INTRODUCTION
As conventional magnetic resonance imaging (MRI) offers excellent anatomic information and has gained considerable success in the disease diagnosis throughout the body, functional assessment becomes available with the rapid development of related techniques. Considering the radiation caused by nuclear medicine examinations and the risk of nephrogenic systemic fibrosis in enhanced MRI scans, unenhanced MRI techniques have gained special clinic interest[1]. Diffusion-weighted imaging (DWI), which uses motion of intrinsic water molecules as genuine contrast was explored, then diffusion tensor imaging (DTI) made a step further and detects diffusion properties along at least six different directions, from which the main diffusion direction and the degree of diffusion anisotropy can be calculated[2]. A frequently used index to measure diffusion anisotropy is the fractional anisotropy (FA), ranges from 0 (isotropic: No preferred direction) to 1 (full anisotropy: Only one direction)[3]. The collected data can be converted to gray-scaled FA-maps, color-coded orientation image, and furthermore, tractography that enables 3D reconstruction[4]. Such data provided unique insight into the tissue microstructure, especially those with highly direction preference.
DTI has been traditionally applied to the brain, where tractography was developed to visualize white matter fiber bundles, and microstructural injuries of white matter fiber tracts were quantified[5]. It has been extensively used in the evaluation of brain tumor, injury, degeneration, etc.[5-7]. Although abdominal and pelvic MRI encountered some problems such as motion artifacts when first introduced, various techniques such as respiratory triggering and breath-hold imaging have sufficiently improved the image quality[8,9]. Accordingly, the utility of DTI is rapidly extending. However, DTI of abdomen and pelvis was relatively inadequately understood. Furthermore, DTI of various organs may have different clinical emphasis and challenges due to these distinct structures and physiologies. This review aims to give a systematic overview of clinical application of DTI in abdominal and pelvic organs such as liver, pancreas, kidneys, uterus, prostate, etc., and discuss potential future research directions of the above-mentioned issues.
DTI OF THE LIVER
Technical perspectives
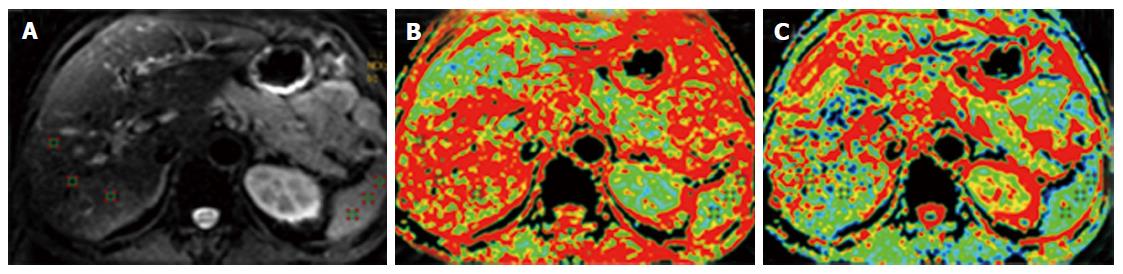
Both 1.5T and 3T MRI devices have been used in liver DTI. Usually, a preliminary T2-weighted single-shot turbo spin echo sequence was required for anatomical localization. The coronal DTI was then acquired using the in-built single-shot echo planar imaging (EPI) sequence. Fat-saturation with the spectral adiabatic inversion recovery approach can be employed to avoid chemical-shift artefacts. Li et al[10] reported that good image quality, acceptable scan time and reasonable FA/ADC values were acquired using diffusion-encoding directions of 9 with b-value of 0.300 s/mm2. The effect of respiratory and cardiac motion in liver DTI was assessed[11]. Although some study reported that respiratory motion tended to increase DTI metrics (mainly mean diffusivity), some researchers also suggest to acquire DTI during free breathing and to adjust respiratory mismatch by post-processing[12,13]. The left lobe appeared to present increased FA and mean diffusivity during systole, but the right lobe was less affected by cardiac motion[12]. Regarding the repeatability of diffusion-related parameters, both apparent diffusion coefficient (ADC, measured in DWI) and FA had relatively low degrees of variation on both intra-session and inter-session basis in general, but the right lobe values were considered more reliable than left lobes[11]. The normal images of hepatic DTI are presented in Figure 1.
Figure 1 Normal images of hepatic diffusion tensor imaging.
