Published online Apr 26, 2017. doi: 10.13105/wjma.v5.i2.63
Peer-review started: October 8, 2016
First decision: November 29, 2016
Revised: February 17, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: April 26, 2017
Processing time: 204 Days and 16.4 Hours
To assess mucin expression in pancreatic premalignant and malignant states, and to establish its role as a prognostic marker.
English Medical literature searches were conducted for “mucin” and “pancreas”. Observational studies were included. Meta-analysis was performed by using Comprehensive meta-analysis software. Pooled odds ratios and 95%CIs were calculated.
Out of 949 eligible papers we found 20 according to the inclusion criteria, including 4262 patients, published till May 31, 2016. Mucin expression increased in pancreatic lesions with OR 10.206 (95%CI: 4.781-21.781, P < 0.0001). Measure of heterogeneity was high: Q = 296.973, df (Q) = 55.00, I2 = 81.48%. We found a significant increase in the expression of MUC2, MUC4 and MUC5AC, 13.39, 118.43 and 13.91 times respectively, in pancreatic lesion in comparison with normal pancreatic tissue, and decreased expression of MUC5B.
Mucin expression may serve as prognostic marker for transformation of intraductal papillary mucinous neoplasms to ductal adenocarcinoma, for aggressiveness of the pancreatic tumor, and as targets for potential therapy.
Core tip: There is a higher mucin expression in intraductal papillary mucinous neoplasms (IPMN) and ductal pancreatic cancer. Mucin expression may be a bad prognostic factor. MUC2, MUC4, MUC5AC and probably MUC1, are expressed in IPMN advanced to ductal adenocarcinoma. These mucins are also bad prognostic factors for ductal adenocarcinoma.
- Citation: Niv Y. Mucin expression and the pancreas: A systematic review and meta-analysis. World J Meta-Anal 2017; 5(2): 63-70
- URL: https://www.wjgnet.com/2308-3840/full/v5/i2/63.htm
- DOI: https://dx.doi.org/10.13105/wjma.v5.i2.63
Mucins are high-molecular-weight glycoproteins, heavily glycosylated, synthesized and secreted by all mucosal surfaces of the human body and have an important role in healthy state and malignant diseases[1-3]. Change in mucins synthesis and secretion may be primary event or secondary to carcinogenesis or inflammation.
There are 21 known mucin genes in the human genome, encode for 2 types of mucins: Secreted and membrane-bound[4]. Membrane-bound mucins are involved in cell signaling and have a role in cellular processes such as growth, immune modulation, motility and adhesion.
Pancreatic carcinogenesis is associated with genetic and epigenetic changes, than may affect MUCs (mucin genes). MUCs may be expressed de novo during carcinogenesis. Mucins have potential value for diagnosis and follow-up of pancreatic neoplasms and for therapeutic interventions[5]. Mucin expression patterns may serve as a criterion for classification of intraductal papillary mucinous neoplasms (IPMN).
Several studies looked at mucins expression, comparing pancreatic lesions with normal pancreatic tissue. MUC1, membrane-bound mucin, is expressed in normal pancreatic tissue, but there is no detectable MUC2, MUC4 and MUC5AC[6,7].
Secretion of MUC1 is associated with adenocarcinoma and high grade dysplasia in pancreatic intraepithelial neoplasia (PanIN)[8-10]. MUC1 is rarely expressed in IPMN.
Positive expression of MUC2 in IPMN (intestinal type) indicates progression to carcinoma with secretion of MUC1[8,11]. Absence of MUC2 expression (gastric type) implies benign phenotype. MUC1 is rarely expressed in mucinous cyst in one study, while in another study mucinous cysts were found positive for MUC1/DF3[12,13].
MUC4 secretion correlates to the severity of dysplasia in PanIN and a poor prognosis in patients with adenocarcinomas, but results are somewhat inconsistent in different studies[14-17]. Expression of MUC4 in pancreatic cancer cell line was associated with increased proliferation, motility, adhesion, aggregation and metastasis[18].
The 2015 American Gastroenterological Association guidelines define 3 high-risk features of pancreatic cyst for developing cancer: Cyst size > 3 cm, dilated pancreatic duct and mural nodule[19]. There are no characteristics of mucin expression in the cyst fluid or the epithelial lining, as a marker for carcinogenesis.
