Published online Aug 26, 2016. doi: 10.13105/wjma.v4.i4.95
Peer-review started: March 14, 2016
First decision: April 14, 2016
Revised: April 28, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: August 26, 2016
Processing time: 167 Days and 22.1 Hours
To perform a meta-analysis assessing the value of gadoxetic acid-enhanced magnetic resonance imaging (Gd-EOB-MRI) in detecting small hepatocellular carcinoma (HCC) (≤ 2.0 cm) in patients with chronic liver disease.
Databases, including MEDLINE and EMBASE, were searched for relevant original articles published from January 2008 to February 2015. Data were extracted, and summary estimates of diagnostic accuracy indexes such as sensitivity, specificity, diagnostic odds ratio, predictive value, and areas under summary receiver operating characteristic curve were obtained using a random-effects model, with further exploration employing meta-regression and subgroup analyses.
In 10 studies evaluating 768 patients, pooled per-lesion sensitivity of Gd-EOB-DTPA was 91% (95%CI: 83%-95%), with a specificity of 95% (95%CI: 87%-98%). Overall positive likelihood ratio was 18.1 (95%CI: 6.6-49.4), for negative likelihood ratio (NLR) of 0.10 (95%CI: 0.05-0.19) and diagnostic odds ratio of 182 (95%CI: 57-581). Subgroup analysis suggested that diagnostic performance of Gd-EOB-MRI for sub-centimeter HCC (≤ 1.0 cm) detection was low, with a sensitivity of 69% (95%CI: 59%-78%). In studies with both Gd-EOB-MRI and diffusion-weighted imaging (DWI) performed, Gd-EOB-MRI/DWI combination was more sensitive than Gd-EOB-DTPA alone, whether for small lesions (86% vs 77%) or sub-centimeter ones (80% vs 56%).
A limited number of small studies suggested that Gd-EOB-MRI has good diagnostic performance in the detection of small HCC (≤ 2.0 cm) among patients with chronic liver disease, but relatively lower performance for detection of sub-centimeter HCC (≤ 1.0 cm). Combination of Gd-EOB-MRI and DWI can improve the diagnostic sensitivity of MRI.
Core tip: Although studies have shown that gadoxetic acid-enhanced magnetic resonance imaging (Gd-EOB-MRI) had good diagnostic performance in detecting hepatocellular carcinoma (HCC), the results about small HCC have been limited thus far by a small number of included patients, especially for subcentimeter lesion (≤ 1.0 cm). Therefore, we performed a systematic review and meta-analysis to obtain updated diagnostic performance values of Gd-EOB-MRI for the detection of small HCC in terms of different size (≤ 2.0 cm vs≤ 1.0 cm), different technique (Gd-EOB-MRI alone vs combined diffusion weighted imaging).
- Citation: Shan Y, Gao J, Zeng MS, Lin J, Xu PJ. Gadoxetic acid-enhanced magnetic resonance imaging for the detection of small hepatocellular carcinoma (≤ 2.0 cm) in patients with chronic liver disease: A meta-analysis. World J Meta-Anal 2016; 4(4): 95-104
- URL: https://www.wjgnet.com/2308-3840/full/v4/i4/95.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i4.95
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related deaths worldwide[1]. Despite important advances in multidisciplinary therapies, complete curative treatment of early-stage small HCC (≤ 2.0 cm, including hypervascular and hypovascular HCC) remains the only option for long-term patient survival. Studies indicated that the smaller the HCC, the less likely the occurrence of microvascular invasion[2].The International Consensus Group for Hepatocellular Neoplasia also stated that early HCC, well differentiated HCC with a vaguely nodular appearance and less than 2 cm in size, should be considered a carcinoma in situ, and is characterized by an indistinct margin without capsule formation, vascular invasion or intrahepatic metastasis[3,4]. In addition, the smaller the HCC, the more likely it is for local ablation to be complete[5,6]. It is therefore important to perform early diagnosis of HCC when the tumor is still as small as possible. However, in small nodules (≤ 2.0 cm), an atypical vascular profile is not uncommon, which constitutes a challenge for definitive radiological diagnosis. These lesions may, in fact, represent either early HCCs or preneoplastic lesions, such as high-grade dysplastic nodules[3,7,8]. They are often hypovascular and lack arterial enhancement or a washout pattern[7,8]. In addition, many small, benign nodules (e.g., cirrhosis-related nodules and arterioportal shunts) can mimic small HCC in patients with cirrhosis.