A: Diffusion image (b value of 0), mainly presenting the anatomic profiles; B: Fractional anisotropy map of the liver, presenting as the pseudo-colored image; C: Apparent diffusion coefficient map of the liver, presenting as the pseudo-colored image as well (Li et al[10], 2015; open access from PlosOne, permission confirmed).
DTI findings in livers with various pathologies
Diffusion studies in liver have been advocated as a tool to stage liver fibrosis, based on the assumption that fibrosis can be reflected by changes of diffusion properties. Whereas DWI is commonly used for such purposes, few studies reported DTI results. Two studies performed liver DTI on a 1.5T and a 3T scanner respectively, and both reported that conventional DWI performed better than DTI for the diagnosis of fibrosis and inflammation, and concluded that ADC values measured on DWI had the potential for fibrotic and inflammatory grading. The FA showed a trend toward higher levels with increasing inflammatory grade and fibrotic stage, but was of less diagnostic value. It was explained that many other nonspecific pathologic processes could lead to FA elevation as well[14,15].
For focal mass-like lesions assessment, the literature is sparse. Erturk et al[16] reported that metastases tended to have low ADCs and high FA values, cysts had high ADCs and low FA values, and hemangiomas had high ADCs and high FA values. ADC had a better diagnostic performance for discriminating metastases from benign hepatic lesions than FA values (areas under the curve (Az values): 0.88 for ADC and 0.73 for FA), but FA had excellent ability to discriminate cysts from hemangiomas (Az value: 0.96)[16]. Another study revealed that hepatocellular carcinomas presented significantly lower ADC and higher mean FA value than normal liver regions, but diagnostic cutoff values, diagnostic accuracy and the comparison between ADC and FA were not given[10]. In general, DTI can play a supportive role in definition of focal liver lesion pathology.
DTI OF THE PANCREAS
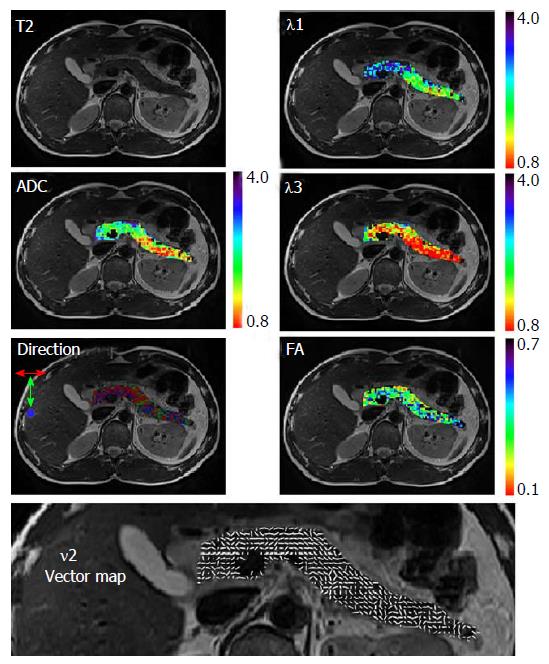
The pancreas is a glandular organ with complex exocrine microstructure and endocrine microvascular physiology, and very few studies reported pancreatic DTI results. The normal images of pancreatic DTI are presented in Figure 2. A small sampled trial by Nissan et al[17] compared pancreatic DTI scans between healthy volunteers and patients with pancreatic-ductal-adenocacinoma. Fat-suppressed, respiratory triggered twice refocused spin-echo sequence, and b-values of 0, 100 and 500 s/mm2 were used. They found a significant reduction in the directional diffusion coefficients and a lower contribution of fast intra-voxel-incoherent-motion (IVIM) component at b ≤ 100 s/mm2 in the malignant lesion[17].
Figure 2 Normal images of pancreatic diffusion tensor imaging.
Maps were overlaid on the corresponding T2 weighted image. The direction map presents in three colors the direction of the 1st principal eigenvector; red: Left to right direction; green: Head to feet direction; and blue: Anterior to posterior direction. Vector map presents in white sticks the direction of the 1st principal eigenvector (Nissan et al[17], 2014; open access from PlosOne, permission confirmed). FA: Fractional anisotropy; ADC: Apparent diffusion coefficient.
Another larger sampled study focused on patients with acute pancreatitis (AP). It reported that both FA and ADC value from DTI could be employed to differentiate AP from normal pancreas, and also differentiate edema AP from necrosis AP. Furthermore, both ADC and FA value of pancreas had a negative correlation with the severity of AP (measured by MRI severity index). The authors discussed that the aggravation of pancreatic microcirculation ischemia, which played a key role in the transition from pancreatic edema to necrosis, could be detected by ADC and FA decreases[18]. DTI may spare the patients from radiation exposure of commonly used CT, and showed the potential to offer additional pathologic information to conventional MRI.
DTI OF THE KIDNEYS
Technical perspectives
Usually, a rapid T2-weighted sequence axial and coronal to the body axis were obtained for morphological evaluation. Then, coronal DTI were obtained with EPI. Several studies explored the optimal imaging parameters for anisotropy measurement. In the study of Kataoka et al[19], five different sequences with different parameters including respiration-triggered acquisition or multiple breath-holding, different slice thicknesses and different numbers of signal averaging and b-values were compared. It determined that the optimal sequence used respiratory triggering including a 3-mm slice thickness, three signal averages, and b-values of 0, 200 or 400 s/mm2, which allowed the largest cortex–medulla difference of FA. Another study reported to have achieved high-resolution scans by free-breathing approaches, using a navigator-triggered sequence[20]. A later study registered DTI images of native and transplanted kidneys, using a multimodal nonrigid registration algorithm, and revealed that respiratory triggering was not necessary to measure diffusion parameters in transplanted kidneys, but still appeared advantageous in native kidneys[21]. However, these studies were small sampled, and based on the strategy to increase acquisition time, which lasted for 6 to 8 min and therefore the effect of respiratory motion needed to be properly handled. But some trials adopting shorter acquisition time appears reasonable to use breath-held, or even free-breathing approaches as well[22-24]. The influence of the number of encoding directions on image quality was explored; a preliminary study reported that the best image quality was visually assessed for images acquired with 15 and 32 encoding directions[25]. DTI at 1.5T and 3T were compared, researchers showed renal DTI with a 3T scanner had significant better signal to noise ratio (SNR) than that with a 1.5T scanner, although FA and ADCs at 3T did not significantly differ from that at 1.5T[26,27]. Indeed, increasing studies are using 3T systems to perform renal DTI.
Normal values and repeatability of renal DTI
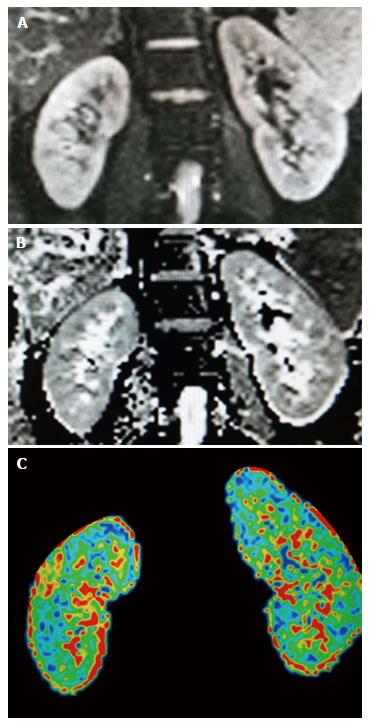
In the renal structures, especially in the renal medulla, diffusion properties are expected to be anisotropic as a consequence of the radial orientation of tubules, collecting ducts, and blood vessels, which are the basis for the application of DTI[28]. Indeed, kidney becomes the most explored organ to perform DTI among abdominal and pelvic structures up to date. The normal images of renal DTI are presented in Figure 3.
Figure 3 Normal images of renal diffusion tensor imaging.
A: Diffusion image (b-value of 0), mainly presenting the anatomic profiles; B: Apparent diffusion coefficient map of the kidneys; C: Fractional anisotropy map of the kidneys, presenting as the pseudo-colored image.
The medullary FA value (ranged from 0.32 to 0.45) was reported to be higher than the cortical FA value (ranged from 0.24 to 0.28), whereas the cortical ADC (ranged from 2.21 to 2.39 × 10-3 mm2/s) was higher than the medullary ADC (ranged from 1.87 to 1.99 × 10-3 mm2/s)[24,29,30]. This can be attributed to a more orientated architecture of the renal medulla than the cortex. One study reported that medullary FA values in women were lower than those in men, and medullary FA positively correlated with estimated glomerular filtration rate (eGFR)[29].
Repeatability is another issue concerned in clinical practice. Studies exhibited that the intra- and inter-observer measurements correlated well: No significant difference was noted between different observers, and the within-subject coefficient of variation obtained for both the ADC and FA values were less than 10% in the analyses[9,20,24]. Factors that may potentially influence the diffusion parameters include hydration status and renal blood flow, etc. The study by Müller et al[31] showed significantly decreased ADC values in the kidneys of dehydrated healthy volunteers that increased upon rehydration, which could be attributed to the increase in GFR and osmotically driven water motion in the kidney. To reduce the inter-individual variation, fluid intake was suggested to be restricted prior to the scans. It has also been reported that ADC of the renal cortex and FA values of the renal medulla tended to elevate as the blood flow increased, which should be considered when interpreting DTI data[9].
DTI findings in kidneys with various pathologies
Diffused renal diseases such as chronic kidney disease usually lead to renal function impairment. Studies showed the feasibility of DTI to assess such conditions, inferring that the reduction of diffusion parameters was possibly caused by tubular atrophy, interstitial fibrosis, cellular infiltration, and the scarring of glomeruli. The parenchymal ADC and FA of kidneys was showed significantly lower in patients than healthy controls, regardless of whether eGFR was reduced. Both the renal ADC and FA values (both cortical and medullary) correlated with eGFR, and inversely correlated with serum creatinine and blood urea nitrogen[22,32]. Another article proposed the medullary FA value to be the main parameter for assessing renal function damage[33]. Relations between diffusion property changes and specific pathologic processes were investigated. The study of Liu et al[32] found that renal FA values (both cortical and medullary) negatively correlated with glomerular lesion and tubulointerstitial injury. Whereas another study investigating chronic glomerulonephritis concluded that cortical FA negatively correlated with the percentage of glomerulosclerosis, and medullary FA negatively correlated with tubulointerstitial fibrosis[34]. Some studies employed DTI to assess diabetic nephropathy (DN), reporting that both mean medullary FA and ADC were significantly lower in diabetics (even with eGFR > 60 mL/min per 1.73 m2) than in controls, indicating the possibility of early identification of diabetics at risk of DN progression by DTI[35]. More recently, DTI was used to evaluate renal function in the follow-up of patients with autosomal dominant polycystic kidney disease[36].
For focal lesions in kidneys, the literature is sparse. A recent study used DTI to differentiate clear-cell renal cell carcinoma from low-fat renal angiomyolipomas (RAML). With the b-value of 0, 800 s/mm2 and the cutoff FA value of 0.254, DTI reached a sensitivity of 100%, and a specificity of 73.3% for the differentiation[37]. However, other tumors were poorly reported so far.
DTI for the evaluation of transplanted kidneys
Renal transplantation has been established as the widely-accepted treatment for patients with end-stage renal diseases. Given the shortage of clinically available donor kidneys and the significant incidence of allograft dysfunction, noninvasive and accurate assessment of the allograft renal function becomes critical to ensure the success of transplantation. Several studies explored the utility of DTI for the functional assessment. The medullary FA exhibited a high correlation with eGFR, and was proposed as a valuable indicator of allograft function[38,39]. Lanzman et al[38] also found that the corticomedullary difference in FA values was lower in functionally impaired renal allografts. Hueper et al[40] compared the DTI parameters between allografts with initial graft function and delayed graft function, and both FA and ADC exhibited an inverse correlation with the amount of renal fibrosis. These results indicated that DTI might become a sensitive biomarker of allograft function with the information of anisotropy.
DTI OF THE PROSTATE
Technical perspectives
The introduction of ultra-fast EPI sequences and parallel imaging techniques enabled the application of DTI to the prostate[41]. Both 1.5T and 3T scanners were used to perform prostatic DTI, but it is commonly accepted that 3T or even higher magnetic field systems can lead to higher SNR, improved spatial and temporal resolution[42]. Several different numbers of diffusion-encoding directions were tested, and all resulted satisfactory imaging quality in the study by Kim et al[43]. Reduced field-of-view acquisition was attempted in another study, generating high-resolution DTI of the prostate, which could potentially enable a more accurate detection of focal tumors[44].
Normal values of prostatic DTI
Prostate gland consists of various vascular, neural, and other anisotropic water paths, and there are distinct structural arrangements among central gland (CG), peripheral zone (PZ) and periprostatic neurovascular tracts, which make the DTI potentially applicable. Besides, the prostate is also an organ under the effect of the hormones and has dynamic changes as the age grows. In 2004, in vivo prostatic DTI were performed on six subjects, reporting a relatively high FA value of the PZ and CG of around 0.4. Later studies recorded lower FA values, and observed significant differences in the FA value between PZ (ranged from 0.16 to 0.21) and CZ (ranged from 0.26 to 0.37)[44,45]. Age-related changes of normal prostate were also assessed. The FA and ADC values in the normal prostatic PZ showed an age-dependent pattern, as FA decreases and ADC increases with age. Whereas the FA and ADC in CZ did not appear to be significantly age-related[46].
DTI for the diagnosis of prostate cancer and evaluation of periprostatic neurovascular bundles
Despite recent advances in MRI, the extent and aggressiveness of prostate cancer remain diagnostic challenges for radiologists. As the reliance on DWI increases in the clinical practice, DTI with additional anisotropic information was explored. An earlier study observed decreased FA values in the peripheral cancerous tissues compared with the normal peripheral portion[47]. Whereas no significant differences between the FA values for cancerous and normal PZ tissues in the study by Xu et al[48]. By contrast, later studies mostly reported increased FA values in cancerous tissues (ranged from 0.29 to 0.38) compared with non-cancerous tissues (ranged from 0.15 to 0.31), regardless of the location of the neoplastic tissue (either in PZ or CZ)[41,49,50]. Another study investigated the relation between gleason scores (GS) and DTI parameters in prostate cancer in PZ, revealing that FA values in the cancerous tissues were positively correlated with the GS (r = 0.48; P < 0.001), while the ADC values were negatively correlated with the GS (r = -0.54; P < 0.001)[51]. The authors claimed that increased FA were compatible with the hypercellular nature of prostate tumors[52]. The variation in the FA values reported by the above-mentioned studies may be related to the different grades of malignant changes, as each study included relatively small sample with various cancer grades. In addition, Xu et al[48] discussed that the measured anisotropy could be biased with the increased image noise in the cancerous region where shorter T2 led to more significantly lowered SNR than in the normal regions. In general, the reliability of FA measurement still need to be improved, and DTI needs to be better integrated to multi-modality assessment of prostatic lesions.
Several studies investigated the evaluation of periprostatic neurovascular bundles by DTI, but the results varied. Some studies optimistically showed that 3D-DTI allowed effective description of the entire plexus of the periprostatic fibers in all directions[53,54]. But 3 of 8 subjects had no tracts detected with conventional DTI acquisition in another small-sampled study, in which zoomed DTI improved the detection rate[55]. A recent study even doubted whether the visualized DTI tracts really represent nerve tracts or neurovascular bundles, because DTI tract profiles were significantly dependent on FA thresholds and tended to present non-specificity[56]. Therefore, the utility of DTI for this indication remains controversial.
DTI OF THE UTERUS
The uterus is a fibromuscular organ with layered structures. The overall arrangement of muscle fibers is highly directional, which is the basis of DTI application. Specially, this organ is under the effect of hormones and has physiological (menstrual cycle, menopausal period) fluctuations, which may influence the diffusion properties. In 2006, three-dimensional fiber architecture of the normal human uterus based on DTI was evaluated in five samples ex vivo, revealing two basic fiber directions: Circular and longitudinal oriented[57]. The study by Fiocchi et al[58] confirmed such results in vivo, and showed that two third of the Caesarean-scarred uteri had altered fiber structure (lower fiber number and density) in the scarred anterior isthmus. They inferred that DTI may detect significant caesarean scars which may lead to subsequent placental complications. Diffusion of different layers were measured in the study by Fujimoto et al[59], showing that FA was the highest for junctional zone (0.297), followed by outer myometrium (0.257) and endometrium (0.186); fibers were the longest in outer myometrium (42.0 mm), followed by junctional zone (34.2 mm) and endometrium (20.0 mm). This presented detailed and quantified data for the understanding of the layered structure of uterus. The study by He et al[60] revealed that uterine FA and ADC values had dynamic changes during menstrual cycle, which includes several different phases: Menstrual phase (MP), follicular phase (FP), ovulatory phase (OP) and luteal phase (LP). Specifically, endometrial FA values significantly declined, whereas ADC values increased during MP, and ADC values of myometrium significantly increased from MP to LP. Variation of FA values between MP-FP, MP-OP was found correlated moderately with serum oestradiol levels, and the authors inferred that higher serum oestradiol levels were accompanied by larger increase of the endometrium with higher isotropy of water diffusion directionality[60].
The DTI data of diseased uterus is quite scarce. Toba et al[61] investigated the feasibility of DTI for evaluating the myometrial invasion of endometrial cancer ex vivo. Myometrial infiltration of endometrial tumor may be detected with the disruption of the anisotropic layer, as the FA values of the tumors (0.21 ± 0.05) were significantly lower than the inner layer of the myometrial zone (0.44 ± 0.01) and exterior myometrium (0.32 ± 0.08)[61]. More pathologies need to be included to expand the understanding of DTI in uterus with abnormality.
DTI OF OTHER PELVIC STRUCTURES
DTI, especially with tractography, has been used to depict the fiber orientation of skeletal muscles to some extent. The female pelvic floor, which has a multilayered complex anatomy and includes several closely aligned muscles, were investigated by Zijta et al[62,63] with small samples. The assumption was to use DTI with fiber tractography to depict normal pelvic floor anatomy, and to further evaluate pelvic organ prolapse. Several clinically relevant pelvic structures (including the perineal body, anal sphincter complex, internal obturator muscle and the puboperineal muscle) were satisfactorily and reliably identified, but no significant differences in DTI parameters were found between the prolapse group and the asymptomatic group overall[62,63].
One study has investigated the anal canal structures by DTI so far. FA values of various layers of normal anal canal were measured, and good inter-rater agreement and test reproducibility were achieved[64]. However, further data remains lacked.
FUTURE DIRECTIONS OF RESEARCH
DTI scans of abdominal and pelvic organs still confront with technical limitations: Not only common issues related to abdominal and pelvic MRI such as motional artifacts due to breathing and peristalsis, but also additional problems related to DTI such as the low SNR of EPI sequence due to gradient eddy currents, B0 field inhomogeneity and susceptibility difference[17]. It seems state of art to balance the longer acquisition time and less motional artifacts by either improved sequence or motional triggering protocols. Further studies can explore better techniques to increase SNR, reduce geometrical distortions and assess the contribution of IVIM.
Another issue is the methodology of post-processing and parameter measurements, which would influence the reliability and repeatability of scan results. Factors influencing the DTI parameter measurements, such as hydration state and blood flow of targeted organs, should be further understood in order to standardize patient conditions[24,65]. Besides, consensus is needed on the placement of regions of interest (ROIs), where mean FA was calculated, since ROIs were manually delineated along the outlines of the targeted organs in some studies, but were placed in representative regions of the organ or tissue in other studies[17,22].
In general, DTI is more applied in diffused diseases of solid organs than in focal lesions. Organs with more directionally arranged structures have been explored to a greater extent, such as the kidney. In the circumstances that DTI parameters such as FA was reported of less diagnostic value, it should be noted that the underlying pathologic conditions that led to a final presentation of disease (such as renal function impairment or liver fibrosis) can be heterogeneous[14,38]. Future studies can explore the potential of DTI to differentiate between these pathologic conditions.
In focal lesion assessment, it is suggested that FA might reflect the histology of tumors, in particular of tumor cell density, and it is expected that locations with higher FA would have higher tumor cellularity, as well as a relatively higher Ki-67 labeling index, and therefore a higher malignant potential[16,52,66]. Future studies could apply DTI in lesions with prominent cellularity changes. For example, it is known that the density of gastrointestinal stromal tumor metastases decrease after cytostatic chemotherapy, and FA might be shown of value in these group of patients[67].
The last but not the least, DTI scan should be further integrated into the current multi-modality evaluation in clinical practice. For instance, it is mentioned that the differentiation of hepatic masses such as cysts, hemangiomas, and metastases can be performed easily by well-established imaging criteria. In this regard, performing DTI might seem unnecessary[16]. Another example is the diagnosis of prostate cancer, where multi-modality functional imaging has been researched extensively. Combined algorithm can be designed in future studies with the purpose of a more precise and full-scaled assessment.
CONCLUSION
In conclusion, DTI has been explored and applied in abdominal and pelvic organs such as liver, pancreas, kidneys, prostate, uterus, etc. Technical advances enabled generally satisfactory image quality and measurement repeatability. DTI appears to be more valuable in the evaluation of diffused diseases of organs with highly directionally arranged structures, such as the assessment of function impairment of native and transplanted kidneys. However, the utility of DTI to diagnose focal lesions remains limited. It is suggested that DTI parameters might potentially depict certain pathologic characterization such as cell density. Furthermore, DTI should be better integrated into the current multi-modality evaluation in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shin C S- Editor: Ji FF L- Editor: A E- Editor: Li D