The aim of this metaanalysis and systematic review is to assess the knowledge about mucin expression in pancreatic premalignant and malignant states, and to understand the possible role of mucin expressions as prognostic markers.
Searches were conducted for “mucin” and “pancreas” through May 31, 2016, using MEDLINE, PubMed, Scopus, EMBASE, and CENTRAL. Hand searches of articles references were also performed. Only fully published human studies in English were included (Figure 1).
Observational studies about mucin expression in pancreatic tissue of cysts and adenocarcinoma were included. PRISMA guidelines for systematic reviews were strictly followed.
Author, country, year of publication, number of patients, and the number of positive staining were extracted. Data was stratified according to lesions (ductal adenocarcinoma, IPMN, mucinous cyst) and according to the mucin expressed (Table 1).
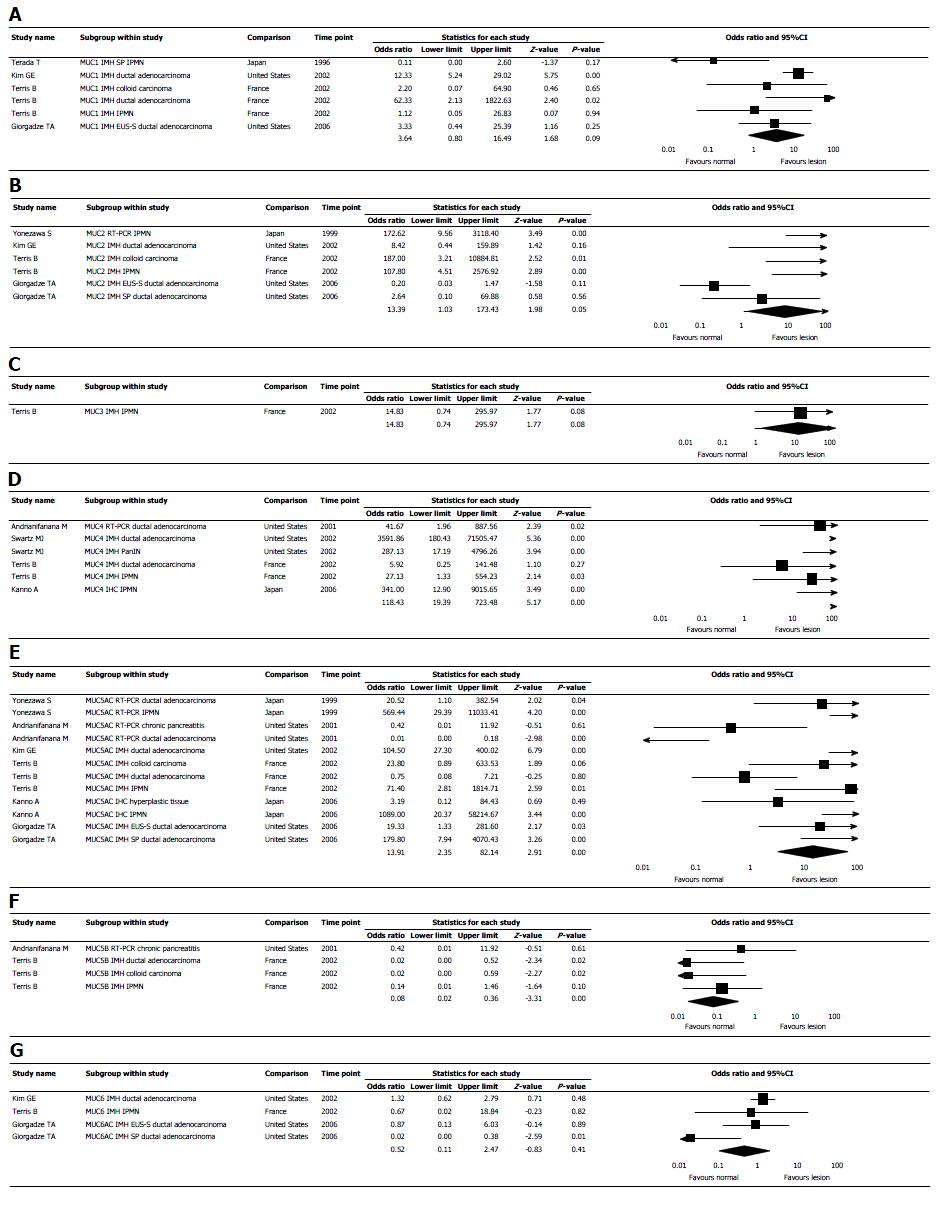
| Mucin gene | OR of mucin expression | P |
| MUC1 | 3.64 | 0.09 |
| MUC2 | 13.39 | 0.05 |
| MUC3 | 14.33 | 0.08 |
| MUC4 | 118.43 | < 0.001 |
| MUC5AC | 13.91 | < 0.001 |
| MUC5B | 0.08 | < 0.001 |
| MUC6 | 0.52 | 0.41 |
| MUC7 | 0 | NA |
| Total mucin | 9.99-21.72 | < 0.001 |
Metaanalysis was performed by using Comprehensive metaanalysis software (Version 3, Biostat Inc., Englewood, NJ, United States). Pooled odds ratios and 95%CIs were calculated for mucin expression in pre-malignant and malignant pancreatic lesions. In all methods used (IMH, ISH or RT-PCR) OR represents quantitatively the number of patients with higher expression.
