Published online Nov 26, 2014. doi: 10.13105/wjma.v2.i4.171
Revised: August 7, 2014
Accepted: August 27, 2014
Published online: November 26, 2014
Processing time: 223 Days and 9.3 Hours
AIM: To compare two treatments for ruptured cerebral aneurysm with reference to the relative risk of developing hydrocephalus.
METHODS: We reviewed the English language literature on the risk of developing hydrocephalus after aneurysm treatment. Data were divided by type of study (randomized controlled trial, cohort trial, nonrandomized comparison, prospectively- and retrospectively-collected observational study). They were also divided by type of aneurysm treatment (microvascular - clipping, or endovascular - coiling). Additional predictive variables collected for each publication were average age, gender distribution, measures of hemorrhage volume and subarachnoid hemorrhage severity, aneurysm locations, time to treatment, duration of follow-up and date of publication. We employed meta-analysis to calculate pooled risk ratios of developing hydrocephalus in cases receiving aneurysm clipping vs those receiving coiling. Meta-regression was used to correct pooled results for covariates.
RESULTS: Because indications for the two treatments are different, there is little clinical equipoise for treating most cases. The single randomized, controlled trial dealt with a small subset of ruptured aneurysms. Neither this nor pooled values from other studies which compared the two treatments had the power to demonstrate significant differences between the two treatments. Nor was there an apparent difference when observational data were meta-analytically pooled. However, when meta-regression was used to correct for predictive variables known to differ between the two treatment groups, a highly-significant difference appeared. Coiling is used more commonly in older, sicker patients with aneurysms in certain locations. These cases are more likely to develop hydrocephalus. When corrected for these covariates, the risk of hydrocephalus was found to be significantly lower in coiled vs clipped cases (P = 0.014).
CONCLUSION: Pooled observational data were necessary to demonstrate that coiling ruptured cerebral aneurysms is associated with a lower risk of developing hydrocephalus than is clipping.
Core tip: Several treatment comparisons in clinical medicine are not amenable to randomized controlled trials. The conditions may be too rare for trials to obtain adequate statistical power. There may be a lack of clinical equipoise on the part of patients or clinicians. Comparisons of different treatments are nevertheless still important and can only be addressed by pooling observational data.
- Citation: Lang SS, Sanborn MR, Ju C, Premjee A, Stein SC, Smith MJ. Hydrocephalus after subarachnoid hemorrhage: A meta-analytic comparison of aneurysm treatments. World J Meta-Anal 2014; 2(4): 171-178
- URL: https://www.wjgnet.com/2308-3840/full/v2/i4/171.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i4.171
Cerebral aneurysms are small spherical outpouchings of the arteries that run along the surface of the brain. Affecting approximately 3.2% of adults[1], they are usually clinically silent and cause no symptoms. However, as its weakened wall stretches, the aneurysm is prone to rupture, bleeding into the subarachnoid space that surrounds the brain[2]. This subarachnoid hemorrhage occurs annually in about 9 per 100000 population worldwide, many countries experiencing much higher rates[3]. The most common cause of nontraumatic subarachnoid hemorrhage (SAH), ruptured cerebral aneurysm, is associated with high mortality and morbidity, and is considered an important cause of disabling stroke. One common complication of SAH is hydrocephalus, enlarging of the brain’s fluid spaces[4]. This is thought to be caused by the blood and the inflammation it incites clogging the pathways and preventing circulation and absorption of cerebrospinal fluid (CSF). Hydrocephalus is extremely common in the first days after SAH; as many as 30% of hydrocephalus cases are permanent and require long-term treatment.
Ruptured aneurysms are commonly treated acutely, in order to prevent repeat hemorrhage. One standard treatment is craniotomy with application of a spring-loaded clip across the neck of the aneurysm. Usually this procedure is performed with the aid of an operative microscope and is termed “microvascular” or “clipping”. The other technique involves threading a catheter within the artery from the groin to the aneurysm and filling the aneurysm sac with tiny platinum coils. Termed “endovascular” or “coiling”, this approach induces the blood within the aneurysm to clot. Both approaches are usually successful in preventing aneurysms from bleeding again. The choice of which procedure is most appropriate often varies according to the characteristics of the aneurysm and the patient[5,6]. Recent guidelines have been published to establish the relative strengths and weaknesses of both approaches[7].
One unresolved issue is whether one approach is more successful in preventing chronic hydrocephalus. Each has its strong points. Microvascular surgery promotes dissipation of the blood surrounding the aneurysm. On the other hand, the brain exposure and manipulation involved in microvascular surgery may itself predispose to hydrocephalus, a problem the endovascular approach avoids.
Clinical trials reporting incidence of hydrocephalus after each aneurysm treatment have reported wide variations in the incidence of chronic hydrocephalus. Studies reporting outcomes from both approaches have measured conflicting relative risks. Since there are few controlled clinical trials, it is apparent that meta-analytic pooling of results is necessary to predict the relative risks associated with the two aneurysm treatments. Point estimates of the actual probability of hydrocephalus are often required for decision analyses and cost-effectiveness determinations. Since these values may be difficult to determine from meta-analytically pooled risk ratios, Einarson proposed meta-analysis of observational data[8]. However, this and similar approaches to meta-analysis have been criticized as precise but not necessarily valid[9,10]. Our study compares analysis of trials, in which both treatments are compared, with observational data to predict relation between treatment and risk of chronic hydrocephalus
We performed a structured search in Medline, EMBASE and the Cochrane database of articles published in English before March 2013 containing a combination of the terms “subarachnoid”, “hemorrhage/haemorrhage”, “intracranial aneurysm”, “treatment” and “hydrocephalus,” either as keywords or in the text or title. We supplemented our search using the “Related Citations” feature of PubMed and manual reviews of the bibliographies of selected articles. Abstracts of returned articles were examined for a likelihood of yielding useful data. Unlikely abstracts were excluded; the others were reviewed by at least two authors. We excluded series reporting fewer than 10 treated cases. We also excluded publications that reviewed or duplicated data from other reports or dealt only with special cases, such as giant or pediatric aneurysms. For purposes of this study, we defined “hydrocephalus” as the placement of a permanent ventricular shunt at any time in the acute or follow-up period. The articles were divided into two groups, those treated by microvascular clip occlusion and those with endovascular coil occlusion. If an article reported on both approaches, it was included in the analysis only if results of the two treatment groups were reported separately. We included only cases in which a permanent CSF shunt was placed; acute but transient hydrocephalus was not included.
In addition to treatment and hydrocephalus incidence, the following categories of data were also extracted: (1) number of cases surviving acute care; (2) median Fisher grade (FG) a measure of hemorrhage volume, median Hunt-Hess grade (HHG) a measure of the hemorrhage’s clinical severity; (3) mean age; (4) gender distribution; (5) ruptured aneurysm location and diameter; (6) proportion of microvascular cases with attempted clot removal; (7) mean time to treatment; (8) duration of follow-up; and (9) date of publication.
We used meta-analytic technique to calculate pooled risk ratios from randomized controlled trials (RCTs), non-randomized cohort studies, and retrospective reviews of case series that reported results from both treatment approaches. In addition we pooled observational data meta-analytically, creating pooled means and distributions for each variable {Einarson, 1997 #36}, calculating risk ratios from the pooled results.
To assess the effects of follow-up length, date of publication and other covariates on results, we employed meta-regression {Thompson, 2002 #39}. Statistical associations between a predictor variable such as type of aneurysm treatment and hydrocephalus incidence assume that treatment was independent of other influences. If there were other predictor variables that might interact with treatment (covariates), their contributions were analyzed using multiple regression analysis. In the case of pooled data, this required meta-regression. Meta-analytic pooling, meta-regressions and statistical analyses were done using Stata 12 (StataCorp LP; College Station, Texas).
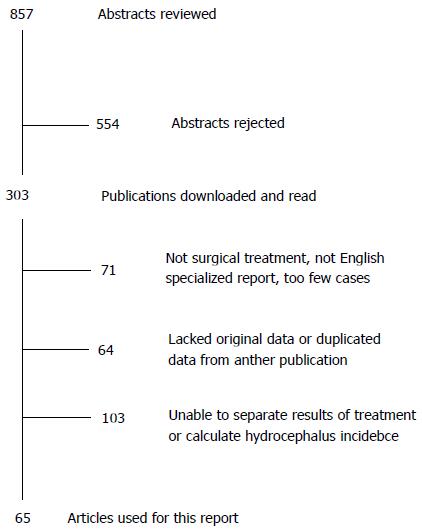
The search returned 857 abstracts of which 303 were relevant to our study. Some published series were excluded from our analysis since they reported only on elderly, pediatric or other specialized groups of patients. Figure 1 illustrates our search results, and Table 1 summarizes the publications used in our analysis, along with the study type, treatments used and incidence of hydrocephalus. In all, there were 65 publications reporting 38081 cases surviving acute care. Note that 14 papers (termed “comparative studies”) reported outcomes for both clipped and coiled patients separately. There was only one RCT[11] and one prospectively collected cohort study[12]. The other 12 papers involved retrospective data review of cases having one procedure or the other. This suggests that the bulk of the evidence was Level 4[13]. The incidence of hydrocephalus varied from 1 to 96% in individual series.
| Series | Trial type | Treatment | No. of cases | Hydrocephalus incidence |
| Akyuz et al[21] | Ret | Clip | 145 | 6.9% |
| Andaluz et al[22] | Pro | Clip | 106 | 6.6% |
| Auer et al[23] | Ret | Clip | 138 | 6.5% |
| Bailes et al[24] | Ret | Clip | 27 | 96.3% |
| Chan et al[25] | Ret | Clip | 89 | 57.3% |
| de Oliveira et al[26] | Ret | Clip | 212 | 17.5% |
| Dehdashti et al[12] | Cohort | Both | 245 | 13.9% |
| Diaz et al[27] | Ret | Coil | 31 | 15.5% |
| Dorai et al[28] | Ret | Clip | 651 | 23.3% |
| Fanning et al[29] | Ret | Coil | 178 | 1.7% |
| Ferch et al[30] | Ret | Clip | 73 | 53.4% |
| Fujimura et al[31] | Ret | Clip | 39 | 17.9% |
| Fukuhara et al[32] | Pro | Clip | 62 | 9.7% |
| Gao et al[33] | Ret | Coil | 221 | 4.1% |
| Gilsbach et al[34] | Ret | Clip | 138 | 23.2% |
| Graff-Radford et al[35] | Ret | Clip | 3521 | 8.9% |
| Gruber et al[36] | Ret | Both | 187 | 21.4% |
| Harrigan et al[37] | Ret | Both | 349 | 41.2% |
| Hernesniemi et al[38] | Ret | Clip | 57 | 31.6% |
| Hirashima et al[39] | Ret | Clip | 114 | 34.2% |
| Hoh et al[40] | Ret | Both | 20585 | 5.4% |
| Hornyak et al[41] | Ret | Clip | 92 | 20.7% |
| Jartti et al[42] | Ret | Both | 209 | 30.1% |
| Joakimsen et al[43] | Pro | Clip | 47 | 10.6% |
| Kaku et al[44] | Ret | Both | 100 | 18.0% |
| Kang et al[45] | Pro | Coil | 76 | 5.3% |
| Kasuya et al[46] | Ret | Clip | 108 | 29.6% |
| Kolluri et al[47] | Ret | Clip | 500 | 4.2% |
| Komotar et al[48] | Ret | Clip | 582 | 10.3% |
| Krayenbühll et al[49] | Ret | Clip | 218 | 10.1% |
| Kung et al[50] | Ret | Coil | 102 | 30.4% |
| Kwon et al[51] | Ret | Coil | 107 | 12.1% |
| Liew et al[52] | Ret | Clip | 13 | 69.2% |
| Lin et al[53] | Ret | Clip | 147 | 15.6% |
| Milhorat et al[54] | Ret | Clip | 29 | 20.7% |
| Moriyama et al[55] | Ret | Clip | 58 | 32.8% |
| Nam et al[56] | Ret | Both | 736 | 19.7% |
| Natarajan et al[57] | Ret | Both | 192 | 14.3% |
| Ohwaki et al[58] | Ret | Clip | 74 | 33.8% |
| O'Kelly et al[59] | Ret | Both | 3120 | 18.7% |
| Park et al[60] | Ret | Clip | 94 | 13.8% |
| Park et al[61] | Ret | Coil | 118 | 5.9% |
| Pinsker et al[62] | Ret | Clip | 31 | 48.4% |
| Raimondi et al[63] | Ret | Clip | 30 | 13.3% |
| Säveland et al[64] | Pro | Clip | 247 | 1.2% |
| Schmieder et al[65] | Ret | Clip | 138 | 10.9% |
| Sethi et al[66] | Ret | Both | 100 | 6.0% |
| Sindou et al[67] | Ret | Clip | 197 | 12.7% |
| Spallone et al[68] | Ret | Clip | 125 | 7.2% |
| Stenhouse et al[69] | Ret | Clip | 27 | 11.1% |
| Sundt et al[70] | Ret | Clip | 616 | 9.4% |
| Taha et al[71] | Ret | Both | 133 | 22.5% |
| Tapaninaho et al[72] | Ret | Clip | 835 | 9.7% |
| Theander et al[73] | Ret | Clip | 16 | 6.3% |
| Tomasello et al[74] | Pro | Clip | 47 | 4.3% |
| Tsementzis et al[75] | Pro | Clip | 61 | 6.6% |
| Vale et al[76] | Ret | Clip | 90 | 20.0% |
| Vanninen et al[11] | RCT | Both | 106 | 13.2% |
| Varelas et al[77] | Ret | Both | 163 | 7.3% |
| Vassilouthis et al[78] | Ret | Clip | 181 | 6.1% |
| Widenka et al[79] | Ret | Clip | 283 | 18.4% |
| Williams et al[80] | Ret | Clip | 19 | 42.1% |
| Yang et al[81] | Ret | Both | 88 | 25.0% |
| Yasui et al[82] | Ret | Clip | 82 | 46.3% |
| Yoshioka et al[83] | Ret | Clip | 576 | 37.3% |
We compared the risk ratios (endovascular/microvascular) reported by the single RCT, the cohort study, the pooled values of the 14 comparative studies and pooled value of all 65 publications treated as observational studies. The results are shown in Table 2. The pooled mean incidence of chronic hydrocephalus, calculated from all sources, is 0.191 (± 0.023). The probability of hydrocephalus was lower after coiling than clipping (0.161 ± 0.199 and 0.209 ± 0.115, respectively). This corresponds to a risk ratio of 0.770 (relative risk = 0.726, absolute difference 0.048), the difference being non-significant (P = 0.289) on the Student’s t test.
| Study type | Number of studies | Risk ratio(coil/clip) | 95%CI |
| RCT | 1 | 0.286 | 0.008-1.011 |
| Cohort study | 1 | 1.512 | 0.956-2.068 |
| All comparative studies | 14 | 1.150 | 0.982-1.318 |
| Observational studies | 65 | 0.770 | 0.539-1.005 |
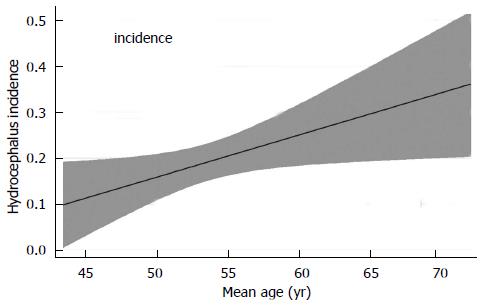
For the observational analysis we also investigated the influence of variables other than treatment on hydrocephalus incidence. Table 3 reports the results of simple linear meta-regression for the associations between hydrocephalus incidence and the other measured variables, demonstrating several other significant differences between the two groups. These include HHG, time to treatment, and patient age, the last of which is shown in Figure 2. Of note, there was no significant association between date of publication and hydrocephalus incidence, suggesting no progress has been made over time to lower its risk. In addition, neither sex distribution nor the mean FG appeared to influence hydrocephalus occurrence. Other variables, such as the presence of vasospasm, intraventricular hemorrhage (IVH), removing the subarachnoid clot surgically or with antifibrinolytics, acute hydrocephalus and aneurysm diameter lacked sufficient pooled data to permit meaningful analysis.
| Variable | B coefficient | 95%CI | P value |
| Treatment1 | -0.048 | -0.131-0.034 | 0.249 |
| Mean hunt-hess grade | 0.146 | 0.064-0.228 | 0.001 |
| Mean fisher grade | 0.015 | -0.106-0.136 | 0.805 |
| Mean age (yr) | 0.010 | 0.002-0.018 | 0.019 |
| % Female patients | -0.047 | -0.544-0.451 | 0.852 |
| % Vertebrobasilar aneurysms | 0.001 | -0.002-0.004 | 0.434 |
| Time to treatment | -0.011 | -0.021-0.0004 | 0.042 |
| Duration of follow-up | -0.000 | -0.004-0.003 | 0.833 |
| Year of publication | 0.001 | -0.002-0.005 | 0.455 |
We cannot assume that all the predictor variables for hydrocephalus are independent. Since the treatment was not chosen randomly, we must consider whether characteristics of the patients or the hemorrhages influenced choice of treatment. Statistical comparisons revealed significant differences between clipped and coiled cases regarding patient age (P = 0.045), % females (P = 0.038), and % vertebrobasilar aneurysms (P = 0.018). In all cases, the higher values were in the coiled group. Although the lower risk of hydrocephalus in coiled patients was not significant in single regression, adjusting with multivariable meta-regression for these covariates (age, sex, aneurysm location) revealed that treatment became a highly significant independent predictor of hydrocephalus (P = 0.014). HHG (P = 0.001) and age (P = 0.032) remained the only other independent significant predicting variables. Correcting the comparative series for covariates did not alter the non-significant associated between treatment type and hydrocephalus incidence.
Meta-analytic techniques to pool observational data and correct for covariates enabled us to demonstrate a lower risk of hydrocephalus following endovascular aneurysm repair compared to microvascular treatment. This knowledge may aid aneurysm surgeons in selecting the ideal approach to individual patients after subarachnoid hemorrhage.
The task is complicated by a strong bias in selecting patients for each surgical approach. For example, older and sicker patients and those harboring aneurysms in certain locations are more likely to be selected for endovascular over microvascular surgery. The single RCT[11] was limited to the small subset of cases in which clinical equipoise was thought to exist. Not only is this report of limited generalizability to the ruptured aneurysm population, it is too small to demonstrate significance. Nor is a significant difference obtained by pooling results from non-randomized trials in which the two approaches are compared. Only the larger numbers obtained by treating all studies as observational allowed the underlying pattern to emerge. Surgeons are more likely to employ coiling to treat aneurysms in older, sicker patients and those whose aneurysms are in the vertebrobasilar circulation, all of which have been shown to predispose to hydrocephalus. Correcting for these covariates allowed us to demonstrate that the risk of hydrocephalus was significantly lower after endovascular coiling than microvascular clipping. Among attempts to extend the utility of meta-analysis in clinical medicine[14,15], employing observational data has been reported as a valuable addition[8,16-18]. Not only can it be used to generate pooled probabilities, but, as here, to explore possible causal relationships in situations in which RCTs have not or cannot be done.
This study has several important limitations. It could be argued that possible selection bias creates two overlapping but distinct populations, for which any statistical comparison is invalid. The role of meta-analysis for observational data has been criticized as introducing possible confounding and heterogeneity[9], and there are situations in which it creates misleading conclusions[10]. Nevertheless, guidelines have been established in reporting observational meta-analyses[19] along the lines of the QUOROM specifications[20].
In a conclusion, we propose that analysis of observational data is necessary to display a significant association between treatment choice for subarachnoid hemorrhage and subsequent hydrocephalus. We used meta-analysis to create pooled estimates of frequency and meta-regression to correct for covariates.
Cerebral aneurysms are common causes of hemorrhagic stroke, and hydrocephalus is a common complication of a ruptured aneurysm. Aneurysm treatment usually consists of occlusion by intracranial clipping or intravascular coiling. There is lack of consensus about which treatment has the lower risk of hydrocephalus. However, because of the different indications for the two treatments, different patient ages and other discordances, a suitable trial has not been done.
Authors’ present an approach to the problem which employs observational meta-analysis and meta-regression to compare the two procedures in reference to the risk of hydrocephalus and to correct for covariates, such as subject age and aneurysm location.
Previous comparisons of relative risks and treatment effects were generally limited to randomized clinical trials.
This approach permits comparison of risks and effectiveness of treatment strategies from observational data in the absence of randomized clinical trials.
Cerebral aneurysms: small spherical outpouchings of the arteries that run along the surface of the brain. Aneurysm rupture: tear in the thinned wall of the aneurysm. Subarachnoid hemorrhage: bleeding resulting from aneurysm rupture. Hydrocephalus: enlargement of the fluid spaces of the brain secondary to impairment of fluid flow or absorption.
This paper presented a very well-written meta-analysis of published literature aiming to determine whether endovascular coiling or (open) surgical clipping of (cerebral) aneurysm affects incidence of chronic hydrocephalus.
P- Reviewer: Algin O, Chu D, Leonardi M, Lesley WS S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 4. | Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, Shi X, Tang Y, Peng Y. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | van der Schaaf I, Algra A, Wermer M, Molyneux A, Clarke M, van Gijn J, Rinkel G. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2005;CD003085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 2394] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 8. | Einarson TR. Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin Ther. 1997;19:559-569; discussion 538-539. [PubMed] |
| 9. | Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140-144. [PubMed] |
| 10. | Smith GD, Egger M. Meta-analyses of observational data should be done with due care. BMJ. 1999;318:56. [PubMed] |
| 11. | Vanninen R, Koivisto T, Saari T, Hernesniemi J, Vapalahti M. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils--a prospective randomized study. Radiology. 1999;211:325-336. [PubMed] |
| 12. | Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg. 2004;101:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Available from: http://www.cebm.net/index.aspx?o=1025. Accessed March 30, 2014. |
| 14. | Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769-773. [PubMed] |
| 15. | Goodman SN. Meta-analysis and evidence. Control Clin Trials. 1989;10:188-204. [PubMed] |
| 16. | Binder DA. Methodological Issues in the Meta-Analysis of Observational Studies: Discussion (Statistical Society of Canada – Joint Statistical Meeting, 2010. Accessed April 11, 2014). Available from: http://www.amstat.org/sections/srms/proceedings/y2010/.../205375_56394.pdf. |
| 17. | Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913. [PubMed] |
| 18. | Monroe J. Meta-Analysis for Observational Studies: Statistical Methods for Heterogeneity, Publication Bias and Combining Studies. Los Angeles: Statistics, UCLA 2007; . |
| 19. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] |
| 20. | Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896-1900. [PubMed] |
| 21. | Akyuz M, Tuncer R. The effects of fenestration of the interpeduncular cistern membrane arousted to the opening of lamina terminalis in patients with ruptured ACoA aneurysms: a prospective, comparative study. Acta Neurochir (Wien). 2006;148:725-733; discussion 731-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Andaluz N, Zuccarello M. Fenestration of the lamina terminalis as a valuable adjunct in aneurysm surgery. Neurosurgery. 2004;55:1050-1059. [PubMed] |
| 23. | Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990;26:804-808; discussion 808-809. [PubMed] |
| 24. | Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 221] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Chan M, Alaraj A, Calderon M, Herrera SR, Gao W, Ruland S, Roitberg BZ. Prediction of ventriculoperitoneal shunt dependency in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2009;110:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, Raabe A. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery. 2007;61:924-933; discussion 933-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Diaz RJ, Wong JH. Clinical outcomes after endovascular coiling in high-grade aneurysmal hemorrhage. Can J Neurol Sci. 2011;38:30-35. [PubMed] |
| 28. | Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763-769; discussion 769-771. [PubMed] |
| 29. | Fanning NF, Willinsky RA, ter Brugge KG. Wall enhancement, edema, and hydrocephalus after endovascular coil occlusion of intradural cerebral aneurysms. J Neurosurg. 2008;108:1074-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Ferch R, Pasqualin A, Barone G, Pinna G, Bricolo A. Surgical management of ruptured aneurysms in the eighth and ninth decades. Acta Neurochir (Wien). 2003;145:439-345; discussion 445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Fujimura M, Sugawara T, Seki H, Oku T, Niimura K, Otawara Y, Higuchi H. Perivascular coating with fibrin glue of cerebral arteries in patients with aneurysmal subarachnoid hemorrhage; incidence of chronic hydrocephalus. Tohoku J Exp Med. 1996;179:267-272. [PubMed] |
| 32. | Fukuhara T, Shimizu T, Namba Y. Limited efficacy of endoscopic third ventriculostomy for hydrocephalus following aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2009;49:449-455. [PubMed] |
| 33. | Gao X, Liang G, Li Z, Wei X, Hong Q. Complications and adverse events associated with Neuroform stent-assisted coiling of wide-neck intracranial aneurysms. Neurol Res. 2011;33:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Gilsbach JM, Harders AG, Eggert HR, Hornyak ME. Early aneurysm surgery: a 7 year clinical practice report. Acta Neurochir (Wien). 1988;90:91-102. [PubMed] |
| 35. | Graff-Radford NR, Torner J, Adams HP, Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol. 1989;46:744-752. [PubMed] |
| 36. | Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B. Chronic shunt-dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;44:503-509; discussion 509-512. [PubMed] |
| 37. | Harrigan MR, Rajneesh KF, Ardelt AA, Fisher WS. Short-term antifibrinolytic therapy before early aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurgery. 2010;67:935-939; discussion 939-940. [PubMed] |
| 38. | Hernesniemi J, Vapalahti M, Niskanen M, Kari A. Management outcome for vertebrobasilar artery aneurysms by early surgery. Neurosurgery. 1992;31:857-861; discussion 861-862. [PubMed] |
| 39. | Hirashima Y, Hamada H, Hayashi N, Kuwayama N, Origasa H, Endo S. Independent predictors of late hydrocephalus in patients with aneurysmal subarachnoid hemorrhage--analysis by multivariate logistic regression model. Cerebrovasc Dis. 2003;16:205-210. [PubMed] |
| 40. | Hoh BL, Kleinhenz DT, Chi YY, Mocco J, Barker FG. Incidence of ventricular shunt placement for hydrocephalus with clipping versus coiling for ruptured and unruptured cerebral aneurysms in the Nationwide Inpatient Sample database: 2002 to 2007. World Neurosurg. 2011;76:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Hornyak M, Gilsbach J, Harders A. Clinical significance of computed tomography in early aneurysm surgery. Neurochirurgia (Stuttg). 1991;34:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Jartti P, Karttunen A, Isokangas JM, Jartti A, Koskelainen T, Tervonen O. Chronic hydrocephalus after neurosurgical and endovascular treatment of ruptured intracranial aneurysms. Acta Radiol. 2008;49:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Joakimsen O, Mathiesen EB, Monstad P, Selseth B. CSF hydrodynamics after subarachnoid hemorrhage. Acta Neurol Scand. 1987;75:319-327. [PubMed] |
| 44. | Kaku Y, Yamashita K, Kokuzawa J, Hatsuda N, Andoh T. Treatment of ruptured cerebral aneurysms - clip and coil, not clip versus coil. Acta Neurochir Suppl. 2010;107:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Kang HS, Han MH, Lee TH, Shin YS, Roh HG, Kwon OK, Kwon BJ, Kim SY, Kim SH, Byun HS. Embolization of intracranial aneurysms with hydrogel-coated coils: result of a Korean multicenter trial. Neurosurgery. 2007;61:51-58; discussion 58-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage: a retrospective analysis of 108 patients. Neurosurgery. 1991;28:56-59. [PubMed] |
| 47. | Kolluri VR, Sengupta RP. Symptomatic hydrocephalus following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1984;21:402-404. [PubMed] |
| 48. | Komotar RJ, Olivi A, Rigamonti D, Tamargo RJ. Microsurgical fenestration of the lamina terminalis reduces the incidence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51:1403-112; discussion 1403-112;. [PubMed] |
| 49. | Krayenbühl HA, Yaşargil MG, Flamm ES, Tew JM. Microsurgical treatment of intracranial saccular aneurysms. J Neurosurg. 1972;37:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Kung DK, Policeni BA, Capuano AW, Rossen JD, Jabbour PM, Torner JC, Howard MA, Hasan D. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J Neurosurg. 2011;114:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Kwon BJ, Chang HW, Youn SW, Kim JE, Han MH. Intracranial aneurysm perforation during endosaccular coiling: impact on clinical outcome, initial occlusion, and recanalization rates. Neurosurgery. 2008;63:676-672; discussion 682-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Liew D, Ng PY, Ng I. Surgical management of ruptured and unruptured symptomatic posterior inferior cerebellar artery aneurysms. Br J Neurosurg. 2004;18:608-612. [PubMed] |
| 53. | Lin CL, Kwan AL, Howng SL. Acute hydrocephalus and chronic hydrocephalus with the need of postoperative shunting after aneurysmal subarachnoid hemorrhage. Kaohsiung J Med Sci. 1999;15:137-145. [PubMed] |
| 54. | Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987;20:15-20. [PubMed] |
| 55. | Moriyama E, Matsumoto Y, Meguro T, Kawada S, Mandai S, Gohda Y, Sakurai M. Combined cisternal drainage and intrathecal urokinase injection therapy for prevention of vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 1995;35:732-736. [PubMed] |
| 56. | Nam KH, Hamm IS, Kang DH, Park J, Kim YS. Risk of Shunt Dependent Hydrocephalus after Treatment of Ruptured Intracranial Aneurysms : Surgical Clipping versus Endovascular Coiling According to Fisher Grading System. J Korean Neurosurg Soc. 2010;48:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Natarajan SK, Sekhar LN, Ghodke B, Britz GW, Bhagawati D, Temkin N. Outcomes of ruptured intracranial aneurysms treated by microsurgical clipping and endovascular coiling in a high-volume center. AJNR Am J Neuroradiol. 2008;29:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Ohwaki K, Yano E, Nakagomi T, Tamura A. Relationship between shunt-dependent hydrocephalus after subarachnoid haemorrhage and duration of cerebrospinal fluid drainage. Br J Neurosurg. 2004;18:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | O’Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Wallace MC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: incidence, predictors, and revision rates. Clinical article. J Neurosurg. 2009;111:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Park BE. Spontaneous subarachnoid hemorrhage complicated by communicating hydrocephalus: epsilon amino caproic acid as a possible predisposing factor. Surg Neurol. 1979;11:73-80. [PubMed] |
| 61. | Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, Kassam A. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005;26:506-514. [PubMed] |
| 62. | Pinsker MO, Gerstner W, Wolf S, Trost HA, Lumenta CB. Surgery and outcome for aneurysmal subarachnoid hemorrhage in elderly patients. Acta Neurochir Suppl. 2002;82:61-64. [PubMed] |
| 63. | Raimondi AJ, Torres H. Acute hydrocephalus as a complication of subarachnoid hemorrhage. Surg Neurol. 1973;1:23-26. [PubMed] |
| 64. | Säveland H, Hillman J, Brandt L, Edner G, Jakobsson KE, Algers G. Overall outcome in aneurysmal subarachnoid hemorrhage. A prospective study from neurosurgical units in Sweden during a 1-year period. J Neurosurg. 1992;76:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Schmieder K, Koch R, Lücke S, Harders A. Factors influencing shunt dependency after aneurysmal subarachnoid haemorrhage. Zentralbl Neurochir. 1999;60:133-140. [PubMed] |
| 66. | Sethi H, Moore A, Dervin J, Clifton A, MacSweeney JE. Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg. 2000;92:991-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Sindou M. Favourable influence of opening the lamina terminalis and Lilliequist’s membrane on the outcome of ruptured intracranial aneurysms. A study of 197 consecutive cases. Acta Neurochir (Wien). 1994;127:15-16. [PubMed] |
| 68. | Spallone A, Pastore FS, Rizzo A, Guidetti B. Low-dose tranexamic acid combined with aprotinin in the pre-operative management of ruptured intracranial aneurysms. Neurochirurgia (Stuttg). 1987;30:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Stenhouse LM, Knight RG, Longmore BE, Bishara SN. Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J Neurol Neurosurg Psychiatry. 1991;54:909-914. [PubMed] |
| 70. | Sundt TM, Kobayashi S, Fode NC, Whisnant JP. Results and complications of surgical management of 809 intracranial aneurysms in 722 cases. Related and unrelated to grade of patient, type of aneurysm, and timing of surgery. J Neurosurg. 1982;56:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 194] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Taha MM, Nakahara I, Higashi T, Iwamuro Y, Iwaasa M, Watanabe Y, Tsunetoshi K, Munemitsu T. Endovascular embolization vs surgical clipping in treatment of cerebral aneurysms: morbidity and mortality with short-term outcome. Surg Neurol. 2006;66:277-284; discussion 284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Tapaninaho A, Hernesniemi J, Vapalahti M, Niskanen M, Kari A, Luukkonen M, Puranen M. Shunt-dependent hydrocephalus after subarachnoid haemorrhage and aneurysm surgery: timing of surgery is not a risk factor. Acta Neurochir (Wien). 1993;123:118-124. [PubMed] |
| 73. | Theander S, Granholm L. Sequelae after spontaneous subarachnoid haemorrhage, with special reference to hydrocephalus and Korsakoff's syndrome. Acta Neurol Scand. 1967;43:479-488. [PubMed] |
| 74. | Tomasello F, d'Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999;45:827-831; discussion 831-832. [PubMed] |
| 75. | Tsementzis SA, Hitchcock ER, Meyer CH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms. A double-blind placebo-controlled study. Acta Neurochir (Wien). 1990;102:1-10. [PubMed] |
| 76. | Vale FL, Bradley EL, Fisher WS. The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Varelas P, Helms A, Sinson G, Spanaki M, Hacein-Bey L. Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care. 2006;4:223-228. [PubMed] |
| 78. | Vassilouthis J, Richardson AE. Ventricular dilatation and communicating hydrocephalus following spontaneous subarachnoid hemorrhage. J Neurosurg. 1979;51:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Widenka DC, Wolf S, Schürer L, Plev DV, Lumenta CB. Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurol Neurochir Pol. 2000;34:56-60. [PubMed] |
| 80. | Williams JP, Pribram HF, Lynde RH, Sharpe AR. Isotope cisternography in the evaluation of patients with subarachnoid hemorrhage. J Nucl Med. 1970;11:592-596. [PubMed] |
| 81. | Yang TC, Chang CH, Liu YT, Chen YL, Tu PH, Chen HC. Predictors of shunt-dependent chronic hydrocephalus after aneurysmal subarachnoid haemorrhage. Eur Neurol. 2013;69:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Yasui N, Nathal E, Fujiwara H, Suzuki A. The basal interhemispheric approach for acute anterior communicating aneurysms. Acta Neurochir (Wien). 1992;118:91-97. [PubMed] |
| 83. | Yoshioka H, Inagawa T, Tokuda Y, Inokuchi F. Chronic hydrocephalus in elderly patients following subarachnoid hemorrhage. Surg Neurol. 2000;53:119-24; discussion 124-125. [PubMed] |