The hepatocyte-specific magnetic resonance imaging (MRI) contrast agent gadoxetic acid (Gd-EOB-DTPA; Bayer Healthcare, Berlin, Germany) can provide, in a single examination, comprehensive hemodynamic information during early dynamic phases and improved lesion detection in the hepatobiliary phase (HBP)[9-11]. HBP images better depict HCC, which appears as a hypointense lesion, compared with conventional dynamic gadolinium-enhanced images, on which small HCCs frequently show only arterial enhancement without early washout[12-14].
Although studies have compared gadoxetic acid-enhanced MRI (Gd-EOB-MRI) with multidetector computed tomography (MDCT) and Gd-DTPA-enhanced MRI for detecting small HCC, and shown that HBP imaging provides a slight improvement in the diagnosis of small HCC[10,11,15-22], the results were limited thus far by the small numbers of included patients, especially for sub-centimeter lesions (≤ 1.0 cm). Therefore, we performed a systematic review and meta-analysis of the literature published in the past few years, to obtain updated diagnostic performance values of Gd-EOB-MRI for detecting small HCC in patients with chronic liver disease.
A comprehensive literature search of studies evaluating human subjects was performed by two investigators (Yan Shan and Peng-Ju Xu) to identify articles on diagnostic performance of Gd-EOB-MRI in detecting small HCC in patients with chronic liver disease. The PubMed and EMBASE databases were searched from January 2008 to February 2015, for English articles with the following keywords: (Gd-EOB-DTPA or gadoxetic acid or gadoxetate disodium or Gd-EOB-MRI) and (hepatocellular carcinoma or liver neoplasms) and (sensitivity or specificity or false negative or false positive or diagnosis or detection or accuracy). Other databases, such as Web of Science, Scopus and the Cochrane Database of systematic review, were also searched for relevant articles. All review articles, comments, case reports, letters, and unpublished articles were eliminated. Articles found to be eligible based on title, and subsequently abstract, were then selected to determine further suitability for inclusion in this study.
Studies were included if, in addition, all the following inclusion criteria were met: (1) articles reported in English; (2) Gd-EOB-MRI with HBP performed to evaluate small HCC in patients with chronic liver disease; (3) histopathology analysis and/or cross-sectional imaging follow-up used as the reference standard; (4) data based on per-lesion basis; and (5) sufficient data reported to construct 2 × 2 contingency tables. Authors of studies with insufficient published data were contacted personally in an effort to retrieve the missing data. Studies were excluded if either of the following exclusion criteria were applicable: (1) fewer than 10 patients; or (2) multiple reports published for the same study population (in this case, the publication with the most details and/or most recently published was selected).
The methodological quality of the included studies was assessed independently by the same two investigators using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool in Review Manager 5.3, which evaluates the risk of bias for four domains and clinical applicability for three domains of study characteristics. The QUADAS-2 tool was used as provided by the QUADAS-2 group[23]. Meanwhile, relevant data were also extracted from each study, including author, publication year, sample size, number of lesion, description of study population (age and gender), study design (case series, case control, cohort study, and randomized controlled trial), patient enrollment (consecutive or not), etiology of liver disease, magnetic field strength, dose of Gd-EOB-DTPA, number of experts who assessed and interpreted Gd-EOB-MRI data, and mean time interval between Gd-EOB-MRI and histopathology. Any mention Gd-EOB-MRI measurement blinding to histopathologic and clinical results and/or other diagnostic methods used was also recorded. For each study, the number of true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) findings was recorded for Gd-EOB-MRI in detecting small HCC in patients with chronic liver disease. Disagreements were resolved by discussion between the two investigators.
Diagnostic accuracy: Data regarding diagnostic performance of Gd-EOB-MRI were combined quantitatively across eligible studies. In addition, bivariate random-effects model and hierarchical summary receiver operating characteristic (ROC) were used to obtain summary estimates of sensitivity and specificity[24]. Diagnostic odds ratio (DOR) and likelihood ratios are also metrics that combine both sensitivity and specificity in calculations.
Heterogeneity exploration and subgroup analysis: Heterogeneity was assessed by likelihood χ2 tests and I2. The I2 index is a measure of the percentage of total variation across studies due to heterogeneity beyond chance. Values of 30%-60%, 50%-90%, and 75%-100% may represent moderate, substantial and considerable heterogeneity, respectively[25]. In likelihood ratio χ2 tests, P < 0.05 was regarded as indicative of apparent heterogeneity. The threshold effect is an important extra source of variation in meta-analysis. If there is a threshold effect, an inverse correlation appears; in this case, combining study results involving fitting a ROC curve was better than pooling sensitivities and specificities. To assess threshold effect existence, sensitivity and specificity for Gd-EOB-MRI were plotted on an ROC plane[26]. Moreover, Spearman correlation coefficient (between the logit of sensitivity and that of specificity) was determined for Gd-EOB-MRI. In case no threshold effect was found in the meta-analysis, meta-regression analysis with a backward stepwise algorithm was then performed to investigate other sources of heterogeneity for Gd-EOB-MRI. Such factors included the type of study design (case series, case control, cohort study, and randomized controlled trial), use of the same reference standard, enrollment patients, age (year), gender, sample size, number of lesions, diameter of HCC, MRI field strength, dose of Gd-EOB-DTPA, mean time interval between Gd-EOB-MRI and histopathology, reviewers (year of experience), and publication year.
Subgroup analysis was performed according to lesion size (≤ 2.0 cm vs≤ 1.0 cm); We also compared the performance of Gd-EOB-MRI alone with that of its combination with DWI by analyzing studies that used these diagnostic methods in the same patients.
Publication bias: Publication bias was assessed visually using a scatterplot of the inverse of the square root of the effective sample size (1/ESS1/2) against diagnostic log odds ratio, which should have a symmetric funnel shape when no publication bias is present. Formal testing for publication bias was conducted using a regression of diagnostic log odds ratio against 1/ESS1/2 and weighting according to the effective sample size, with P < 0.01 indicating significant asymmetry[27].
Statistical analysis was performed with Stata statistical software Version 12 (StataCorp LP, Texas, United States) and Meta-DiSc statistical software, version 1.4 (Unit of Clinical Biostatistics, Ramo’n y Cajal Hospital, Madrid, Spain). P < 0.05 was considered statistically significant.
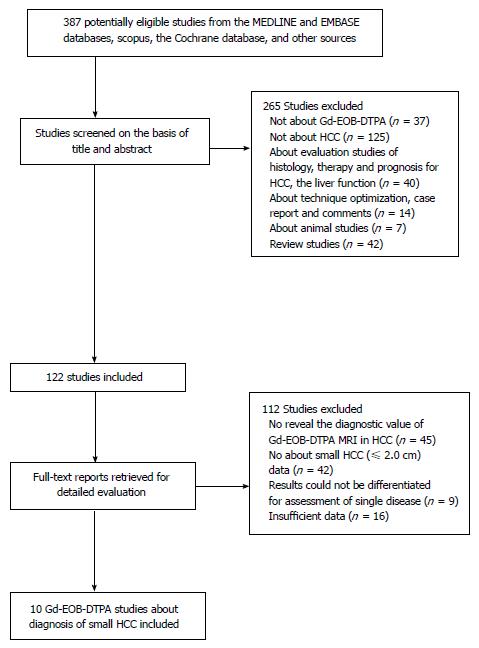
After a comprehensive computerized search was performed, with reference lists extensively cross-checked, this research yielded 387 primary studies; 265 studies were excluded after title and abstract review. One handred twelve articles were excluded after reviewing the full article for the following reasons: (1) study aim did not reveal Gd-EOB-MRI in detecting HCC (n = 45); (2) results were obtained from a combination of HCC, hepatic metastasis and other hepatic diseases that could not be differentiated for assessment of single disease (n = 9); (3) no results regarding Gd-EOB-DTPA in diagnosis of small HCC (n = 42); and (4) too little data reported to allow construction of a 2 × 2 table of TP, FN, FP and TN values (n = 16). Therefore, a total of 10 studies[9-11,17-21,28,29], which fulfilled all inclusion criteria, were considered for the analysis. The detailed procedure of study selection in the meta-analysis is shown in Figure 1.
The important characteristics of the included studies are detailed in Supplement file for review. In brief, there were no cohort or randomized controlled studies. Most studies were case series. Of all 10 studies, 7 enrolled patients retrospectively[9,11,18-21,29], while 3 stated that they were prospective[10,17,28]. All 10 studies enrolled patients in a consecutive manner[9-11,17-21,28,29]. A total of 768 patients were enrolled in the eligible studies.
There were 5 studies with MRI examinations performed with a 1.5 Tesla device[9,10,17,19,20]; 4 studies performed MRI examinations with 3.0 Tesla devices[11,18,21,29]. In the remaining study, MRI examinations were performed with 3.0 Tesla device in comparison with 1.5 Tesla device[28]. One report used a fixed dose of 10 mL of Gd-EOB-DTPA[11], while in the other 9, Gd-EOB-DTPA was administrated according to the manufacturer’s instructions at 0.025 mmol per kilogram body weight. Evaluation of Gd-EOB-DTPA results was carried out in a blinded fashion in all 10 studies[9-11,17-21,28,29]. The reference standard depended solely on explanted livers in only two studies[20,21].
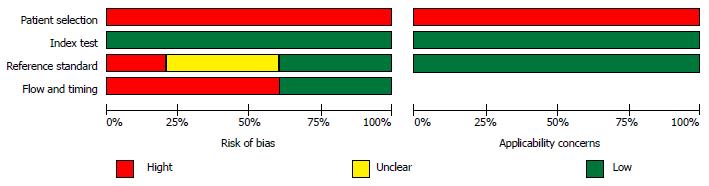
Study quality assessment data obtained with the QUADAS-2 tool are summarized in Figure 2. There were no studies considered to be at low risk of bias for all domains. The included studies being case series or of case-control design, a high risk of bias for patient selection was introduced. The substantial risk of bias regarding patient flow and timing mainly arose from that more than half of these studies used a combination of histopathologic findings and cross-section imaging follow-up as reference standards; this may result in verification bias. There was also a considerable risk of bias regarding the reference standard, as 2 studies reported that the pathologist was not blinded to imaging test results, while 4 others did not mention pathologist blinding to index test results.
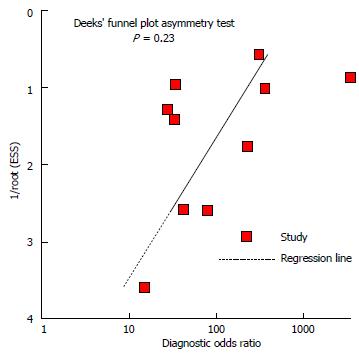
A nonsignificant slope was obtained for Deeks’ funnel plot asymmetry tests (Figure 3), indicating that no significant bias was found (P = 0.23).
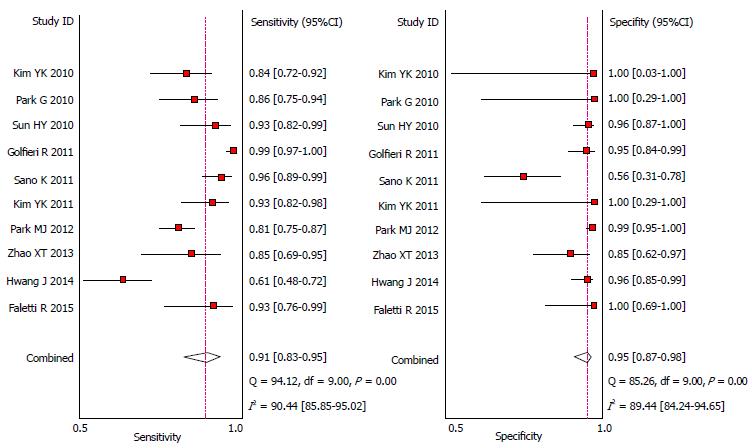
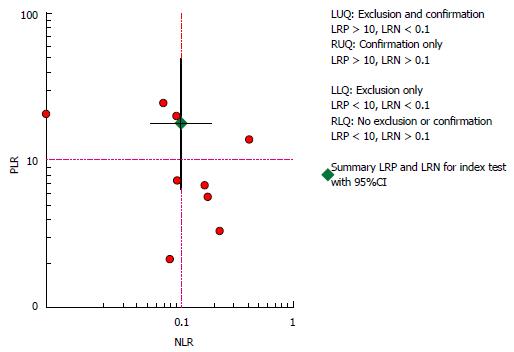
Overall small HCC (≤ 2.0 cm): When studies used multiple readers, giving a range of accuracy, we selected the average result for analysis. Pooled sensitivity of Gd-EOB-MRI was 0.91 (95%CI: 0.83-0.95), for a specificity of 0.95 (95%CI: 0.87-0.98). DOR was 182 (95%CI: 57-581). The detailed sensitivity and specificity data, with 95%CIs for each individual study are provided as a Forest plot in Figure 4. Likelihood ratio syntheses yielded an overall positive likelihood ratio (PLR) of 18.1 (95%CI: 6.6-49.4) and negative likelihood ratio (NLR) of 0.10 (95%CI: 0.05-0.19). The scattergram of PLR and NLR is shown in Figure 5.
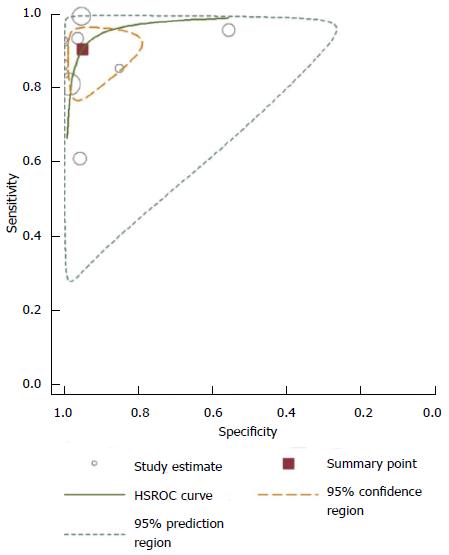
Hierarchical summary receiver operator characteristic (HSROC) curves (Figure 6) showed good diagnostic performance for Gd-EOB-MRI for all the studies combined. The area under the curve of the HSROC was 0.97 (95%CI: 0.96-0.99).
There were three studies with reported results concerning Gd-EOB-MRI for diagnostic performance of sub-centimeter HCC (≤ 1.0 cm)[17,18,21]. For the sub-centimeter HCC subgroup, pooled sensitivity and specificity were 0.69 (95%CI: 0.59-0.78) and 0.94 (95%CI: 0.88-0.98), respectively. Sensitivity for sub-centimeter lesions (0.69) was relatively low than values obtained for all small HCCs (0.91).
Gd-EOB-MRI used alone and in combination with DWI were compared for performance by analyzing 3 studies that employed these diagnostic methods for the same patients[18,21,29]. The results suggested that Gd-EOB-MRI combined DWI was more sensitive compared with Gd-EOB-MRI alone, whether for small HCC or sub-centimeter lesions (Table 1).
| Diagnostic methods compared | Lesion size | Ref. | Summary sensitivity, % (95%CI) | Summary specificity, % (95%CI) |
| Gd-EOB-DTPA MRI alone | ≤ 2.0 cm | [18,21,28] | 0.77 (0.71-0.82) | 0.97 (0.93-0.99) |
| Combined Gd-EOB-DTPA MRI with DWI | 0.86 (0.82-0.90) | 0.92 (0.88-0.96) | ||
| P value | 0.0047 | 0.975 | ||
| Gd-EOB-DTPA MRI alone | ≤ 1.0 cm | [18,21] | 0.56 (0.45-0.69) | 0.96 (0.90-0.99) |
| Combined Gd-EOB-DTPA MRI with DWI | 0.80 (0.68-0.88) | 0.94 (0.87-0.98) | ||
| P value | 0.0013 | 0.709 | ||
The heterogeneity of sensitivity and specificity tests was highly significant (P < 0.05 and I2 > 75%) (Figure 4). This was strong evidence of between-study heterogeneity. Sensitivity and specificity for Gd-EOB-MRI were plotted on an ROC plane, and no curvilinear pattern was found. In addition, Spearman correlation coefficient (between the logit of sensitivity and that of specificity) for Gd-EOB-MRI was 0.237, with a P value of 0.51. No threshold effect was found in this meta-analysis. Meta-regression analysis showed that study design contributed significantly to heterogeneity (P = 0.04). However, other factors did not significantly contribute to study heterogeneity (P > 0.05).
Our results confirmed that Gd-EOB-MRI accurately detects small HCC. Previous reports showed that most HCCs appear as relatively low signal intensity lesions in HBP imaging because of inexistent gadoxetic acid uptake. Therefore, gadoxetic acid is expected to enable excellent lesion detection and characterization for both hypervascular and hypovascular HCCs by arterial phase and HBP imaging, respectively[11,14,16,17,30]. Several studies suggested that hypointensity in HBP imaging, even in the absence of arterial phase hyper-enhancement, is highly predictive of pre-malignant or malignant lesions[7,9,20]. Furthermore, early HCC is essentially hypovascular, with no dominant arterial blood supply. It is not surprising that conventional arterial phase imaging techniques are inefficient in evaluating early HCCs, with Gd-EOB-MRI HBP imaging being the only technique that successfully depicts early HCCs[19]. Previous findings confirmed that arterial hypervascularization delineation in HCC by gadoxetic acid is comparable to that by conventional Gd-DTPA[9]. Furthermore, sensitivity for hypervascular HCC detection is sufficiently high, and HBP images provide an added value to sensitivity, when Gd-EOB-MRI is applied[9,17,19]. However, previous studies found that HBP imaging is almost the only technique that successfully depicts hypovascular HCCs[17,19]. Dynamic contrast-enhanced MRI reveals hypervascular HCCs based on altered arterial vascularity due to the development of unpaired arteries and sinusoidal capillarisation[31]. A pathological explanation of arterial enhancement absence is the weak development of nontriadal arteries in hypovascular nodules (including early HCC), which make their characterization based on dynamic MR phases impossible[3,4,32]. However, hypovascular nodules usually show organic anion-transporting polypeptide under-expression, which begins prior to changes in hemodynamics. Therefore, they appear hypointense in HBP images[33].
We hypothesized that Gd-EOB-MRI and DWI combination has superior diagnostic performance over Gd-EOB-MRI alone, as it provides multi-parametric data such as vascular changes, hepatocyte function and cellular density[20,21,34]. In addition, given the importance of HBP imaging in the detection of small hypovascular HCCs, a considerable number of small HCCs are easily overlooked in the HBP set, particularly the lesions located adjacent to vessels. Thus, hyperintensity on DWI could contribute to improving the detection of small HCCs by helping reduce the number of mischaracterized lesions and allowing more accurate characterization of equivocal lesions[16,18,20,21].
With regard to tumor size in HCC, confident diagnosis of HCC in sub-centimeter hepatic nodules has been considered unfeasible[14,35]. Although per-lesion sensitivity estimates for MR imaging in sub-centimeter HCCs may be further increased with Gd-EOB-DTPA use, it is still relatively low[18,21]. The results of this meta-analysis showed the relatively low per-lesion sensitivity estimates for sub-centimeter HCCs. One possible explanation is that HBP ability to detect malignancies might be reduced in decompensated cirrhosis because gadoxetic acid uptake and metabolism are related to hepatocyte function. Previous studies showed a trend toward decreased sensitivity of Gd-EOB-MRI for detecting small HCC with increasing cirrhosis severity[21,36]. It is clear that a cirrhotic liver shows restricted diffusion in line with hepatic fibrosis severity[37]. Thus, it remains difficult to identify HCC in severely cirrhotic liver in any imaging studies; this limits the usefulness of both Gd-EOB-MRI and DWI in patients with decompensated liver cirrhosis[18,20,21,36], especially for sub-centimeter HCCs.
Investigation of reasons for heterogeneity rather than computation of a single summary measure is an important purpose of meta-analysis[38]. Significant heterogeneity was found in pooled analysis of the included 10 studies. Spearman correlation analysis demonstrated there was no significant threshold effect. This work suggested that study design may affect diagnostic accuracy. These findings corroborated a recently published report[39], which showed that case series studies have significantly higher per-lesion sensitivity than case-control studies. Therefore, it is important that future studies adopt study designs that better control biases and provide higher levels of evidence such as cohort studies and randomized controlled trials.
In seven previous meta-analyses[40-46], investigators evaluated the detection of HCC of any size by Gd-EOB-DTPA, three of which yielded a subgroup analysis for small HCCs[40-42]. In a recent meta-analysis, Kierans et al[47] evaluated the diagnostic performance of dynamic contrast-enhanced MRI for the detection of small HCC with subgroup analysis of Gd-EOB-MRI, whose results were consistent with our findings[40-42,47]. However, compared with the above reports, this study has the following characteristics: All cases in the included literatures had a history of chronic liver disease; subgroup analysis for the diagnostic performance of Gd-EOB-MRI and DWI combination in the detection of sub-centimeter HCC was performed. In addition, in two recent meta-analyses[39,48], investigators compared the diagnostic performance of ultrasonography, CT and MRI in the detection of HCC of any size without subgroup analysis. Therefore, in comparison with the above previous meta-analyses, we expanded the evaluation to combined Gd-EOB-MRI and DWI, and detectability for sub-centimeter HCC.
Our meta-analysis has several limitations. First, data were collected in a prospective manner, with a limited number of studies (only three studies), which resulted in a major methodologic limitation of including many studies with retrospective patient data collection. Pooling such suboptimal retrospective results may have caused a bias toward increased diagnostic sensitivity[49]. Second, participants in included studies were both patients diagnosed with HCC based on findings prior imaging tests or other clinical data and those suspected of having HCC, which might have caused selection bias. In addition, limited numbers of lesions were diagnosed during liver transplantation (only two studies), which might have resulted in an overestimation of the diagnostic performance of Gd-EOB-MRI by decreasing the number of false-negative lesions. Finally, considerable heterogeneity was observed with per-lesion analysis. For example, whether or not interpretation of pathology data was blinded from Gd-EOB-MRI seemed to be a common weakness, and only 4 studies used the same reference standard. Furthermore, we found substantial variation in the way Gd-EOB-MRI findings were used for the identification of HCC, indicating a lack of consensus regarding diagnostic criteria and thresholds. To overcome the heterogeneity of the present data, we used both the hierarchical summary ROC model and the random-effects model. Because the 95%CIs were not substantially wide, we believe that the present results are valuable. However, heterogeneity in this type of diagnostic study remains a point of concern.
In conclusion, our meta-analysis showed that Gd-EOB-MRI has good diagnostic performance in the detection of small HCC (≤ 2.0 cm) among patients with chronic liver disease, but relatively lower performance for the detection of sub-centimeter HCC (≤ 1.0 cm). Combination of Gd-EOB-MRI and DWI can improve the diagnostic sensitivity of MRI for the detection of small HCC.
In recent years, gadoxetic acid-enhanced magnetic resonance imaging (Gd-EOB-MRI) has shown that hepatobiliary phase imaging provides improvement in the diagnosis of small hepatocellular carcinoma (HCC ≤ 2.0 cm). However, the results are limited thus far by small numbers of included patients with chronic liver disease, especially for sub-centimeter lesions (≤ 1.0 cm). In addition, no consensus is available regarding diagnostic performance of combined Gd-EOB-MRI and diffusion weighted imaging (DWI) in the detection of small HCC.
Despite important advances in multidisciplinary therapies, complete curative treatment of early-stage small HCC remains the only option for long-term patient survival. Thus, the importance of early detection of HCC has been emphasized, especially with the application of noninvasive multi-modality imaging.
In this study, the authors investigated the value of Gd-EOB-MRI for the diagnosis of sub-centimeter HCC. It is also believed to be the first meta-analysis evaluating combined Gd-EOB-MRI and DWI in the detection of small HCC.
This meta-analysis showed that Gd-EOB-MRI has relatively lower performance for the detection of sub-centimeter HCC, and combination of Gd-EOB-MRI and DWI can improve diagnostic sensitivity. In clinical practice, the addition of DWI to routine protocol of Gd-EOB-MRI may help increase sensitivity in the detection of small HCC, especially for sub-centimeter lesion.
This is a meta-analysis evaluating Gd-EOB-MRI for the detection of small HCC in patients with chronic liver disease, showing that Gd-EOB-MRI has good diagnostic performance for the detection of small HCC; in addition, Gd-EOB-MRI and DWI combination improves the diagnostic sensitivity of MRI for detecting small HCC. The methods used in this study are state of the art, and data are well presented and discussed in the light of the current literature.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Paumgartner G, Temel HE S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 2. | Rosencher N, Vassilieff N, Guigonis V, Toulon P, Conseiller C. Comparison of effects of Elohes and albumin on hemostasis in orthopedic surgery. Ann Fr Anesth Reanim. 1992;11:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 4. | Desmet VJ. East-West pathology agreement on precancerous liver lesions and early hepatocellular carcinoma. Hepatology. 2009;49:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Zech CJ, Bartolozzi C, Bioulac-Sage P, Chow PK, Forner A, Grazioli L, Huppertz A, Laumonier H, Min Lee J, Murakami T. Consensus report of the Fifth International Forum for Liver MRI. AJR Am J Roentgenol. 2013;201:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Park G, Kim YK, Kim CS, Yu HC, Hwang SB. Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinomas: comparison with gadopentetate dimeglumine. Br J Radiol. 2010;83:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Kim YK, Kim CS, Han YM, Park G. Detection of small hepatocellular carcinoma: can gadoxetic acid-enhanced magnetic resonance imaging replace combining gadopentetate dimeglumine-enhanced and superparamagnetic iron oxide-enhanced magnetic resonance imaging? Invest Radiol. 2010;45:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, Han JK, Choi BI. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (& lt; or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010;45:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, Choi BI. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:W482-W489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Park MJ, Kim YS, Lee WJ, Lim HK, Rhim H, Lee J. Outcomes of follow-up CT for small (5-10-mm) arterially enhancing nodules in the liver and risk factors for developing hepatocellular carcinoma in a surveillance population. Eur Radiol. 2010;20:2397-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [PubMed] |
| 15. | Hwang J, Kim SH, Lee MW, Lee JY. Small (≤ 2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-multirow detector CT. Br J Radiol. 2012;85:e314-e322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (and lt; = 2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013;54:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 20. | Faletti R, Cassinis MC, Fonio P, Bergamasco L, Pavan LJ, Rapellino A, David E, Gandini G. Multiparametric Gd-EOB-DTPA magnetic resonance in diagnosis of HCC: dynamic study, hepatobiliary phase, and diffusion-weighted imaging compared to histology after orthotopic liver transplantation. Abdom Imaging. 2015;40:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Hwang J, Kim YK, Kim JM, Lee WJ, Choi D, Hong SS. Pretransplant diagnosis of hepatocellular carcinoma by gadoxetic acid-enhanced and diffusion-weighted magnetic resonance imaging. Liver Transpl. 2014;20:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, Choi BI. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9570] [Article Influence: 683.6] [Reference Citation Analysis (0)] |
| 24. | Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 25. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46497] [Article Influence: 2113.5] [Reference Citation Analysis (3)] |
| 26. | Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 846] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 27. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [PubMed] |
| 28. | Kim YK, Kim CS, Han YM, Yu HC, Choi D. Detection of small hepatocellular carcinoma: intraindividual comparison of gadoxetic acid-enhanced MRI at 3.0 and 1.5 T. Invest Radiol. 2011;46:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Zhao XT, Li WX, Chai WM, Chen KM. Detection of small hepatocellular carcinoma using gadoxetic acid-enhanced MRI: Is the addition of diffusion-weighted MRI at 3.0T beneficial? J Dig Dis. 2014;15:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Morinaga S, Imada T, Shimizu A, Akaike M, Sugimasa Y, Takemiya S, Takanashi Y. Angiogenesis in hepatocellular carcinoma as evaluated by alpha smooth muscle actin immunohistochemistry. Hepatogastroenterology. 2001;48:224-228. [PubMed] |
| 32. | Kogita S, Imai Y, Okada M, Kim T, Onishi H, Takamura M, Fukuda K, Igura T, Sawai Y, Morimoto O. Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol. 2010;20:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 34. | Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:W758-W765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 36. | Kim AY, Kim YK, Lee MW, Park MJ, Hwang J, Lee MH, Lee JW. Detection of hepatocellular carcinoma in gadoxetic acid-enhanced MRI and diffusion-weighted MRI with respect to the severity of liver cirrhosis. Acta Radiol. 2012;53:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Saxena R, Aisen AM. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am J Roentgenol. 2009;193:1556-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 40. | Liu X, Zou L, Liu F, Zhou Y, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e70896. [PubMed] |
| 41. | Chen L, Zhang L, Bao J, Zhang J, Li C, Xia Y, Huang X, Wang J. Comparison of MRI with liver-specific contrast agents and multidetector row CT for the detection of hepatocellular carcinoma: a meta-analysis of 15 direct comparative studies. Gut. 2013;62:1520-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhu J, Zhang W, Hu J. Is liver-specific gadoxetic acid-enhanced magnetic resonance imaging a reliable tool for detection of hepatocellular carcinoma in patients with chronic liver disease? Dig Dis Sci. 2013;58:3313-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Li X, Li C, Wang R, Ren J, Yang J, Zhang Y. Combined Application of Gadoxetic Acid Disodium-Enhanced Magnetic Resonance Imaging (MRI) and Diffusion-Weighted Imaging (DWI) in the Diagnosis of Chronic Liver Disease-Induced Hepatocellular Carcinoma: A Meta-Analysis. PLoS One. 2015;10:e0144247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Ye F, Liu J, Ouyang H. Gadolinium Ethoxybenzyl Diethylenetriamine Pentaacetic Acid (Gd-EOB-DTPA)-Enhanced Magnetic Resonance Imaging and Multidetector-Row Computed Tomography for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Medicine (Baltimore). 2015;94:e1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Junqiang L, Yinzhong W, Li Z, Shunlin G, Xiaohui W, Yanan Z, Kehu Y. Gadoxetic acid disodium (Gd-EOBDTPA)-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. J Magn Reson Imaging. 2014;39:1079-1087. [PubMed] |
| 46. | Chen L, Zhang L, Liang M, Bao J, Zhang J, Xia Y, Huang X, Wang J. Magnetic resonance imaging with gadoxetic acid disodium for the detection of hepatocellular carcinoma: a meta-analysis of 18 studies. Acad Radiol. 2014;21:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Kierans AS, Kang SK, Rosenkrantz AB. The Diagnostic Performance of Dynamic Contrast-enhanced MR Imaging for Detection of Small Hepatocellular Carcinoma Measuring Up to 2 cm: A Meta-Analysis. Radiology. 2016;278:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 48. | Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, Zakher B, Pappas M, Graham E, Sullivan SD. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;162:697-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 49. | Deeks J. Systematic reviews of evaluations of diagnostic and screening tests. Systematic reviews in health care: meta-analysis in context. London, England: BMJ Books 2001; 248-282. |