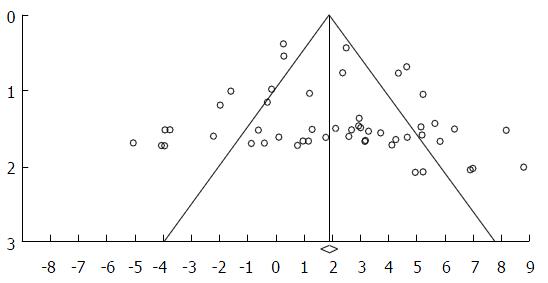
Heterogeneity was evaluated by Cochran Q-test, and considered to be present when Q-test P < 0.10. I2 statistic was used to measure the proportion of inconsistency. We calculated publication bias using funnel plot of standard error by log odds ratio. Even distribution of the studies denied significant publication bias.
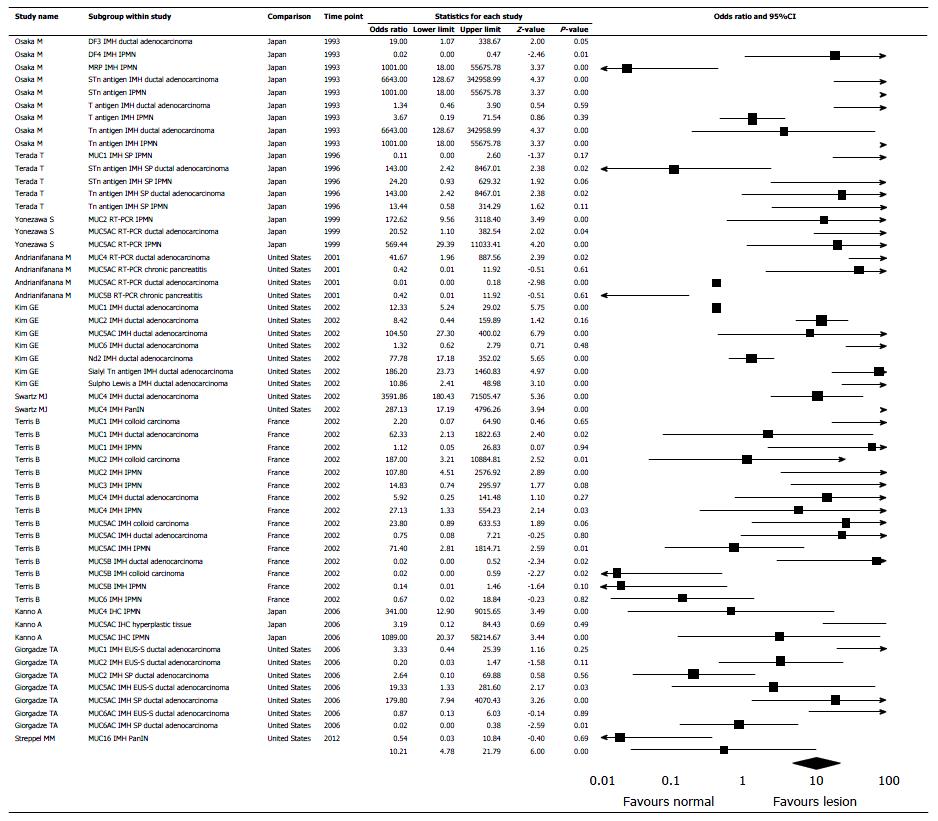
Out of 949 eligible papers we found 20 according to the inclusion criteria, including 4262 patients, published till May 31, 2016 from 4 countries (Japan 10, United States 9, France 1, Norway 1)[6,8,9,11-15,20-32] (Figure 1). There are 134 sub-studies (stratifying data according to mucin types and lesions). In 104 sub-studies immunohistochemistry (IMH) has been used, in 20 sub-studies RT-PCR for RNA, and in10 histochemistry. Eleven studies and 84 sub-studies had also results of normal pancreatic tissue for comparison with the neoplastic lesion. Ductal adenocarcinoma was examined in 14 studies and 60 sub-studies (2206 cases); IPMN was examined in 12 studies and 46 sub-studies (1691 cases). There were 365 cases of mucinous or colloid carcinoma, mucinous cystic neoplasm, hyperplastic pancreatic lesion, chronic pancreatitis and pseudo cysts. Funnel plot denies a significant publication bias (Figure 2).
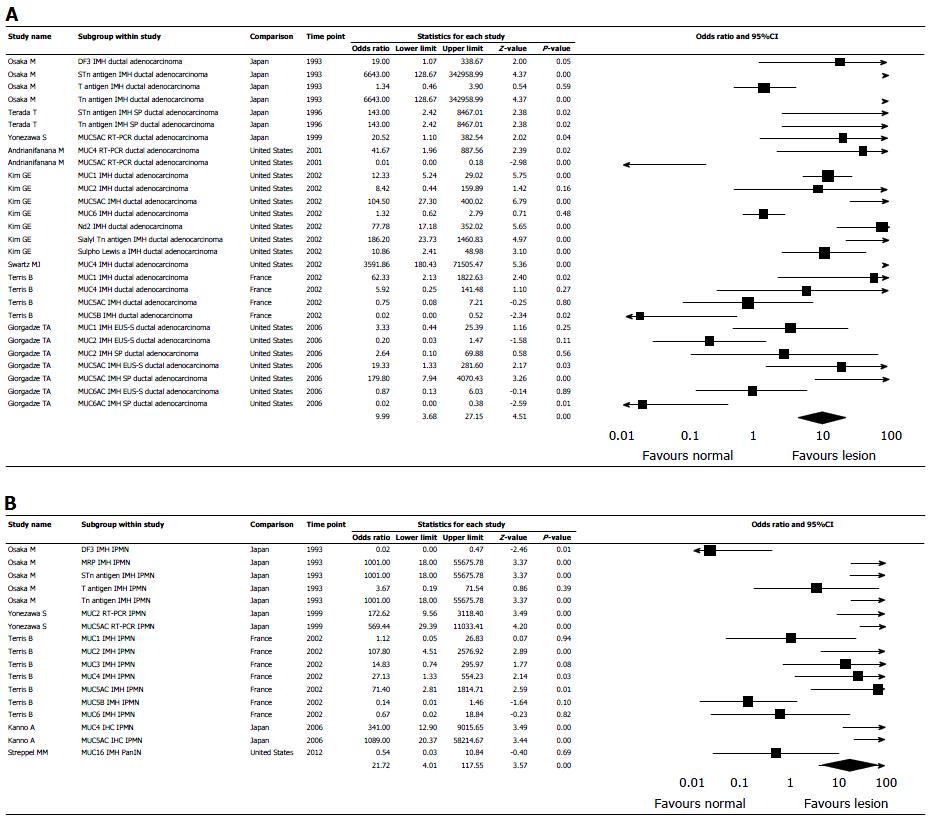
In the random-effect model, mucin expression was significantly higher in pancreatic lesions than in normal pancreatic tissue with OR 10.206 (95%CI: 4.781-21.781, P < 0.0001) (Figure 3). Measure of heterogeneity was high, demonstrated in the included studies: Q = 296.973, df (Q) = 55.00, I2= 81.48%. OR for mucin expression in pancreatic ductal adenocarcinoma and IPMN was 9.99 with 95%CI: 3.68-27.15, P < 0.001, and 21.72 with 95%CI: 4.01-117.55, P < 0.001, respectively (Figure 4). OR for expression in pancreatic lesion of MUC1- 4, MUC5AC, MUC5B, MUC6 and MUC7, was 3.64 with 95%CI: 0.80-16.49, P = 0.09; 13.39 with 95%CI: 1.03-173.43, P = 0.05; 14.33 with 95%CI: 0.742-95.97, P = 0.08; 118.43 with 95%CI: 19.39-723.48, P < 0.001; 13.91 with 95%CI: 2.35-82.14, P < 0.001; 0.08 with 95%CI: 0.02-0.36, P < 0.001; 0.52 with 95%CI: 0.11-2.47, P = 0.41; respectively (Figure 5). MUC7 was never expressed in pancreatic lesion or normal tissue (Table 1).
Yamada et al[20] using histochemical methods compared the mucin expression between malignant and benign tumors of the pancreas. They found significant higher expression of sialomucin (> 50% of glands) in malignant tumors and higher expression of neutral mucin (> 50% of glands) in benign tumors. Osako et al[21] demonstrated a significant contrast between expression of mammary type mucin and intestinal type mucin in carcinomas and intraductal papillary tumor. The oncogenic mucin antigens, Tn and sialyl Tn (STn), were expressed in malignant and premalignant states but not in normal pancreatic mucosa. Incomplete glycosylation of mucins that results in expression of T, Tn, and sialyl-Tn antigens in pancreatic adenocarcinoma was described by Terada et al[13,32]. They found increased expression of Tn antigen and STn antigen in comparison with normal pancreatic tissue, but the same expression of MUC1 and T antigen. Similar findings were described for IPMN, which support the sequence of events from IPMN to adenocarcinoma. Yonezawa et al[8] found higher expression of MUC1 in ductal adenocarcinoma than IPMN, and lower expression of MUC2. Invasive growth areas of IPMN had MUC1 expression similar to adenocarcinoma. The same group demonstrated up regulation of MUC5AC mRNA in IPMN cases with a favorable prognosis, whereas such expression was not found in ductal adenocarcinoma cases with a poor prognosis[22].
Andrianifahanana et al[23] described a significant higher MUC4 expression in adenocarcinoma tissue than in chronic pancreatitis or normal pancreatic tissue. Lüttges et al[9] found expression of MUC2 in all IPMN and mucinous carcinoma cases of the pancreas but in only one of 35 of ductular adenocarcinoma cases. MUC1 expression was only demonstrated in ductular adenocarcinoma tissue. The same group also found strong expression of MUC5AC and MUC2 in mucinous cystic neoplasms of the pancreas, but no such expression of MUC1 and MUC6[12]. Kim et al[24] found a significant higher expression of MUC1, MUC5AC, Md2, STn antigen and sulpho Lewis a antigen in ductal adenocarcinoma of the pancreas than in normal pancreatic tissue. Swartz et al[14] found higher expression of MUC4 in invasive ductal adenocarcinoma of the pancreas than in PanIN. Expression was not demonstrated in normal pancreatic tissue. Nakamura et al[11] described 2 kinds of IPMN, according to MUC2 expression with higher invasive property for MUC2 positive than negative tumors. Terris et al[25] found increased expression of MUC5AC and MUC2 in IPMN, similar to colloid carcinoma, and different from ductal adenocarcinoma where MUC1 expression was increased. Horinouchi et al[6] found higher expression of MUC1 and MUC5AC in ductal adenocarcinoma than in IPMN. MUC2 was only expressed in IPMN of “dark phenotype”. Saitou et al[15] found a positive correlation between the strength of MUC4 expression in ductal adenocarcinoma of the pancreas and aggressive behavior. Such a correlation could not be demonstrated for MUC1. Kanno et al[26] found MUC4 and MUC5AC expression in adenoma and IPMN but not in normal or hyperplastic pancreatic tissue. Giorgadze et al[27] reviewed pancreatic 56 EUS-FNA specimens and 26 pancreatectomy specimens for expression profiles of MUC1, MUC2, MUC5AC and MUC6. MUC5AC expression was significantly higher in adenocarcinoma than in normal tissue both in EUS-FNA specimens and surgical specimens. Westgaard et al[28] found that in adenocarcinoma MUC1 and MUC4 expression was associated with a poor prognosis. Gonzalez Obeso et al[29] used alcian blue and mucicarmine stains in 11 pseudo cysts and 42 IPMNs or mucinous cysts aspirates. They could not demonstrate a significant difference in mucin staining between the various types of cysts. Streppel et al[30] found MUC16 (CA125) expression in 81.5% of 200 pancreatic adenocarcinoma tissues, in comparison with none of 29 IPMN cases and in 2% of normal pancreatic tissues. Kitazono et al[31] looked at the expression rates of MUC4 in intestinal-type IPMNs and gastric-type IPMNs using monoclonal antibodies 8G7 and 1G8. The expression rate of MUC4 in the intestinal-type IPMNs was higher than in the gastric-type IPMNs. Maker et al[33] examined 40 cases of pancreatic IPMN comparing mucin expression in cases with high risk IPMN (with high grade dysplasia or carcinoma) and cases with low risk IPMN (with low grade dysplasia). They found a significant increase in MUC2 and MUC4 expression (10.0 ± 3.0 ng/mL and 20.6 ± 10.6 ng/mL vs 4.4 ± 1.2 ng/mL and 4.5 ± 1.4 ng/mL, P = 0.03, respectively). No change was demonstrated for MUC1 and MUC5AC. This study is not included in the metaanalysis since numerical data is absent and only means of mucin expression are given.
Mucin is an important component of the mucus layers protecting epithelial surfaces of the respiratory, digestive, urinary and reproductive organs, and as such was studied intensively. The role of mucin in exocrine/endocrine gland such as the pancreas is less understood. Most of the studies about pancreatic mucin expression involved malignant transformation and characteristics of pancreatic cysts. In Table 1 we summarized the knowledge about mucin expression in the pancreas, including the findings of our metaanalysis.
In our metaanalysis we found a significant increase in the expression of MUC2, MUC4, and MUC5AC, 13.39, 118.43 and 13.91 times respectively, in pancreatic lesion in comparison with normal pancreatic tissue (Table 1), and decreased expression of MUC5B. The results for MUC1, MUC3, MUC6, Tn and STn were not statistically significant.
Exploring individual studies some different and inconsistent finding are presented, but it is obvious that higher malignant behavior of IPMN and transfer into ductal adenocarcinoma is characterized by increased expression of MUC2, MUC4 and MUC5AC[9,12-15,20,21,23,26-28,33]. The expression of these mucins in the ductal adenocarcinoma implied a bad prognosis. MUC1 expression, even though did not reach significance in the metaanalysis, was also a marker for bad prognosis in ductal adenocarcinoma[8,24,28].
IPMN could be originates from the pancreatic main duct, or side-branches, being of gastric type (MUC5AC is expressed in dark cells) or of intestinal type (MUC2 is expressed in clear cells). Gastric IPMNs are MUC1 and MUC2 negative, usually located in the branch small ducts, and rarely develop into cancer. Intestinal IPMNs are MUC1 negative but MUC2 positive. However, when they transform into cancer, the MUC1 becomes positive. They are mostly located in the main duct. MUC4 expression in IPMNs may help to distinguish intestinal IPMNs from the safer gastric-type IPMNs.
Our meta-analysis has some limitations, since the methods of mucin expression measurement, and the definition of the pancreatic lesion may be inaccurate. There is heterogeneity regarding detection of mucin expression and disease classification. In some studies immunohistochemistry was used for protein detection and in other RT-PCR or in situ hybridization for RNA detection. The definition of pancreatic mucinous cyst and side-branch or main-duct IPMN (Previously IPMT) also was changed during the last decade, and the results of different mucins expression in different lesions should be taken with caution. Also PanIN (pancreatic intra epithelial neoplasia), the pancreatic gland equivalent of adenomatous change or dysplasia, has been never studied in the context or mucin genes expression.
In conclusion, expression of MUC2, MUC4, MUC5AC and probably MUC1, may serve as prognostic marker for transformation of IPMN to ductal adenocarcinoma, for aggressiveness of the pancreatic tumor, and as targets for potential therapy. Further studies are needed to establish these observations.
Pancreatic carcinogenesis is associated with genetic and epigenetic changes, that may affect mucin genes. Certain mucins are expressed during carcinogenesis, while specific patterns have been recognized in pre-malignant and malignant lesions.
Assessing mucin expression in pancreatic premalignant and malignant states, and to establish its role as a prognostic marker.
Mucin expression was higher in pancreatic lesions than in healthy pancreatic tissue: OR 10.206 (95%CI: 4.781-21.781, P < 0.0001).
The author found a significant increase in the expression of MUC2, MUC4 and MUC5AC, 13.39, 118.43 and 13.91 times respectively, in pancreatic lesion in comparison with normal pancreatic tissue, and decrease expression of MUC5B.
This is a very good paper, with a large amount of interesting data and work. The analysis is conducted respecting the protocols of meta-analysis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Biondi-Zoccai G, Kleeff J, Luchini C, Moyana TN, Rosemurgy AS S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589-594. [PubMed] |
| 4. | Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77-99. [PubMed] |
| 5. | Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472-2481. [PubMed] |
| 6. | Horinouchi M, Nagata K, Nakamura A, Goto M, Takoo S, Sakamoto M, Fukushima N, Miwa A, Irimura T, Imai K. Expression of membrane mucin (MUC1) and secretory mucin (MUC2, MUC5AC, and MUC6) in pancreatic neoplasms. Acta Histochem Cytochem. 2003;36:443-453. |
| 7. | Klöppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006;44:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, Takao S, Aiko T, Yamamoto M, Irimura T. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319-322. [PubMed] |
| 9. | Lüttges J, Zamboni G, Longnecker D, Klöppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942-948. [PubMed] |
| 10. | Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, Imai K, Kim YS, Sato E, Yonezawa S. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466-471. [PubMed] |
| 13. | Terada T, Ohta T, Sasaki M, Nakanuma Y, Kim YS. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol. 1996;180:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791-796. [PubMed] |
| 15. | Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. [PubMed] |
| 17. | Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, Gum JR, Batra SK, Rousseau K, Swallow DM. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48-e54. [PubMed] |
| 18. | Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622-630. [PubMed] |
| 19. | Vege SS, Ziring B, Jain R, Moayyedi P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 755] [Article Influence: 75.5] [Reference Citation Analysis (1)] |
| 20. | Yamada M, Kozuka S, Yamao K, Nakazawa S, Naitoh Y, Tsukamoto Y. Mucin-producing tumor of the pancreas. Cancer. 1991;68:159-168. [PubMed] |
| 21. | Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191-2199. [PubMed] |
| 22. | Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45-54. [PubMed] |
| 23. | Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Büchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033-4040. [PubMed] |
| 24. | Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052-1060. [PubMed] |
| 25. | Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Fléjou JF. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Kanno A, Satoh K, Kimura K, Hirota M, Umino J, Masamune A, Satoh A, Asakura T, Egawa S, Sunamura M. The expression of MUC4 and MUC5AC is related to the biologic malignancy of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2006;33:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Giorgadze TA, Peterman H, Baloch ZW, Furth EE, Pasha T, Shiina N, Zhang PJ, Gupta PK. Diagnostic utility of mucin profile in fine-needle aspiration specimens of the pancreas: an immunohistochemical study with surgical pathology correlation. Cancer. 2006;108:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Westgaard A, Schjølberg AR, Cvancarova M, Eide TJ, Clausen OP, Gladhaug IP. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54:337-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Gonzalez Obeso E, Murphy E, Brugge W, Deshpande V. Pseudocyst of the pancreas: the role of cytology and special stains for mucin. Cancer. 2009;117:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, Goggins MG, Van Seuningen I, Maitra A, Montgomery EA. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Kitazono I, Higashi M, Kitamoto S, Yokoyama S, Horinouchi M, Osako M, Shimizu T, Tabata M, Batra SK, Goto M. Expression of MUC4 mucin is observed mainly in the intestinal type of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Terada T, Nakanuma Y. Expression of mucin carbohydrate antigens (T, Tn and sialyl Tn) and MUC-1 gene product in intraductal papillary-mucinous neoplasm of the pancreas. Am J Clin Pathol. 1996;105:613-620. [PubMed] |
| 33. | Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, Jarnagin WR, Brennan MF, Allen PJ. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |