after completion of the first dosing can increase platelet counts to within safe limits[15]. Saab et al[16] have recently reported that repeated dosing of avatrombopag in 4 patients with CLD and thrombocytopenia led to a 2.3-fold mean increase in platelet count after the first dosing and 2.6-fold mean increase after the second dosing. The patients underwent procedures within 9-13 d after starting avatrombopag, with repeat dosing performed at least 30 d after completing the first dose. None of the patients required platelet transfusions or rescue therapy, or had TEAEs, and repeated dosing did not reduce the efficacy of avatrombopag[16]. Avatrombopag dosing has the ability to limit the magnitude and duration of increase in patient platelet counts and this predictable pharmacokinetic/pharmacodynamic profile could potentially be contributing to the limited risk of thromboembolic events associated with this treatment[14].
Lusutrombopag is an orally administered, small-molecule non-peptide TPO agonist that activates the signal transduction pathways in a manner similar to endogenous TPO (while not competing for the same binding site), leading to increased platelet production[6-7]. Lusutrombopag is metabolized via the β- and ω-oxidation pathways and glucuronidation. The cytochrome P450 enzymes are mainly responsible for its metabolism, including CYP4A11[13]. The mean maximum platelet count following treatment with 3 mg/d lusutrombopag was 86.9 × 109/L and the median time to reach this was 12 d[7].
Data from two Phase 3 double-blind studies, L-PLUS-1 and L-PLUS-2, have shown lusutrombopag to be effective in raising platelet counts in patients with CLD and thrombocytopenia prior to undergoing an elective invasive procedure. The primary endpoint of both trials was the proportion of patients who avoided pre-procedure platelet transfusions and rescue therapy for bleeding, as assessed from randomization through 7 d post-procedure[17,18]. Key secondary endpoints included the number of patients who required no platelet transfusions during the study, the proportion of patients who achieved a platelet count of ≥ 50 × 109/L with an increase of ≥ 20 × 109/L from baseline at any time during the study, and the number of days during which the platelet count was maintained at ≥ 50 × 109/L[17]. Safety assessments included the incidence of AEs, adverse drug reactions, bleeding-related AEs, and thrombotic events. Patients treated with lusutrombopag perioperatively demonstrated improvements in platelet counts similar to those seen with avatrombopag after 7 d of treatment. In the L-PLUS-1 trial, one serious AE of PVT, possibly related to lusutrombopag treatment, was reported in the lusutrombopag-treated group and one mild incidence of superior mesenteric vein thrombosis was reported in the placebo group that was considered not related to the study drug. As neither event was associated with an extremely high platelet count, and the incidence of thrombotic events were similar in both treatment groups, the authors concluded that no further monitoring for thromboembolic events was required in lusutrombopag-treated patients. In the L-PLUS-2 trial, four TEAEs were reported, two each in the lusutrombopag- and placebo-treated groups, respectively. All events were asymptomatic and deemed to be consistent with the findings of a meta-analysis that suggested the prevalence of PVT in patients with CLD and thrombocytopenia. Table 1 outlines the key safety and efficacy results from these trials.
A recent retrospective report of 1760 cirrhotic patients with low platelet counts[2] found that 66% of patients with platelet counts below 50 × 109/L required platelet transfusions for radiofrequency ablation, 43% for transarterial chemoembolization, and 55% for endoscopic injection sclerotherapy/endoscopic variceal ligation. When 25 of these patients were administered lusutrombopag prior to the procedures, platelet counts increased significantly compared with baseline levels (82 × 109/L vs 41 × 109/L), and of these patients, only 4 needed platelet transfusions before the procedures. The proportion of lusutrombopag-treated patients who required platelet transfusions was significantly lower (16%) compared with patients not treated with lusutrombopag (54%)[2]. Lusutrombopag was also found to be more effective in raising platelet counts in patients with baseline platelet counts above 30 × 109/L compared with patients with baseline platelet counts less than or equal to 30 × 109/L and spleen index greater than or equal to 40 cm2[2]. The authors of this retrospective study reported 1 case of portal thrombosis in the lusutrombopag group, which was revealed via a routine CT scan taken on the 12th d of treatment. The causal relationship of thrombosis with lusutrombopag treatment was unclear[2].
Sato et al[19] were the first to report the efficacy of repeated administration of lusutrombopag (3 mg daily) in a patient with hepatocellular carcinoma who had two planned invasive procedures, radiofrequency ablation and transarterial chemoembolization. In the first instance, lusutrombopag was discontinued after 5 d, as the platelet count increased to 98 × 109/L by day 5, while in the second instance, the patient required the full 7-d treatment to achieve a platelet count above 50 × 109/L. No AEs such as portal thrombus were reported during either of the treatments. More recently, Ishikawa et al[20] reported the safety and efficacy of repeated use of lusutrombopag to increase platelet counts in 8 patients with hepatocellular carcinoma and thrombocytopenia prior to repeated scheduled radiofrequency ablation procedures. Platelet counts increased from 42500 ± 5200/µL at baseline to 103100 ± 22800/µL at day 14 after first treatment, and from 43800 ± 6000/µL at baseline to 110700 ± 17800/µL at day 14 after repeat treatment, demonstrating that the repeated use of lusutrombopag does not decrease its effect on platelet counts. None of the patients developed clinical symptoms such as thrombosis or PVT[20].
Hetrombopag is a small-molecule non-peptide TPO-RA, with a mechanism of action similar to eltrombopag but with an in vivo pharmacological effect that is 8-10 times that of eltrombopag[21]. Based on animal studies, it appears that hetrombopag is primarily metabolized via glucuronidation and hydrolyzed into aglycone following excretion with bile acid[21]. A Phase 1 study demonstrated that hetrombopag is safe and well-tolerated in healthy subjects and can be a potential candidate for the treatment of patients with chronic ITP[21]. It is now in Phase 2 trials for the treatment of ITP. Currently, there are no clinical studies underway to examine the effectiveness and safety of hetrombopag as perioperative treatment of thrombocytopenia in patients with CLD.
Romiplostim and eltrombopag are currently approved for management of chronic ITP but there are studies (like the ones outlined earlier) demonstrating their efficacy in other indications. For instance, romiplostim has demonstrated efficacy in treating chemotherapy-induced thrombocytopenia and myelodysplastic syndromes[22]. Eltrombopag has been shown to be effective in the treatment of hepatitis C infection (where it raises the platelet counts enough to allow continued treatment with ribavirin and pegylated-interferon), myelodysplastic syndrome, acute myeloid leukemia, and severe aplastic anemia[23]. Both avatrombopag and lusutrombopag have been approved for use perioperatively to treat thrombocytopenia in patients with CLD. Recently, a supplemental New Drug Application was filed for avatrombopag as therapy for the treatment of chronic ITP; meanwhile, the trial for avatrombopag as therapy in chemotherapy-induced thrombocytopenia (NCT03471078) is still underway. A trial to study the safety and efficacy of hetrombopag (NCT03222843) for the treatment of chronic ITP is currently underway.
Patients with thrombocytopenia and CLD are at an increased risk of bleeding. Platelet transfusions can be administered in these patients prophylactically, prior to scheduled medical procedures to minimize the risk of bleeding, or therapeutically to control bleeding. The goal of any prophylactic treatment is limiting hemorrhagic events in the peri- and post-operative surgical setting. However, there is uncertainty in prophylactic treatment of thrombocytopenia, as there are no effective tools that can predict bleeding risk in patients, and data on the correlation between thrombocytopenia and the risk of bleeding are equivocal[24,25]. While platelet transfusion is a primary option in these patients, the decision to undergo transfusion can be a complicated one.
There is a lack of consensus among guidelines related to platelet transfusion in patients, primarily due to lack of sufficient and supportive data to substantiate the recommended platelet transfusion triggers[26]. Further, there are no guidelines that provide recommendations for platelet transfusion specifically for patients with CLD prior to an elective procedure. The variations in the recommended platelet threshold values before invasive procedures often depend on the type of patient and the perceived risk of the procedure. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines[27], which are not specific to gastrointestinal bleeding, state that the minimum threshold platelet count for undergoing upper gastrointestinal endoscopy has not been established, but they also point out that there are publications supporting 20 × 109/L and 50 × 109/L as the threshold for platelet counts prior to diagnostic procedures and biopsies, respectively. The American Association for the Study of Liver Diseases guidelines for transcutaneous or transvenous liver biopsy state that platelet transfusions should be considered when platelet counts are less than 50-60 × 109/L[28], and the ASGE guidelines recommend platelet transfusions only in case of severe thrombocytopenia[29]. The current American Society of Hematology guidelines recommend the use of corticosteroids as first-line treatment in newly diagnosed adults with a platelet count less than 30 × 109/L who are symptomatic or have minor mucocutaneous bleeding. In adults with ITP ≥ 3 mo who are corticosteroid-dependent or unresponsive to corticosteroids, the guidelines suggest treatment with TPO-RA eltrombopag or romiplostim. The guidelines recommend second-line treatment to be individualized, based on duration of disease and patient preferences; however, they provide no recommendations regarding the use of therapies prior to undergoing an invasive procedure or treatment[30].
The updated (2019) International Consensus Report on Management of ITP recommends target platelet counts above 50 × 109/L for minor surgery, above 80 × 109/L for major surgery, and above 100 × 109/L for major neurosurgery[31]. The National Institute for Health and Care Excellence guidelines recommend prophylactic platelet transfusions in patients who are having invasive procedures or surgery, in order to raise the platelet counts above 50 × 109/L. They advocate a higher threshold (50-60 × 109/L) for patients with a high risk of bleeding who are having invasive procedures or surgery after taking into account the type of procedure, the cause of thrombocytopenia, any coexisting causes of abnormal hemostasis, and whether the patient’s baseline platelet count is falling. These guidelines also recommend prophylactic platelet transfusions to raise the platelet counts above 100 × 109/L in patients having surgery at critical sites, such as the central nervous system, whereas they do not recommend transfusions for patients having procedures with a low risk of bleeding, such as central venous cannulation or bone marrow aspiration[32]. The American Association of Blood Banks guidelines recommend prophylactic platelet transfusion for minor invasive procedures, such as central venous catheter placement at platelet counts ≤ 20 × 109/L and for major non-neuraxial surgery at platelet counts ≤ 50 × 109/L, but do not make recommendations for bleeding patients with thrombocytopenia[29].
The choice of initiating platelet transfusions can also be complicated due to the associated risks. Platelet transfusions have been associated with a number of blood transfusion reactions, the possibility of fatal complications (i.e. sepsis), proinflammatory responses, febrile non-hemolytic reactions, and acute lung injury[33]. There is also the risk for development of refractoriness that can prevent further platelet transfusions which can, in turn, lead to decreased survival, prolonged hospital stays, and increased healthcare costs[13,34]. A major limitation of platelet transfusions is the short lifespan of platelets, which require use to occur within 4 d of obtainment to prevent bacterial growth[33]. There is also a shortage of platelets currently, due to the decline in blood collection and utilization[35].
While platelet transfusions can effectively increase platelet counts in the perioperative setting, factors such as alloimmunization or personal beliefs (e.g. Jehovah’s Witnesses), suggest a need for alternatives[9].
The treatment of patients with thrombocytopenia who require a medical procedure is variable and uncertain, as there are limited data to inform decisions. The aim of the treating physician is to minimize bleeding and improve clinical outcomes. Even though guidelines have no consensus on the threshold for platelet transfusion, most do support raising the platelet levels (to varying degrees) prior to procedures. This, combined with the risk associated with platelet transfusions, may make TPO-RAs an alternative to platelet transfusions in patients with CLD.
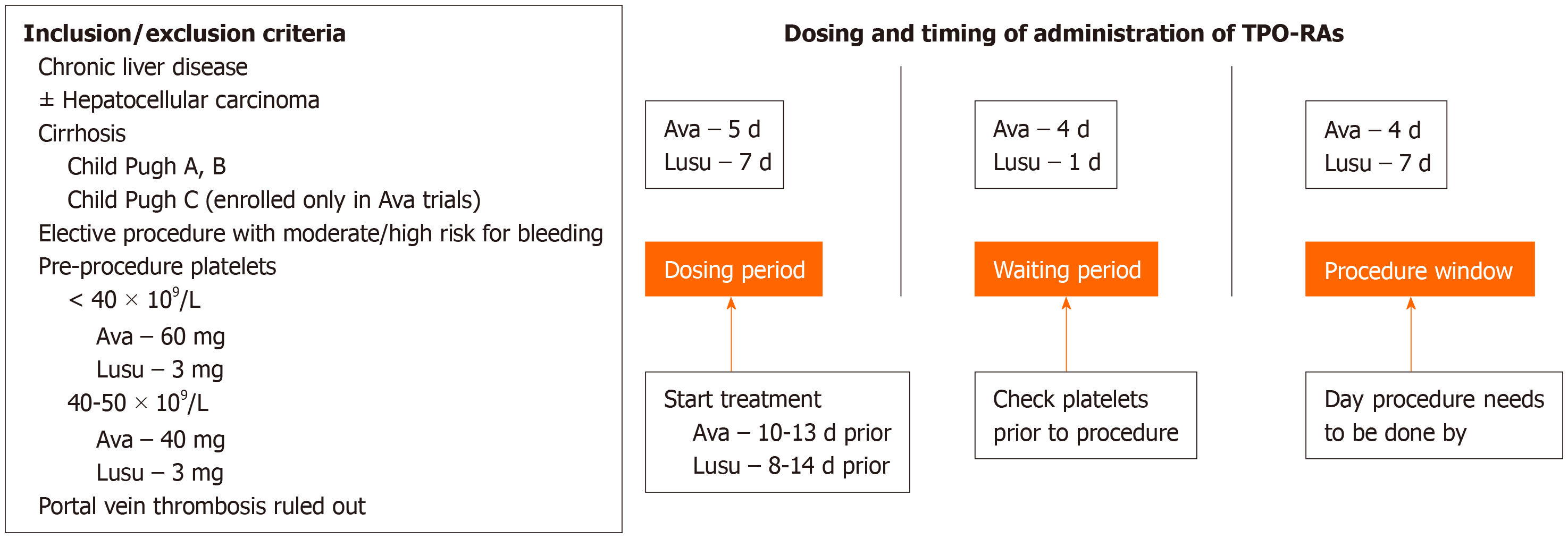
An ideal treatment for thrombocytopenia in patients with CLD undergoing an invasive procedure would have the following characteristics: (1) Effectiveness; (2) Oral bioavailability; (3) Minimal adverse effects; and (4) Cost-effectiveness. Neither platelet transfusions nor TPO-RAs satisfy all these requirements. Table 2 lists the key safety and tolerability information for each TPO-RA approved for use. Unlike platelet transfusions, TPO-RAs are not capable of increasing platelet counts rapidly, as the platelet counts increase approximately 5-7 d after and peak approximately 10-14 d after administration of a TPO-RA[36]. Therefore, one potential disadvantage of TPO-RAs is the lag time between dosage and increased platelet numbers, as it prevents the use of TPO-RAs in emergent situations. The advantages of TPO-RAs include oral bioavailability (except in case of romiplostim, which is administered subcutaneously), their ability to increase platelet counts endogenously, and sustaining the increase over a longer duration compared to that produced by platelet transfusions and thereby reducing the need for rescue therapy while causing fewer procedure-related complications than platelet transfusions. Avatrombopag and lusutrombopag are currently the only TPO-RAs approved for the perioperative treatment of thrombocytopenia in patients with CLD, and compared with romiplostim and eltrombopag, they are associated with a decreased risk of thromboembolic and hepatic events. Nevertheless, platelet counts must be measured during treatment, as counts higher than 200 × 109/L have been shown to be associated with serious AEs, such as PVT. Both avatrombopag and lusutrombopag may be used in cirrhotic patients with low platelet counts scheduled to undergo an elective procedure with bleeding risks and in patients who cannot undergo platelet transfusions (Figure 1). Depending on the pre-procedure platelet counts, patients should be treated with either 40 mg or 60 mg of avatrombopag; the dose of lusutrombopag is always 3 mg. The window for undergoing procedures following dosing with avatrombopag and lusutrombopag is between 9-13 d and 8-14 d, respectively. It must be noted that while clinical trials with avatrombopag and lusutrombopag excluded patients with PVT, a PVT-prescreen is not a requirement as per labeling guidelines for both these TPO-RAs. Patients with liver cirrhosis and high Child-Turcotte-Pugh scores are more likely to have severe thrombocytopenia and also require invasive diagnostic procedures. Extreme caution must be taken when using TPO-RAs in such patients, as they are at high risk of developing PVT; currently, no data on the effects of the TPO-RAs avatrombopag and lusutrombopag in this class of patients are available, as they were excluded from the clinical trials[14,16-18].
| Drug | Indication | Boxed warning | AEs | Use in special populations |
| Romiplostim | Treatment of thrombocytopenia in patients with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy | None | Thrombotic and thromboembolic events; bone marrow fibrosis; most common AEs: Arthralgia, dizziness, insomnia, myalgia, pain in extremity, abdominal pain, shoulder pain, dyspepsia, and paresthesia, headache | Not to be used in pregnancy and during lactation; safety and effectiveness in pediatric patients has not been established; use in geriatric patients with dose adjustment |
| Eltrombopag | Treatment of thrombocytopenia in adult and pediatric patients 1 yr and older with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy; treatment of thrombocytopenia in patients with chronic hepatitis C to allow the initiation and maintenance of interferon-based therapy; treatment of patients with severe aplastic anemia who have had an insufficient response to immunosuppressive therapy | Risk for hepatic decompensation in patients with chronic hepatitis C; risk of hepatotoxicity | Thrombotic and thromboembolic events; hepatoxicity; monitor liver function before and during therapy; increased risk of death and progression of MDS to AML; common AEs in patients with ITP: Nausea, diarrhea, upper respiratory tract infection, vomiting, increased ALT, myalgia and urinary tract infection | Not to be used in pregnancy and during lactation; safety and effectiveness in pediatric patients 1 yr and older with chronic ITP has been established; use in geriatric patients has not been studied in clinical trials; reduced initial dose recommended for patients of East Asian ancestry with ITP |
| Avatrombopag | Treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure | None | Thrombotic and thromboembolic complications: Monitoring platelet counts essential; common AEs: Pyrexia, abdominal pain, nausea, headache, fatigue, and edema peripheral | Not to be used in pregnancy and during lactation; safety and effectiveness in pediatric patients has not been established; use in geriatric patients has not been studied in clinical trials |
| Lusutrombopag | Treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure | None | Thrombotic and thromboembolic complications: Monitoring platelet counts essential; most common AE: headache | Not to be used in lactating patients; use in geriatric patients has not been studied in clinical trials; safety and effectiveness in pediatric patients has not been established |
There are also pricing differences between avatrombopag and lusutrombopag; in the United States, the wholesale acquisition cost (WAC) of avatrombopag for a 5-d course is $2970 for the 40-mg dose and $4455 for the 60-mg dose. The WAC for a 7-d course of lusutrombopag is $8500[37].
Recommendations for procedure-specific minimum platelet counts prior to a scheduled procedure often have inadequate supporting evidence, and the benefit of prophylactic platelet transfusion remains unclear. Physicians typically assess the need for platelet transfusions on a patient-to-patient basis, relying on their own experience and patient comorbidities to inform their decisions. TPO-RAs are a safe and effective alternative treatment option for patients with thrombocytopenia who are undergoing a medical procedure. They are associated with increased platelet counts, decreased bleeding events, and a reduced need for rescue treatments. Recent studies also support the safe and efficacious repeated use of TPO-RAs in patients with CLD. The exact fit of TPO-RAs, as compared with platelet transfusions, in patients with thrombocytopenia prior to undergoing a medical procedure is still unclear. Comparative clinical trial data in patients with thrombocytopenia are required to assess the efficacy, safety, cost-effectiveness, and impact on patient quality of life of TPO-RA use compared with the traditional use of platelet transfusions prior to scheduling a medical procedure. In the meantime, treatment decisions must be individualized by establishing the advantages and disadvantages of both these treatment options on a case-by-case basis.
Conflict of interest statement: Both authors report no conflict of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding MX, Moretti R S-Editor: Wang JL L-Editor: A E-Editor: Qi LL
| 1. | Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin Hematol. 2013;50 Suppl 1:S18-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, Yasui Y, Tamaki N, Takaura K, Komiyama Y, Higuchi M, Kubota Y, Wang W, Okada M, Shimizu T, Watakabe K, Enomoto N, Izumi N. Real-life experience of lusutrombopag for cirrhotic patients with low platelet counts being prepared for invasive procedures. PLoS One. 2019;14:e0211122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Moussa MM, Mowafy N. Preoperative use of romiplostim in thrombocytopenic patients with chronic hepatitis C and liver cirrhosis. J Gastroenterol Hepatol. 2013;28:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Amgen Inc. Nplate [package insert]. Thousand Oaks: Amgen Inc., 2017. |
| 5. | Novartis Pharmaceuticals Corporation. Promacta [package insert]. East Hanover: Novartis Pharmaceuticals Corporation, 2018. |
| 6. | Dova Pharmaceuticals, Inc. Doptelet [package insert]. Durham: Dova Pharmaceuticals, Inc., 2018. |
| 7. | Shionogi, Inc. Mulpleta [package insert]. Florham Park: Shionogi, Inc., 2018. |
| 8. | Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, Mumford AD, Stanworth SJ, Tinegate H; British Committee for Standards in Haematology. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 335] [Article Influence: 37.2] [Reference Citation Analysis (1)] |
| 9. | Al-Samkari H, Marshall AL, Goodarzi K, Kuter DJ. Romiplostim for the management of perioperative thrombocytopenia. Br J Haematol. 2018;182:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Dultz G, Kronenberger B, Azizi A, Mihm U, Vogl TJ, Sarrazin U, Sarrazin C, Zeuzem S, Hofmann WP. Portal vein thrombosis as complication of romiplostim treatment in a cirrhotic patient with hepatitis C-associated immune thrombocytopenic purpura. J Hepatol. 2011;55:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, Campbell F, Hyde D, Brainsky A, Theodore D; ELEVATE Study Group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 12. | Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109:4607-4616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Bankston PC, Al-Horani RA. New small molecule drugs for thrombocytopenia: chemical, pharmacological, and therapeutic use considerations. Int J Mol Sci. 2019;20:3013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, Allen LF, Hassanein T. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Hayes S, Farrell C, Aggarwal K, Vredenburg M, Allen LF. PK simulation of avatrombopag-induced increases in platelet counts with redosing in patients with thrombocytopenia and chronic liver disease. Res Pract Thromb Hemost. 2018;2:665. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Saab S, McDaniel TK, Bau SN, Patel RP. Efficacy of repeat doses of avatrombopag: a case series. Dig Med Res. 2019;2:9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Peck-Radosavljevic M, Simon K, Iacobellis A, Hassanein T, Kayali Z, Tran A, Makara M, Ben Ari Z, Braun M, Mitrut P, Yang SS, Akdogan M, Pirisi M, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, Afdhal N. Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2). Hepatology. 2019;70:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Hidaka H, Kurosaki M, Tanaka H, Kudo M, Abiru S, Igura T, Ishikawa T, Seike M, Katsube T, Ochiai T, Kimura K, Fukuhara T, Kano T, Nagata T, Tanaka K, Kurokawa M, Yamamoto K, Osaki Y, Izumi N, Imawari M. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol. 2019;17:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Sato S, Miyake T, Kataoka M, Isoda K, Yazaki T, Tobita H, Ishimura N, Kinoshita Y. Efficacy of repeated lusutrombopag administration for thrombocytopenia in a patient scheduled for invasive hepatocellular carcinoma treatment. Intern Med. 2017;56:2887-2890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Ishikawa T, Okoshi M, Tomiyoshi K, Kojima Y, Horigome R, Imai M, Nozawa Y, Iwanaga A, Sano T, Honma T, Yoshida T. Efficacy and safety of repeated use of lusutrombopag prior to radiofrequency ablation in patients with recurrent hepatocellular carcinoma and thrombocytopenia. Hepatol Res. 2019;49:590-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Zheng L, Liang MZ, Zeng XL, Li CZ, Zhang YF, Chen XY, Zhu X, Xiang AB. Safety, pharmacokinetics and pharmacodynamics of hetrombopag olamine, a novel TPO-R agonist, in healthy individuals. Basic Clin Pharmacol Toxicol. 2017;121:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Giagounidis A, Mufti GJ, Fenaux P, Sekeres MA, Szer J, Platzbecker U, Kuendgen A, Gaidano G, Wiktor-Jedrzejczak W, Hu K, Woodard P, Yang AS, Kantarjian HM. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120:1838-1846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Gill H, Wong RSM, Kwong YL. From chronic immune thrombocytopenia to severe aplastic anemia: recent insights into the evolution of eltrombopag. Ther Adv Hematol. 2017;8:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, Dienstag JL; HALT–C Trial Group. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 25. | Basili S, Raparelli V, Napoleone L, Talerico G, Corazza GR, Perticone F, Sacerdoti D, Andriulli A, Licata A, Pietrangelo A, Picardi A, Raimondo G, Violi F; PRO-LIVER Collaborators. Platelet count does not predict bleeding in cirrhotic patients: results from the PRO-LIVER study. Am J Gastroenterol. 2018;113:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 26. | Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program. 2013;2013:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | ASGE Standards of Practice Committee, Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (2)] |
| 28. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 29. | Laine L. Treatment of thrombocytopenic patients with GI bleeding. Gastrointest Endosc. 2018;88:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, Kühne T, Kuter DJ, Lim W, McCrae KR, Pruitt B, Shimanek H, Vesely SK. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829-3866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 800] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 31. | Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ, Grainger J, Hou M, Kruse C, McDonald V, Michel M, Newland AC, Pavord S, Rodeghiero F, Scully M, Tomiyama Y, Wong RS, Zaja F, Kuter DJ. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780-3817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 695] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 32. | National Institute for Health and Care Exellence. Blood transfusion. Available from: https://www.nice.org.uk/guidance/ng24. |
| 33. | Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017;23:3228-3239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Brown RS. Review article: a pharmacoeconomic analysis of thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Ellingson KD, Sapiano MRP, Haass KA, Savinkina AA, Baker ML, Chung KW, Henry RA, Berger JJ, Kuehnert MJ, Basavaraju SV. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017;57 Suppl 2:1588-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Nagrebetsky A, Al-Samkari H, Davis NM, Kuter DJ, Wiener-Kronish JP. Perioperative thrombocytopenia: evidence, evaluation, and emerging therapies. Br J Anaesth. 2019;122:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Elsevier. ProspectoRx. Available from: https://www.elsevier.com/solutions/prospectorx. |
| 38. | Basu PP NT, Farhat S, James Shah N, Jafri M, Foustin S. Single use of romiplostim thrombopoietin analogue in severe thrombocytopenia for outpatient percutaneous liver biopsy in patients with chronic liver disease-A randomized double blinded prospective trial. J Hepatol. 2012;56:S38. [DOI] [Full Text] |
| 39. | Marshall AL, Goodarzi K, Kuter DJ. Romiplostim in the management of the thrombocytopenic surgical patient. Transfusion. 2015;55:2505-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |