Published online Feb 28, 2020. doi: 10.13105/wjma.v8.i1.27
Peer-review started: June 24, 2019
First decision: August 27, 2019
Revised: September 26, 2019
Accepted: October 20, 2019
Article in press: October 20, 2019
Published online: February 28, 2020
Processing time: 252 Days and 8.4 Hours
Many clinical studies for the long-term survival or efficacy of capecitabine plus oxaliplatin (XELOX) in colon cancer have already been studied, but its clinical benefit is controversial.
To evaluate the long-term efficacy of XELOX regimen in comparison with other adjuvant chemotherapy protocols in colon cancer.
By searching the PubMed, EMBASE and Cochrane databases, a total of 12 randomized controlled trials involving 6698 stage III colon cancer cases (XELOX protocol: n = 3298 cases; other adjuvant chemotherapy protocol: n = 3268 cases) were included. The parameter outcomes included the overall survival and the disease-free survival. The quality control of selected literature was based on the Jadad scale and the GRADE system.
In comparison to other adjuvant chemotherapy regimen, XELOX regimen showed a better overall survival (odds ratio = 1.29, 95% confidence interval: 1.15-1.44, P < 0.0001) and a better disease-free survival (odds ratio = 1.32, 95% confidence interval: 1.18-1.46, P < 0.0001) for colon cancer patients, suggesting the XELOX regimen can be a good option for postoperative treatment of stage III colon cancer.
The XELOX regimen can be a preferred option for adjuvant treatment of stage III colon cancer after surgery.
Core tip: Many clinical studies for the long-term survival of patients or the efficacy of capecitabine plus oxaliplatin (XELOX) in colon cancer have already been studied, but its clinical benefit is controversial. The long-term efficacy of the XELOX regimen in comparison with other adjuvant chemotherapy protocols in colon cancer was evaluated. By searching the PubMed, EMBASE and Cochrane databases, a total of 12 randomized controlled trials involving 6698 stage III colon cancer cases were included. Our findings showed that XELOX regimen had a better overall survival and a better disease-free survival. The XELOX regimen can be a preferred option for adjuvant treatment of stage III colon cancer after surgery.
- Citation: Fu HT, Xu YY, Tian JJ, Fu JX, Nie SL, Tang YY, Chen P, Zong L. Long-term efficacy of capecitabine plus oxaliplatin chemotherapy on stage III colon cancer: A meta-analysis. World J Meta-Anal 2020; 8(1): 27-40
- URL: https://www.wjgnet.com/2308-3840/full/v8/i1/27.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i1.27
Colorectal cancer (CRC) accounts for 9% of all cancers worldwide. It is the second most common cancer in women and the third most frequent cancer in men. More than 70% of the deaths associated with CRC are caused by metastasis to the liver. Although surgery may be potentially curable, less than 25% of cases can be managed with a recurrence rate of up to 70%[1]. The purpose of colon cancer treatment is to cure locally and to prevent metastasis and recurrence. Therefore, in the local excision of colon cancer at the same time, the treatment should be according to individual condition, and chemotherapy is an important method that is based on the patient’s condition, surgical situation and clinical stage of appropriate postoperative adjuvant chemotherapy.
Many clinical studies for the long-term survival benefit or efficacy of capecitabine plus oxaliplatin (XELOX) in colon cancer have already been studied[2-4], but its clinical benefit is controversial[5]. Since the 1990s, the introduction of irinotecan or oxaliplatin has extended the spectrum of therapeutic options. The combination of oxaliplatin or irinotecan with 5-fluorouracil (5-FU) plus leucovorin (LV or FA) has been considered the standard regimen for first-line treatment of metastatic CRC. However, this is an inconvenient therapeutic option due to the requirement for continuous vascular infusion of 5-FU. A retrospective study on XELOX plus bevacizumab vs LV plus 5-FU plus irinotecan (also known as FOLFIRI) plus bevacizumab treatment for metastatic colon cancer reported that XELOX plus bevacizumab was more effective in response rate and overall survival (OS) compared with LV plus 5-FU plus irinotecan plus bevacizumab[6].
Capecitabine is an orally administered fluoropyrimidine that was rationally designed to generate 5-FU preferentially at the tumor site. Capecitabine demonstrated a safety profile superior to that of 5-FU/LV, with a significantly lower incidence of diarrhea, stomatitis, nausea, alopecia and grade 3/4 neutropenia. Also, oral administration of capecitabine simplifies chemotherapy and provides convenient outpatient therapy. Because capecitabine has been adopted as a substitute for infused 5-FU/LV to overcome the inconvenience of 5-FU, subsequent data have found XELOX (also known as CAPOX) to be a comparable therapeutic regimen to infused 5-FU/LV plus oxaliplatin (known as FOLFOX-4 or FUOX). In the Loree et al[7] study, XELOX and FOLFOX were compared in the treatment of colon cancer. The results showed that XELOX may be associated with improved disease-free survival (DFS) despite greater toxicities and reduced adjuvant chemotherapy duration to 3 mo. In a safety analysis of adjuvant chemotherapy for stage III colon cancer after radical resection of stage III colon cancer, mFOLFOX6/XELOX regimens are acceptable[8].
Randomized phase III trials demonstrated that outcomes using first-line XELOX are comparable with those achieved using continuous infusion of 5-FU and FOLFOX. There are many chemotherapy options for advanced CRC, and the long-term benefit is uncertain. Combining XELOX is advantageous for the reasons as follows: Synergistic effects, no overlapping toxicities, easy to administer and outpatient management[9-12]. XELOX has been studied extensively in rectal cancer where the standard therapy is XELOX plus radiation therapy. To determine the efficacy of XELOX in colon cancer, the long-term efficacy of capecitabine combined with oxaliplatin (XELOX regimen) in comparison with other adjuvant chemotherapy protocols was evaluated.
This meta-analysis is in terms of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 declaration.
Two researchers independently retrieved randomized controlled trials (RCT) articles involved in oxaliplatin combined with capecitabine in CRC published in PubMed, EMBASE, Cochrane, web of science, clinical trial and China National Knowledge Infrastructure databases from 1991 to August 2017. The retrieval languages were Chinese and English. The retrieval was performed using the following keywords. English search terms for: (PubMed): Search (((Colonic Neoplasms [MeSH Terms]) OR ((((((((((Colonic Neoplasm [Title/Abstract]) OR Colon Neoplasm* [Title/Abstract]) OR Neoplasm*, Colonic) OR Neoplasm*, Colon [Title/Abstract]) OR Cancer of Colon [Title/Abstract]) OR Cancer of the Colon [Title/Abstract]) OR Colonic Cancer* [Title/Abstract]) OR Cancer*, Colonic [Title/Abstract]) OR Colon Cancer* [Title/Abstract]) OR Cancer*, Colon [Title/Abstract]))) AND (((((((((((ECX) [Title/Abstract] OR XELOX) [Title/Abstract] OR Xeloda) [Title/Abstract] OR Capecitabine [Title/Abstract])) ORN (4) - pentyloxycarbonyl – 5’ - deoxy- 5- fluorocytidine [Title/Abstract]) OR Capecitabine)) OR Capecitabine [MeSH Terms]) AND (((oxaliplatin [MeSH Terms]) OR oxaliplatin [Title/Abstract]) OR ((((((((((((((((1, 2 - diamminocyclohexane (trans-1) oxolatoplatinum (II) [Title/Abstract]) OR oxalato- (1,2-cyclohexanediamine) platinum II [Title/Abstract]) OR L-OHPcpd [Title/Abstract]) OR oxaliplatine [Title/Abstract]) OR1,2-diaminocyclohexane platinum oxalate [Title/Abstract]) OR platinum (II)-1,2-cyclohexanediamine oxalate [Title/Abstract]) OR cis-oxalato-(trans-l)-1,2-diaminocyclohexane - platinum (II) [Title/Abstract]) ORoxaliplatin, (SP-4-3-(cis))-isomer [Title/Abstract]) OR oxaliplatin, (SP-4-2-(1R-trans)) - isomer [Title/Abstract]) OR oxaliplatin, (SP - 4 - 2 - (1S-trans)) -isomer [Title/Abstract]) OR ACT-078 [Title/Abstract]) OR ACT-078 [Title/Abstract]) OR Eloxatine [Title/Abstract]) OR Sanofi Synthelabo brand of oxaliplatin [Title/Abstract]) OR Sanofi brand of oxaliplatin [Title/Abstract]) OR Eloxatin [Title/Abstract])))). Chinese search terms for: Capecitabine, oxaliplatin, XELOX, colon cancer.
Literature was retrieved and screened in accordance with the PRISMA guidelines. Two reviewers independently screened literature and abstracts based on predefined inclusion and exclusion criteria and screened the full text if necessary. In the literature that met the inclusion criteria, two reviewers used a unified data extraction table to independently extract data. Disagreements were resolved through consultation or by a third researcher.
The inclusion criteria was: (1) Experimental design for RCT; (2) The research object for the pathological diagnosis of patients with CRC; (3) The experimental group intervention for capecitabine combined with oxaliplatin, the control group for other chemotherapeutic drugs; (4) Observation results for patients with long-term efficacy: OS and DFS; (5) If the study included many cases, then only select the required part; and (6) The selected patients were stage III colon cancer patients and had undergone surgery without neoadjuvant chemotherapy.
Studies were excluded if: (1) Non-RCT; (2) Subjects included rectal cancer; (3) DFS or OS was not compared with two chemotherapy regimens in the same trial; (4) No specific data were provided; and/or (5) Repeated publication.
Data for each article was extracted, including the first author and title of the RCT, sample size, follow-up time, publication time, medication regimen, DFS and OS.
Methodological quality and statistical analysis of the RCTs were evaluated with the following criteria. The offset assessment of a single study was evaluated by two independent researchers. Any disagreements were evaluated by a third researcher. Quality evaluation mainly included random sequence generation, randomization concealment, blindness, withdrawal and withdrawal, and the Jadad scale was used to evaluate the score. We defined 1-3 points as low quality and 4-7 points as high quality. At the same time, the meta-analysis software Review Manager Version 5.3 recommended by the Cochrane library was used to test the heterogeneity and calculate the combination of odds ratio (OR) value and 95% confidence interval (CI).
Statistical detection of P values less than 0.05 were considered statistically significant. The heterogeneity of the study was determined by the t test and the I2 test. The unified random effect model was used for consolidation. I2 < 50% were considered as no statistical heterogeneity among the studies, and a random effect model was used to merge effects. Conversely, the random effect model was used to combine the effects. The output combined the OR value and the 95%CI and tests the merging statistic. The Z test was used. The test level was α = 0.05.
Sensitivity analysis included a sensitivity analysis performed in subgroup analyses. If a document was excluded and the impact on the overall outcome was greater, then the literature was reread and the quality was evaluated. Then it was determined whether it was eventually incorporated.
The literature was included and the GRADE system was used to assess methodological quality. In order to thoroughly reveal the source of heterogeneity, we also conducted meta regression and subgroup analysis. In addition, the funnel plot and application of shear reinforcing method (if the funnel plot was asymmetric or incomplete, then there was publication bias, and shear reinforcing method was applied; symmetry indicates that the publication bias is less likely without using the shear reinforcing method). Begg’s funnel plots and Egger’s tests were conducted to assess potential publication bias. Data analysis was performed using Stata 12.0 edition.
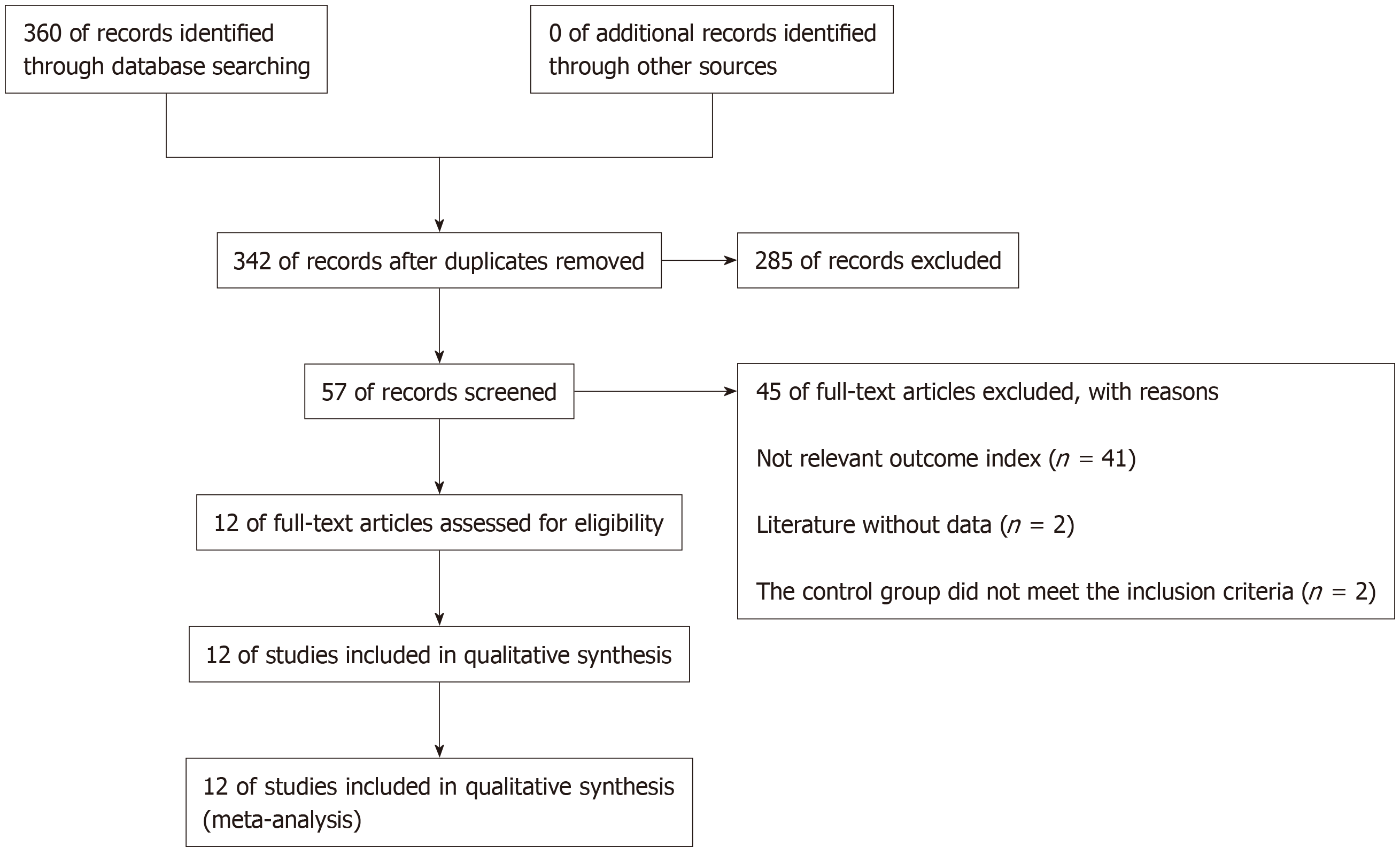
After searching the database, 360 documents were selected. Eighteen repetitive documents were excluded. After reading the title and abstract, 285 papers were excluded from the study of capecitabine combined with oxaliplatin compared with other drugs that affect colon cancer. After reading the full text, 41 articles were excluded because they did not involve relevant outcome indicators. Two articles were excluded because there were no relevant data, and two articles in the control group did not meet the inclusion criteria. Finally, 12 articles met the requirements[13-24] (Figure 1).
Data and quality evaluation were included in a clinical controlled trial (Table 1).
| Study | Year | T event | T no event | T total | C event | C no event | C total | Outcome | The Jadad score |
| Haller et al[14] | 2011 | 643 | 295 | 938 | 573 | 353 | 926 | DFS | 7 |
| 741 | 197 | 938 | 701 | 225 | 926 | OS | |||
| Kubicka et al[16] | 2016 | 541 | 320 | 861 | 482 | 379 | 861 | DFS | 7 |
| 619 | 242 | 861 | 575 | 286 | 861 | OS | |||
| Pectasides et al[20] | 2014 | 169 | 44 | 213 | 160 | 41 | 201 | DFS | 6 |
| 185 | 28 | 213 | 175 | 26 | 201 | OS | |||
| Schmoll et al[22] | 2012 | 594 | 350 | 944 | 527 | 415 | 942 | DFS | 7 |
| 689 | 255 | 944 | 631 | 311 | 942 | OS | |||
| Diao et al[13] | 2008 | 47 | 24 | 71 | 69 | 18 | 87 | DFS | 2 |
| Xun et al[21] | 2016 | 9 | 21 | 30 | 3 | 25 | 28 | OS | 3 |
| Song et al[23] | 2016 | 63 | 10 | 73 | 49 | 6 | 55 | OS | 3 |
| Lei et al[17] | 2016 | 5 | 16 | 21 | 2 | 19 | 21 | OS | 4 |
| Li et al[18] | 2012 | 43 | 17 | 60 | 32 | 28 | 60 | OS | 3 |
| Lian et al[19] | 2016 | 2 | 54 | 56 | 2 | 48 | 50 | OS | 4 |
| Zhang et al[15] | 2011 | 20 | 5 | 25 | 3 | 22 | 25 | OS | 2 |
| Wang et al[24] | 2011 | 8 | 22 | 30 | 3 | 27 | 30 | OS | 3 |
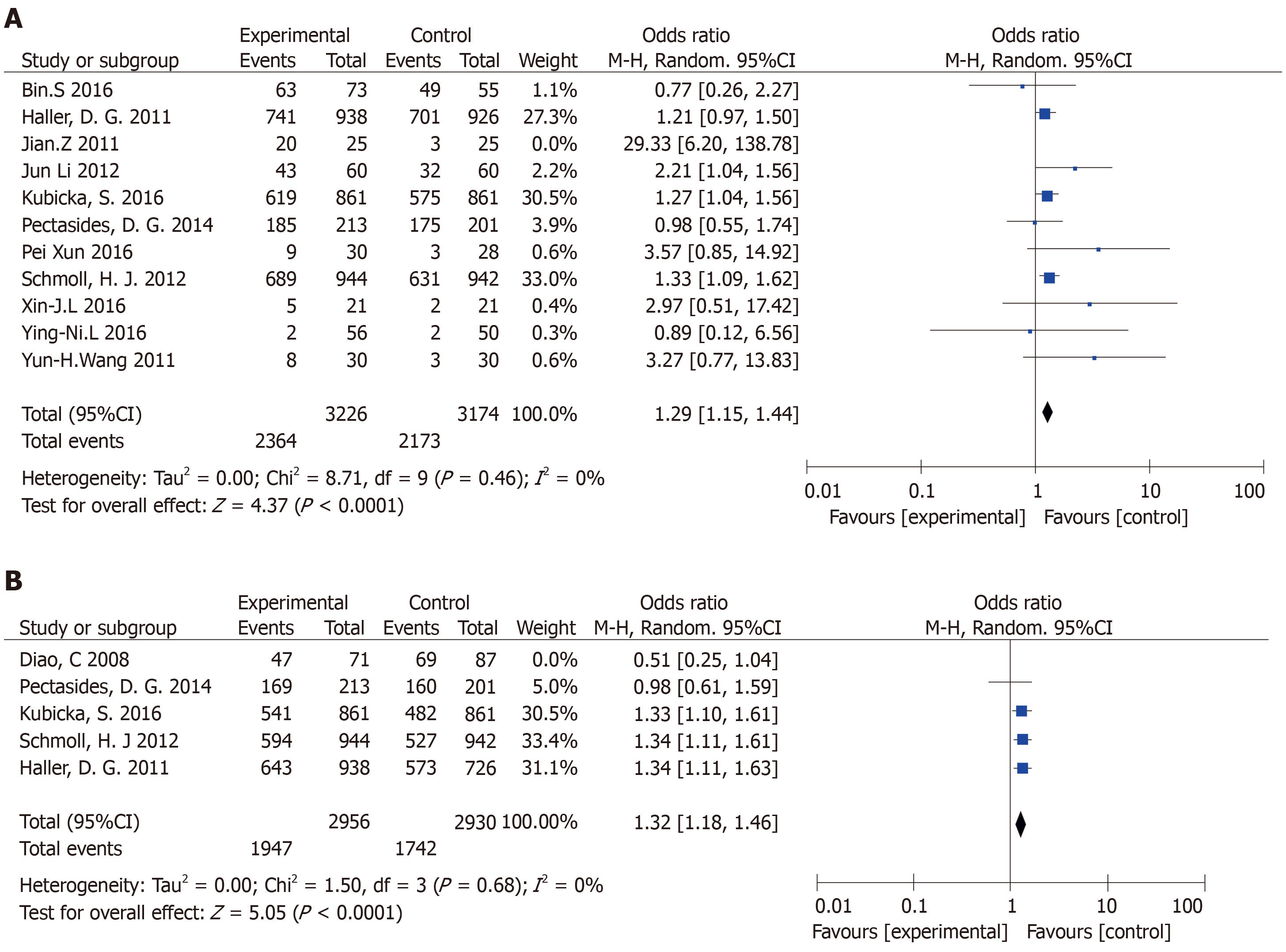
OS: The meta-analysis obtained OS data from 11 articles. Finally, ten papers were included. The combined analysis showed that the XELOX group had longer OS compared with other chemotherapy groups (Figure 2A). The study has statistical significance (OR = 1.29, 95%CI: 1.15-1.44, P < 0.0001). There was no heterogeneity among the study sites (P = 0.46, I2 = 0%).
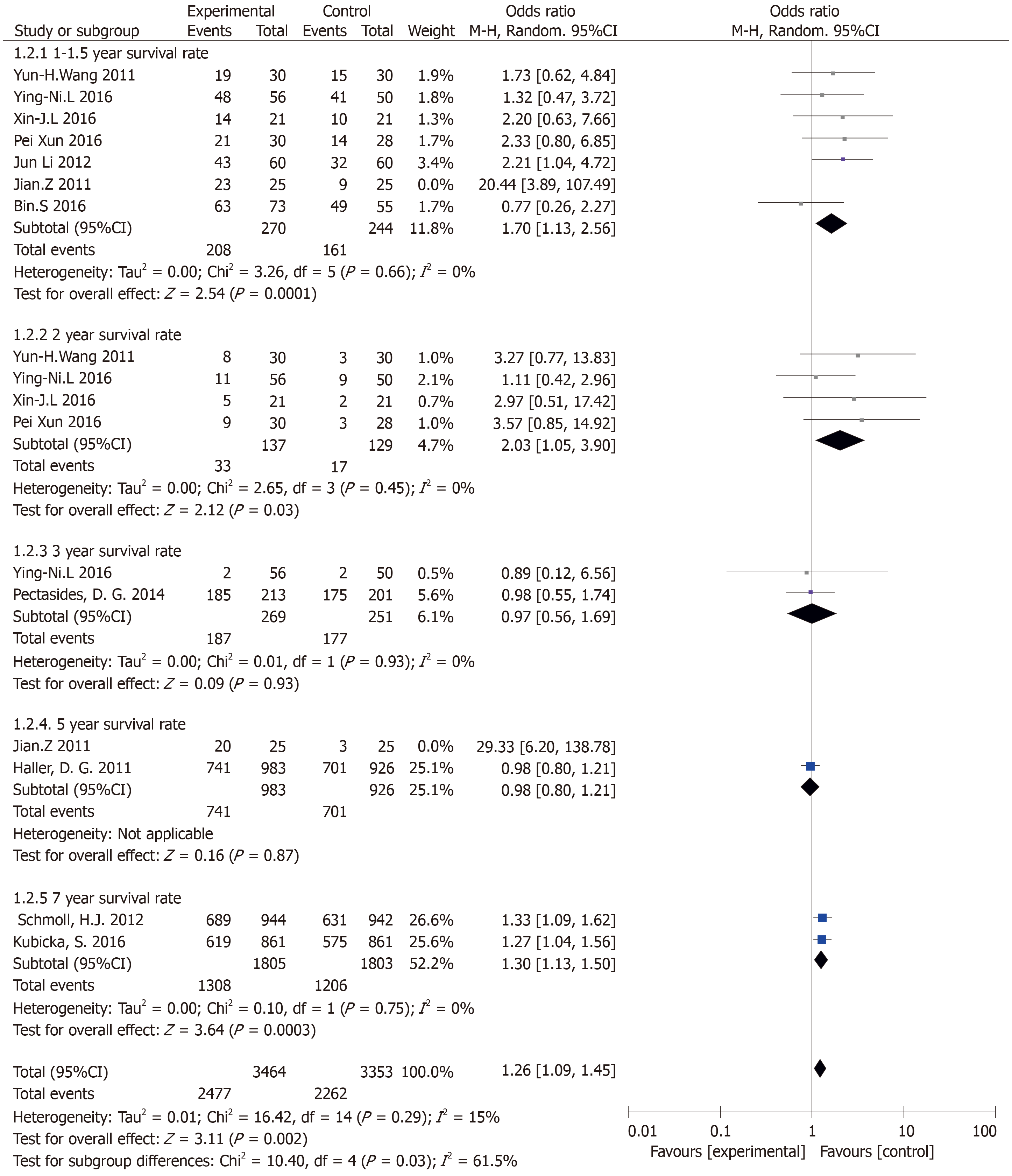
Results of the OS subgroup analysis (subgroup analysis of different follow-up visits) (Figure 3): Annual survival was statistically significant: (OR = 1.70, 95%CI: 1.13-2.56, P = 0.01). There was no heterogeneity in the study (P = 0.66, I2 = 0%). The 2-year survival rate was statistically significant: (OR = 3.30, 95%CI: 1.37-7.97, P = 0.01). There was no heterogeneity in the study (P = 0.99, I2 = 0%). The 3-year survival rate was not statistically significant: (OR = 0.97, 95%CI: 0.56-1.69, P = 0.93). There was no heterogeneity in the study (P = 0.93, I2 = 0%). The 7-year survival rate was statistically significant (OR = 1.30, 95%CI: 1.13-1.50, P = 0.0003). There was no heterogeneity in the study (P = 0.75, I2 = 0%).
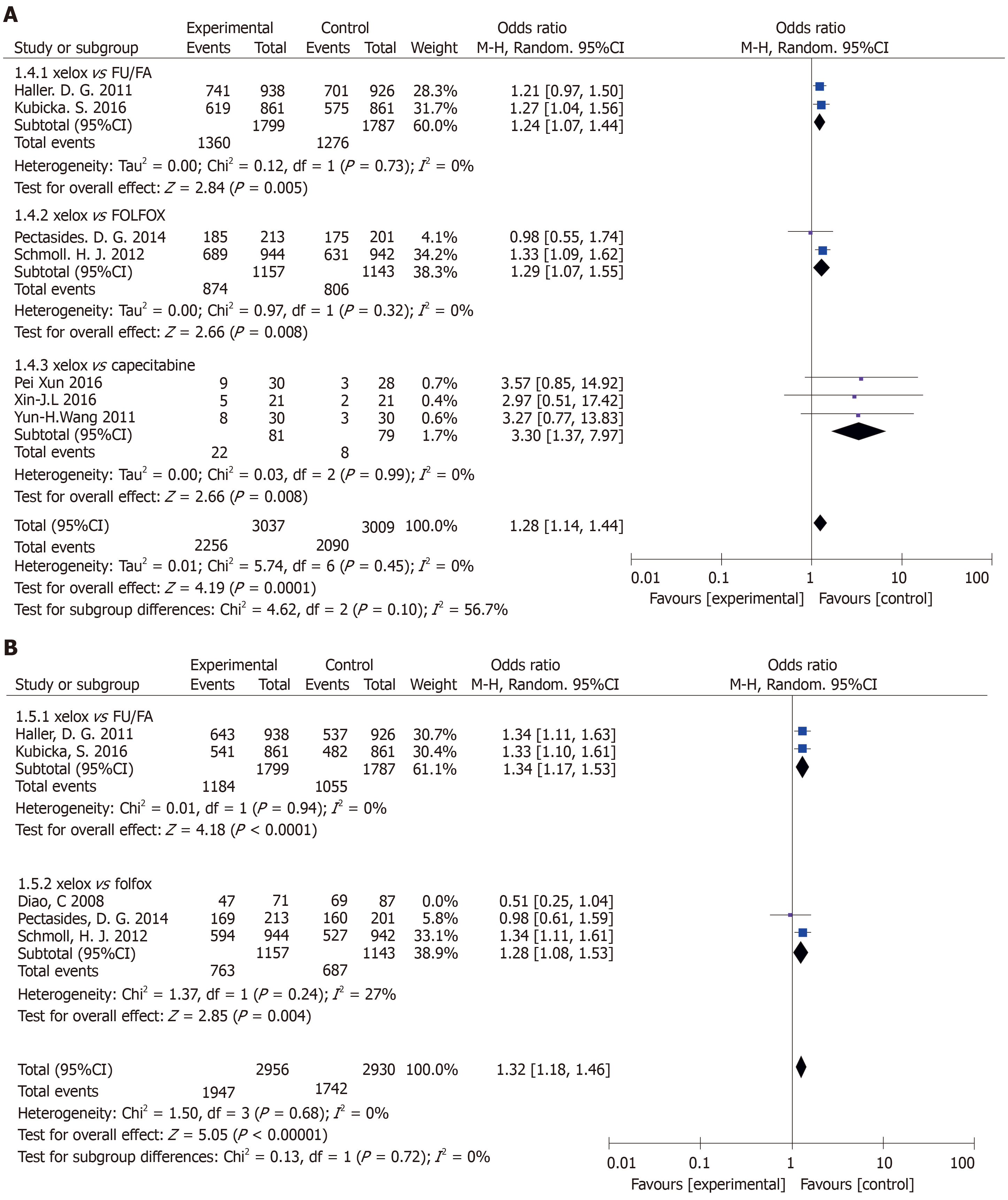
Results of the OS subgroup analysis (XELOX vs other chemotherapy groups) (Figure 4A): XELOX vs FU/FA: The study was statistically significant: (OR = 1.24, 95%CI: 1.07-1.44, P = 0.005). There was no heterogeneity in the study (P = 0.73, I2 = 0%). XELOX vs FOLFOX: The study was statistically significant: (OR = 1.29, 95%CI: 1.07-1.55, P = 0.008). There was no heterogeneity in the study (P = 0.32, I2 = 0%). XELOX vs capecitabine: The study was statistically significant: (OR = 1.28, 95%CI: 1.14-1.44, P = 0.008). There was no heterogeneity in the study (P = 0.99, I2 = 0%).
DFS: In this meta-analysis, DFS data were obtained in five articles, and four articles were finally included. The combined analysis showed that the XELOX group had longer DFS compared with other chemotherapy groups (Figure 2B). The study had significant statistical significance (OR = 1.32, 95%CI: 1.18-1.46, P < 0.0001). There was no heterogeneity in the study (P = 0.68, I2 = 0%).
Results of the DFS subgroup analysis (XELOX and other chemotherapy drug groups (Figure 4B): XELOX vs FU/FA: DFS had significant statistical significance: (OR = 1.34, 95%CI: 1.17-1.53, P < 0.0001). There was no heterogeneity among the study sites (P = 0.94, I2 = 0%). XELOX vs FOLFOX: DFS had statistical significance: (OR = 1.28, 95%CI: 1.08-1.53, P = 0.004). There was low heterogeneity among the studies (P = 0.24, I2 = 27%).
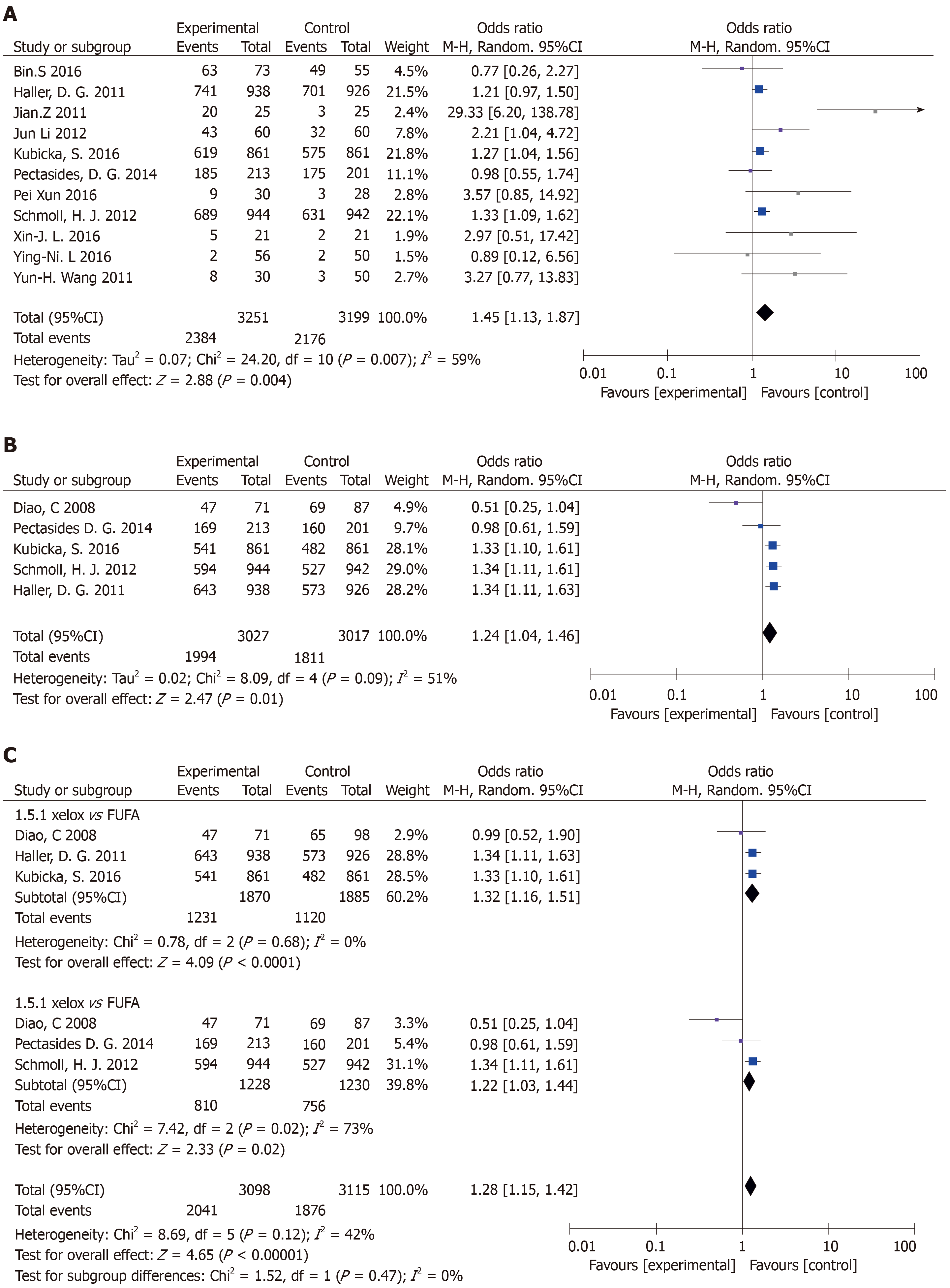
Included 11 articles from OS meta-analysis: There was statistical significance (P = 0.004) with moderate heterogeneity in this meta-analysis (P = 0.007, I2 = 59%) (Figure 5A).
Articles were included in the DFS meta-analysis: There was statistical significance (OR = 1.24, 95%CI: 1.04-1.46, P = 0.01) with moderate heterogeneity in this meta-analysis (P = 0.09, I2 = 51%) (Figure 5B). In its subgroup analysis, there was statistical significance (P = 0.02) with high heterogeneity in this meta-analysis (P = 0.02, I2 = 73%) (Figure 5C).
Sensibility analysis 1: There was heterogeneity in the article published by Zhang et al[15] (Figure 2A). After evaluating the article, the quality was low and samples were excluded. After exclusion (OR = 1.29, 95%CI: 1.15-1.44, P < 0.0001), there was no heterogeneity among the studies (P = 0.46, I2 = 0%).
Sensibility analysis 2: The sensitivity analysis (Figure 2B) showed heterogeneity in the article from Diao et al[13]. After evaluating the article, the quality was low and samples were excluded. After exclusion (OR = 1.32, 95%CI: 1.18-1.46, P < 0.0001), there was no heterogeneity among the studies (P = 0.68, I2 = 0%). After completely excluding Diao et al[13], the study remained statistically significant (P = 0.004), and the heterogeneity of the study was greatly reduced (P = 0.24, I2 = 27%) (Figure 4B).
Bias detection of OS: The funnel plot was conducted to evaluate the publication bias. However, we did not observe clear asymmetry suggesting the results from this study are reliable (data not shown).
Li et al[18] reported that in elderly CRC patients (age above 70-years-old), a reduction in chemotherapy dose did not decrease the DFS with a benefit of less mortality. But, elderly patients receiving 50% of planned cycles had shorter DFS and higher CRC mortality than elderly patients receiving the full planned cycles[25]. Therefore, a reduced dose but full cycles should be considered for elderly CRC patients. Our results are consistent with Kim et al[26] who found that OS was better in patients receiving at least 75% of expected cycles, but a dose reduction did not affect OS. These results suggest that a primary dose reduction in elderly patients may reduce the side effects of chemotherapeutics and help them finish the full planned cycles. The choice between mFOLFOX6 and XELOX should be discussed based on the gene subtypes of colon cancer[27-36].
It is well known that the prognosis of CRC patients has significant association with gene mutations. It is generally accepted that dMMR confers favorable prognosis in patients with resected colon cancer[10,11]. Sinicrope et al[12,37] found that KRAS mutations were associated with adverse prognosis specifically in pMMR tumors, while Blons et al[38] showed that KRAS mutations conferred shorter DFS in patients with left colon primaries.
Patients with poor compliance may affect the accuracy of the results[39]. Cancer patients are generally expected to have higher adherence to treatment than other patients because they are highly motivated by the gravity of their disease[40,41]. However, studies have shown cancer patients to have similar adherence rates to patients with other diseases[42-45]. Treatment duration plays a role in adherence to the regimen: when medication is continued over a longer period of time, patients become less adherent[46].
For oral cytotoxic agents, which require close monitoring of side effects and regular patient visits. There is no gold-standard measurement, and all methods have limitations[42,47]. Previous studies of oral cytotoxic chemotherapeutic drugs have mainly used self-reported questionnaires[48], which tend to overestimate adherence because patients are inclined to over-report to please their doctors.
The side effects of commonly used adjuvant chemotherapy regimens FOLFOX is more serious[49]. The annoying neurotoxicity side effect of FOLFOX appears at the 8-10th cycle of administration. This time period is the critical time to gain or lose survival benefits. Although treatment series of fewer cycles showed some potential to ameliorate this neurotoxicity[50,51], recent studies failed to show any convincing benefit[52-55], even on a molecular basis[56]. It is still a challenge to be solved. Any “wait and go” policy to reduce side effects needs to be evaluated in a larger cohort of patients[57].
To our knowledge, many studies have indicated that monocyte count is associated with poor survival in patients with many types of cancer, but the potential mechanisms remain unknown[58]. Low monocyte to lymphocyte ratio (MLR) level may help improve the demographic and clinicopathological characteristics[59]. We found that low MLR, low monocyte and high lymphocyte were all associated with better prognosis in advanced gastric cancer patients. Meanwhile, our study indicated that low level MLR and low level neutrophil or high level lymphocyte correlated with better median DFS and OS for all patients. The 5-year DFS and OS rates of patients with low level MLR were higher than those with high level MLR[58,60-67]. We speculate that there may be similar mechanisms in CRC.
Nowadays, tumor molecular pathology assessment serves as a regular part of clinical practice. Treatment effect is unlikely uniform across different molecular subtypes. Molecular pathological epidemiology is an integrative science to determine the molecular pathology in relation to clinical features and outcome in patients and populations. Molecular pathological epidemiology will be a future direction for personal treatment[68,69].
In this study, some of the non-English articles were not included because of the low assessed quality. Secondly, a limited number of RCT trials and small number of included patients may limit the conclusion of this study. Finally, a diversity in genetics, tumor staging and XELOX dose may also influence the results. Therefore, our conclusion needs to be further validated by a large RCT trial in the future.
In conclusion, the XELOX regimen is recommended for stage III colon cancer after surgery.
Colorectal cancer (CRC) accounts for 9% of all cancers in the world. In the last decade, it is the third most common malignant tumor in Europe and the United States. There is an urgent need to establish an effective standard treatment for CRC. In addition, more than 70% of CRC-related deaths are associated with the liver metastasis. A recurrence rate and poor overall survival make CRC a serious public health problem.
The aim of treatment for CRC is to cure locally and prevent metastasis and recurrence. Generally, comprehensive treatment is the focus of CRC, and chemotherapy is one of the important treatment methods. Reasonable and effective chemotherapy can prolong the life span and improve the quality of life of patients. Therefore, local resection of colon cancer should be combined with individual treatment. For patients with CRC, the choice of chemotherapy is very important for their prognosis. In patients with CRC, the purpose of adjuvant chemotherapy is to eliminate the occult micrometastasis during surgery, so as to improve the overall survival.
The purpose of this study was to explore the efficacy of capecitabine plus oxaliplatin (XELOX) regimen over other chemotherapy regimens, specifically XELOX vs 5-fluorouracil plus leucovorin, XELOX vs 5-fluorouracil plus leucovorin plus oxaliplatin, XELOX vs capecitabine and XELOX vs oxaliplatin plus 5-fluorouracil.
By searching the PubMed, EMBASE and Cochrane databases, a total of 12 randomized controlled trials involving 6698 stage III colon cancer cases (XELOX protocol: n = 3298 cases; other adjuvant chemotherapy protocol: n = 3268 cases) were included. The parameter outcomes included the overall survival and the disease-free survival. The quality control of selected literature was based on the Jadad scale and the GRADE system.
In comparison to other adjuvant chemotherapy regimen, the XELOX regimen showed a better overall survival and a better disease-free survival for colon cancer patients.
In clinical application, XELOX and 5-fluorouracil plus leucovorin plus oxaliplatin showed similar efficacy, but different types of patients may have different benefits from treatment. According to our data, in comparison to other adjuvant chemotherapy regimen, XELOX regimen showed a better overall survival and a better disease-free survival for colon cancer patients, suggesting the XELOX regimen can be a good option for postoperative treatment of stage III colon cancer.
The XELOX regimen is recommended for stage III colon cancer after surgery. In addition, our conclusion needs to be further validated by a large RCT trial in future.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, Ogino S S-Editor: Dou Y L-Editor: Filipodia E-Editor: Qi LL
| 1. | Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Tataryn BB, Kryzhanivska AY, Golotyuk VV, Partykevych YD. Chemotherapy in colon cancer. Wiad Lek. 2018;71:1674-1680. [PubMed] |
| 3. | Baretti M, Rimassa L, Personeni N, Giordano L, Tronconi MC, Pressiani T, Bozzarelli S, Santoro A. Effect of Comorbidities in Stage II/III Colorectal Cancer Patients Treated With Surgery and Neoadjuvant/Adjuvant Chemotherapy: A Single-Center, Observational Study. Clin Colorectal Cancer. 2018;17:e489-e498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Chiu J, Tang V, Leung R, Wong H, Chu KW, Poon J, Epstein RJ, Yau T. Efficacy and tolerability of adjuvant oral capecitabine plus intravenous oxaliplatin (XELOX) in Asian patients with colorectal cancer: 4-year analysis. Asian Pac J Cancer Prev. 2014;14:6585-6590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tang J, Wu X, Gao Y, Jiang W, Kong L, Lin J, Wan D, Pan Z, Ding P. Long-Term Outcome of Oxaliplatin and Capecitabine (XELOX) Concomitant with Neoadjuvant Radiotherapy and Extended to the Resting Period in High Risk Locally Advanced Rectal Cancer. J Cancer. 2018;9:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Duran AO, Karaca H, Besiroglu M, Bayoglu IV, Menekse S, Yapici HS, Yazilitas D, Bahceci A, Uysal M, Sevinc A, Hacibekiroglu I, Aksoy A, Tanriverdi O, Arpaci E, Inanc M, Dane F, Ozkan M. XELOX plus bevacizumab vs. FOLFIRI plus bevacizumab treatment for first-line chemotherapy in metastatic colon cancer: a retrospective study of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev. 2014;15:10375-10379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Loree JM, Sha A, Soleimani M, Kennecke HF, Ho MY, Cheung WY, Mulder KE, Abadi S, Spratlin JL, Gill S. Survival Impact of CAPOX Versus FOLFOX in the Adjuvant Treatment of Stage III Colon Cancer. Clin Colorectal Cancer. 2018;17:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kosugi C, Koda K, Ishibashi K, Yoshimatsu K, Tanaka S, Kato R, Kato H, Oya M, Narushima K, Mori M, Shuto K, Ishida H. Safety of mFOLFOX6/XELOX as adjuvant chemotherapy after curative resection of stage III colon cancer: phase II clinical study (The FACOS study). Int J Colorectal Dis. 2018;33:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Munemoto Y, Kanda M, Ishibashi K, Hata T, Kobayashi M, Hasegawa J, Fukunaga M, Takagane A, Otsuji T, Miyake Y, Nagase M, Sakamoto J, Matsuoka M, Oba K, Mishima H. Capecitabine and oxaliplatin combined with bevacizumab are feasible for treating selected Japanese patients at least 75 years of age with metastatic colorectal cancer. BMC Cancer. 2015;15:786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Mouradov D, Domingo E, Gibbs P, Jorissen RN, Li S, Soo PY, Lipton L, Desai J, Danielsen HE, Oukrif D, Novelli M, Yau C, Holmes CC, Jones IT, McLaughlin S, Molloy P, Hawkins NJ, Ward R, Midgely R, Kerr D, Tomlinson IP, Sieber OM. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. Am J Gastroenterol. 2013;108:1785-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt JA, Fuchs CS, Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 12. | Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, Nelson GD, Goldberg RM, Sargent DJ, Alberts SR. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664-3672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 13. | Diao C, Cheng RC, Zhang JM, Wei XP, Su YJ, Liu QY, Xu JB. [Clinical observation of XELOX (Capecitabine puls Oxaliplatin): an adjuvant chemotherapy regimen used in stage III colorectal cancer]. Zhonghua Zhong Liu Za Zhi. 2008;30:147-150. [PubMed] |
| 14. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 15. | Zhang J. Analysis and evaluation of the curative effect of hiroda and oxaliplatin in the treatment of advanced colon cancer. Linchuang Heli Yongyao Zazhi. 2011;36. [DOI] [Full Text] |
| 16. | Kubicka S. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial[German]. Strahlenther Onkol. 2016;192:505-506. |
| 17. | Lei XJ, Zheng KH, Li F, Li J. To observe the curative effect of capecitabine combined with oxaliplatin in the treatment of advanced colorectal cancer. Beifang Yaoxue. 2016;11:31-37. |
| 18. | Li J, Cui BJ. Clinical observation of capecitabine combined with oxaliplatin in the treatment of advanced colorectal cancer. Zhongguo Yaowu Yu Linchunag. 2012;S1:46-47. |
| 19. | Lian YN, Mao JX, Zeng ZJ, Chen YL, Gao TH, Huang JD. The efficacy of XELOX and OLF chemotherapy for the treatment of advanced colorectal cancer in aged patients with k-ras mutation. Zhongguo Xiandai Yixue Zazhi. 2016;2:51-54. |
| 20. | Pectasides DG, Papaxoinis G, Xanthakis I, Kouvatseas G, Kotoula V, Xiros N, Samantas E, Papakostas P, Klouvas G, Varthalitis II, Pentheroudakis G, Fountzilas G, Bafaloukos D. Randomized phase III trial of FOLFOX versus XELOX as adjuvant chemotherapy in patients with early-stage colorectal adenocarcinoma. J Clin Oncol. 2014;32:3617-3617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Xun P. Observation of oxaliplatin and capecitabine combined with clinical efficacy of capecitabine in treatment of advanced colorectal cancer. Zhongguo Shiyong Yiyao. 2016;151-152. [DOI] [Full Text] |
| 22. | Schmoll HJ, Tabernero J, Maroun JA, De Braud FG, Price TJ, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin (XELOX) versus bolus 5-fluorouracil/leucovorin (5-FU/LV) as adjuvant therapy for stage III colon cancer: Survival follow-up of study NO16968 (XELOXA). J Clin Oncol. 2012;30:388-388. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Song B, Liu D, Song WQ, Liu SD, Mao ZJ, Gao ZZ, Long YB, Wang P, Guo XY. The clinical observation of 55 cases of colorectal cancer was postoperatively assisted in the chemoradio regimen. Shaanxi Yixue Zazhi. 2016;45:1507-1509. [DOI] [Full Text] |
| 24. | Wang YH. Capecitabine combined with oxaliplatin in the treatment of advanced colon cance. Hainan Yixueyuan Xuebao. 2011;4:506-507. [DOI] [Full Text] |
| 25. | Lund CM, Nielsen D, Dehlendorff C, Christiansen AB, Rønholt F, Johansen JS, Vistisen KK. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: the ACCORE study. ESMO Open. 2016;1:e000087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Zang DY, Chung IJ, Cho SH, Park KU, Oh HS, Lee KH, Lee BH, Kim MJ, Park CK, Han B, Kim HS, Choi DR, Song HH, Jung JY. A Muti-center, Randomized Phase II Study of Oxaliplatin and S-1 versus Capecitabine and Oxaliplatin in Patients with Metastatic Colorectal Cancer. J Cancer. 2015;6:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Hoeben KW, van Steenbergen LN, van de Wouw AJ, Rutten HJ, van Spronsen DJ, Janssen-Heijnen ML. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol. 2013;24:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Kim CA, Spratlin JL, Armstrong DE, Ghosh S, Mulder KE. Efficacy and safety of single agent or combination adjuvant chemotherapy in elderly patients with colon cancer: a Canadian cancer institute experience. Clin Colorectal Cancer. 2014;13:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, O'Connell M, Twelves CJ, Saltz LB, Haller DG, Sargent DJ. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014;32:2654-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, Köhne CH, Rostoft S, Lemmens V, Mitry E, Rutten H, Sargent D, Sastre J, Seymour M, Starling N, Van Cutsem E, Aapro M. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 32. | Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6:7339-7347. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 773] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 34. | Gajra A, Klepin HD, Feng T, Tew WP, Mohile SG, Owusu C, Gross CP, Lichtman SM, Wildes TM, Chapman AE, Dotan E, Katheria V, Zavala L, Akiba C, Hurria A; Cancer and Aging Research Group (CARG). Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older with solid tumors. J Geriatr Oncol. 2015;6:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Hermosillo-Rodriguez J, Anaya DA, Sada Y, Walder A, Amspoker AB, Berger DH, Naik AD. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J Geriatr Oncol. 2013;4:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Lonardi S, Sobrero A, Rosati G, Di Bartolomeo M, Ronzoni M, Aprile G, Massida B, Scartozzi M, Banzi M, Zampino MG, Pasini F, Marchetti P, Cantore M, Zaniboni A, Rimassa L, Ciuffreda L, Ferrari D, Barni S, Zagonel V, Maiello E, Rulli E, Labianca R; TOSCA (Three or Six Colon Adjuvant) Investigators. Phase III trial comparing 3-6 months of adjuvant FOLFOX4/XELOX in stage II-III colon cancer: safety and compliance in the TOSCA trial. Ann Oncol. 2016;27:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M, Goldberg RM, Mahoney M, Sargent DJ, Alberts SR. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 38. | Blons H, Emile JF, Le Malicot K, Julié C, Zaanan A, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Nørgård-Petersen L, Van Cutsem E, Lepage C, Zawadi MA, Salazar R, Laurent-Puig P, Taieb J; PETACC-8 Study Investigators. Prognostic value of KRAS mutations in stage III colon cancer: post hoc analysis of the PETACC8 phase III trial dataset. Ann Oncol. 2014;25:2378-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Kawakami K, Nakamoto E, Yokokawa T, Sugita K, Mae Y, Hagino A, Suenaga M, Mizunuma N, Oniyama S, Machida Y, Yamaguchi T, Hama T. Patients' self-reported adherence to capecitabine on XELOX treatment in metastatic colorectal cancer: findings from a retrospective cohort analysis. Patient Prefer Adherence. 2015;9:561-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Klein CE, Kastrissios H, Miller AA, Hollis D, Yu D, Rosner GL, Grinblatt DL, Larson RA, Ratain MJ. Pharmacokinetics, pharmacodynamics and adherence to oral topotecan in myelodysplastic syndromes: a Cancer and Leukemia Group B study. Cancer Chemother Pharmacol. 2006;57:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 239] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 43. | Liedholm H, Gullberg B. Refill adherence to repeat prescriptions of cancer drugs to ambulatory patients. Eur J Cancer Care (Engl). 2007;16:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Nilsson JL, Andersson K, Bergkvist A, Björkman I, Brismar A, Moen J. Refill adherence to repeat prescriptions of cancer drugs to ambulatory patients. Eur J Cancer Care (Engl). 2006;15:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Noens L, van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, Martiat P, Mineur P, Van Eygen K, MacDonald K, De Geest S, Albrecht T, Abraham I. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401-5411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 458] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 46. | Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 47. | Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 415] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 48. | Font R, Espinas JA, Gil-Gil M, Barnadas A, Ojeda B, Tusquets I, Segui MA, Margelí M, Arcusa A, Prat A, Garcia M, Borras JM. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. Br J Cancer. 2012;107:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Tsai YJ, Lin JK, Chen WS, Jiang JK, Teng HW, Yen CC, Lin TC, Yang SH. Adjuvant FOLFOX treatment for stage III colon cancer: how many cycles are enough? Springerplus. 2016;5:1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Hosokawa A, Ogawa K, Ando T, Suzuki N, Ueda A, Kajiura S, Kobayashi Y, Tsukioka Y, Horikawa N, Yabushita K, Fukuoka J, Sugiyama T. Preventive effect of traditional Japanese medicine on neurotoxicity of FOLFOX for metastatic colorectal cancer: a multicenter retrospective study. Anticancer Res. 2012;32:2545-2550. [PubMed] |
| 51. | Milla P, Airoldi M, Weber G, Drescher A, Jaehde U, Cattel L. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009;20:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, Kon M, Ogata Y, Takahashi K, Ishida H, Saeki H, Sakaguchi Y, Yamanaka T, Kono T, Tomita N, Baba H, Shirabe K, Kakeji Y, Maehara Y. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015;20:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL. Clinical Course of Oxaliplatin-Induced Neuropathy: Results From the Randomized Phase III Trial N08CB (Alliance). J Clin Oncol. 2015;33:3416-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 54. | Zimmerman C, Atherton PJ, Pachman D, Seisler D, Wagner-Johnston N, Dakhil S, Lafky JM, Qin R, Grothey A, Loprinzi CL. MC11C4: a pilot randomized, placebo-controlled, double-blind study of venlafaxine to prevent oxaliplatin-induced neuropathy. Support Care Cancer. 2016;24:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol. 2014;32:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 56. | Ruzzo A, Graziano F, Galli F, Giacomini E, Floriani I, Galli F, Rulli E, Lonardi S, Ronzoni M, Massidda B, Zagonel V, Pella N, Mucciarini C, Labianca R, Ionta MT, Veltri E, Sozzi P, Barni S, Ricci V, Foltran L, Nicolini M, Biondi E, Bramati A, Turci D, Lazzarelli S, Verusio C, Bergamo F, Sobrero A, Frontini L, Magnani M. Genetic markers for toxicity of adjuvant oxaliplatin and fluoropyrimidines in the phase III TOSCA trial in high-risk colon cancer patients. Sci Rep. 2014;4:6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Kochi M, Ichikawa W, Meguro E, Shibata H, Fukui T, Nagase M, Hoshino Y, Takeuchi M, Fujii M, Nakajima T. Phase II study of FOLFOX4 with "wait and go" strategy as first-line treatment for metastatic colorectal cancer. Cancer Chemother Pharmacol. 2011;68:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Eo WK, Jeong DW, Chang HJ, Won KY, Choi SI, Kim SH, Chun SW, Oh YL, Lee TH, Kim YO, Kim KH, Ji YI, Kim A, Kim HY. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol. 2015;21:2668-2676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Chen L, Hao Y, Zhu L, Li S, Zuo Y, Zhang Y, Song H, Xue Y. Monocyte to lymphocyte ratio predicts survival in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy. Onco Targets Ther. 2017;10:4007-4016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 61. | Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver. 2013;7:385-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Quéro L, Guillerm S, Hennequin C. Neoadjuvant or adjuvant therapy for gastric cancer. World J Gastrointest Oncol. 2015;7:102-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | el Aziz LM. Blood neutrophil-lymphocyte ratio predicts survival in locally advanced cancer stomach treated with neoadjuvant chemotherapy FOLFOX 4. Med Oncol. 2014;31:311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Feng F, Sun L, Zheng G, Liu S, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget. 2017;8:5281-5291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 66. | Brenkman HJ, Haverkamp L, Ruurda JP, van Hillegersberg R. Worldwide practice in gastric cancer surgery. World J Gastroenterol. 2016;22:4041-4048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol. 2015;9:2054-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 69. | Ogino S, Nowak JA, Hamada T, Milner DA, Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |