Published online Jun 18, 2025. doi: 10.13105/wjma.v13.i2.107171
Revised: April 12, 2025
Accepted: May 13, 2025
Published online: June 18, 2025
Processing time: 91 Days and 5.9 Hours
Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder presenting as symptoms of dysphagia, esophageal food impaction, chest pain, and heartburn. After an initial trial of proton pump inhibitor (PPI) therapy, swallowed topical corticosteroids (STC) are effective as induction therapy for EoE. However, out
To systematically evaluate the long-term outcomes and safety of maintenance treatment with STC.
A systematic search of PubMed, EMBASE, Cochrane, and Web of Science was performed from inception to January 2025 for studies comparing long term or maintenance treatment with STC for EoE compared to placebo. We included studies that investigated patients who underwent successful induction therapy. Pooled data was analyzed for histologic recurrence, symptom recurrence, need for repeat esophageal dilation, use of concomitant PPI, and candida infection rates. A random effects model was used, and the data was presented using odds ratios (OR) with 95% confidence intervals (CI).
Three randomized control trials and one observational study were included, involving 303 patients (189 in the STC group, 114 in the placebo-controlled group). Analysis showed that histologic recurrence was significantly lower with STC (OR: 0.04, 95%CI: 0.01-0.28, P < 0.00001, I2 = 78%). Overall symptom recurrence was similar between groups (OR: 0.23, 95%CI: 0.02-3.54, P = 0.29, I2 = 92%). On sensitivity analysis, symptom recurrence was significantly lower in the STC group (OR: 0.05, 95%CI: 0.02-0.17, P = 0.00001, I2 = 39%). Odds of repeat dilation were significantly lower in the STC group (OR: 0.14, 95%CI: 0.02-0.91, P = 0.04, I2 = 0%). Candida infection rates were similar between groups (OR: 6.13, 95%CI: 0.85-44.26, P = 0.07, I2 = 24%). Proportion of concomitant PPI use was similar between groups (OR: 1.64, 95%CI: 0.83-3.21, P = 0.15, I2 = 0%).
For patients who successfully achieved remission of EoE with STC induction therapy, maintaining treatment is effective in sustaining histologic remission, while newer regimens may be effective in preventing symptom recurrence compared to placebo. We found no significant difference for oropharyngeal/esophageal candidiasis with STC maintenance therapy. Future studies with longer follow-up periods are needed.
Core Tip: This meta-analysis seeks to perform a focused review of swallowed topical corticosteroid maintenance therapy for eosinophilic esophagitis. The results showed that the steroid group compared to placebo had significantly lower odds of disease recurrence histologically and lower odds of requiring repeat esophageal dilation. Our sensitivity analysis suggests that newer formulations of swallowed topical corticosteroids have favorable symptom outcomes. The groups were similar in candidal infectious outcomes. Thus, the existing literature shows that swallowed topical corticosteroids are safe and effective as maintenance therapy for eosinophilic esophagitis.
- Citation: Wu YZ, Kudlak M, Garza M, Grieme A, Liu KS, Kwon JJ, Smith ER, Yatsynovich E, Bushe B. Swallowed topical steroid maintenance therapy for eosinophilic esophagitis: A systematic review and meta-analysis. World J Meta-Anal 2025; 13(2): 107171
- URL: https://www.wjgnet.com/2308-3840/full/v13/i2/107171.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i2.107171
Eosinophilic esophagitis (EoE) is an interleukin (IL)-4/IL-13 mediated chronic inflammatory condition of the esophagus characterized by infiltration of eosinophils[1]. Over time, this inflammation can lead to progressive esophageal remodeling, resulting in intra-esophageal fibrosis and the formation of strictures[1]. Clinically, EoE manifests as esophageal dysfunction with symptoms of feeding difficulties and vomiting in infants and children while dysphagia, heartburn, epigastric pain, and chest pain are the hallmarks of symptoms in adults[2]. The incidence and prevalence of EoE have dramatically increased over the past two decades, making it the leading cause of esophageal dysphagia and esophageal food impaction[3]. Consequently, EoE significantly impacts the quality of life for affected individuals, contributing to both social challenges and emotional distress, particularly related to recurrent food impaction[4]. Given these adverse effects, effective treatment is essential for improving patient well-being.
Current treatment strategies include mechanical dilation therapy and anti-inflammatory approaches, such as proton pump inhibitors (PPIs), topical steroids, and elimination diets[5]. Medical therapies have demonstrated improvements in esophageal distensibility[5]. However, if left untreated, mechanical dilation remains the primary intervention for ad
This study aims to evaluate the efficacy of maintenance swallowed topical corticosteroids on preventing histologic and symptomatic recurrence while also exploring long term safety.
This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines[9]. The study was registered on PROSPERO (ID: CRD420250652801).
The search was limited to the English language. The following key terms were used in different combinations: “eosinophilic esophagitis” or “EoE” AND “swallowed topical corticosteroids” or “topical corticosteroid” or “steroid” or “budesonide” or “fluticasone” AND “maintenance” or “maintenance therapy” or “long term.”
The search, data extraction, and study quality assessment were performed independently by pairs of two investigators. Any discrepancy between these two investigators was discussed and resolved with the input of a senior investigator (MG).
Several databases were searched including MEDLINE, EMBASE, Cochrane, and Web of Science from inception until October 2024. Abstracts published at major scientific conferences were also screened.
All the studies were assessed for eligibility by two independent investigators (YW and MK). The inclusion criteria were as follows: Publication as a manuscript or abstract, studies of topical corticosteroid for eosinophilic esophagitis, studies of patients with EoE symptoms and confirmed histological diagnosis, and studies of patients who had successful induction therapy for EoE.
The following types of studies were excluded: Fewer than 10 participants, follow-up < 24 weeks, no control group, animal model studies, studies published in languages other than English, case reports, reviews, editorials and duplicate studies. The full texts of all the published studies were reviewed in an independent manner by two investigators (YW and MK). In cases where a group had multiple publications using data from the same cohort based on overlap in study period, the latest study was included, if otherwise eligible, in the review. This included studies where cases published in the interim as an abstract were later published as a complete manuscript.
The following baseline study characteristics were extracted from the studies: Study design, country where the study was conducted, age of participants, sex of participants, number of patients in each study arm.
The following outcome data were extracted from the included studies: Histologic recurrence, symptomatic recurrence, adverse events, candida infection, and need for repeat dilation. Data obtained from the included studies were systematically recorded in a database using Google sheets.
The Cochrane Risk of Bias 2.0 tool was used to assess study quality. Authors YW and AG performed the quality assessment.
The primary outcomes investigated were histologic recurrence of EoE defined as eosinophils > 15 per high powered field on longitudinal follow up and symptomatic recurrence at long term follow up.
Secondary outcomes were also assessed and included the need for repeat dilation, candida infection rates, and concomitant use of PPIs.
Any discrepancies were resolved by discussion and consensus between the two investigators who performed the data extraction process (YW and MK) in consultation with a senior author (MG).
The effect sizes for analyzed outcomes were reported as mean differences with 95% confidence interval (CI) for numerical or continuous variables and odds ratios (ORs) with 95%CI for categorical data. Where applicable, medians were considered representative of the mean. When applicable, numerical data reported as a range were transformed to mean and standard deviation before analysis using the method described by Hozo et al[10]. Data reported as percentages were transformed into numbers for analysis.
Heterogeneity among the studies was determined by inspection of forest plots, the Cochrane Q test, and the I2 statistic, and classified as low (I2 = 0%–30%), moderate (31%–60%), substantial (61%–75%), and considerable (76%–100%) heterogeneity. Sensitivity analysis was performed for analyses with at least substantial heterogeneity. If more than ten studies were included, publication bias was assessed by visual inspection of funnel plots and the Egger test[11].
Outcome comparisons were analyzed using the Random Effects model[12]. Forest plots were constructed for primary and secondary outcomes with Z values. A two-sided P value ≤ 0.05 was considered statistically significant. Additionally, Tau2, χ2, and degrees of freedom (df) representing heterogeneity were reported. All analyses were performed using Review Manager (RevMan) 5.4.1.
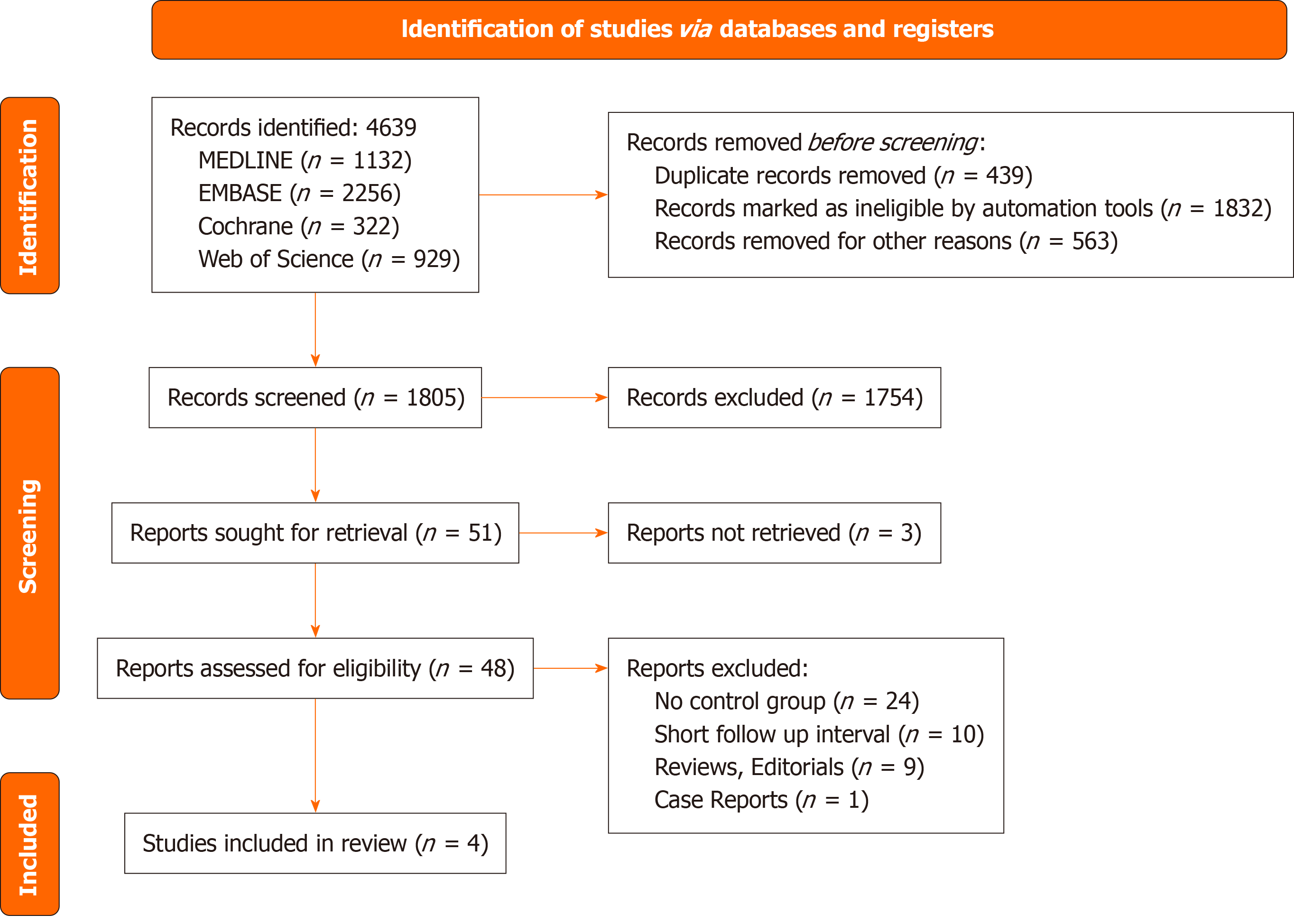
The preliminary search resulted in 4639 studies. After screening for eligibility, 4 manuscripts with 3 RCTs and 1 retrospective study were included in this review[13-16]. These studies were published between 2011 and 2022. Figure 1 shows the details of the selection process according to the PRISMA guidelines. Table 1 shows baseline study characteristics including median follow up interval which ranged from 36 to 50 weeks for the RCTs and was 3 years for the observational trial.
| Ref. | Design | Country | Age (Intervention/Control) | % Male (Intervention/Control) | Intervention/Placebo | Median follow up interval |
| Straumann et al[13], 2011 | RCT | Switzerland | 38.0 ± 11.7/34 ± 13.9 | 92.86/78.57 | 14/14 | 50 weeks |
| Straumann et al[14], 2020 | RCT | Switzerland | 36.5 ± 10.97/36 ± 9.9 | 83.8/80.9 | 136/68 | 48 weeks |
| Schupack et al[16], 2021 | Retrospective | United States | 45 ± 14.2/36.5 ± 11.0 | 50.0/66.7 | 14/9 | 3 years |
| Dellon et al[15], 2022 | RCT | United States | 36.8 ± 14.1/36.1 ± 11.7 | 56.0/69.6 | 25/23 | 36 weeks |
There was heterogeneity in the type of maintenance STC therapy detailed in each study. Straumann et al’s 2011 RCT used an older inhaled budesonide suspension[13]. Straumann et al’s 2020 RCT used oro-dispersible budesonide tablets[14]. Dellon et al’s 2022 RCT used a budesonide oral suspension[15]. The observational study by Schupack et al[16] reported regimens of either budesonide or fluticasone.
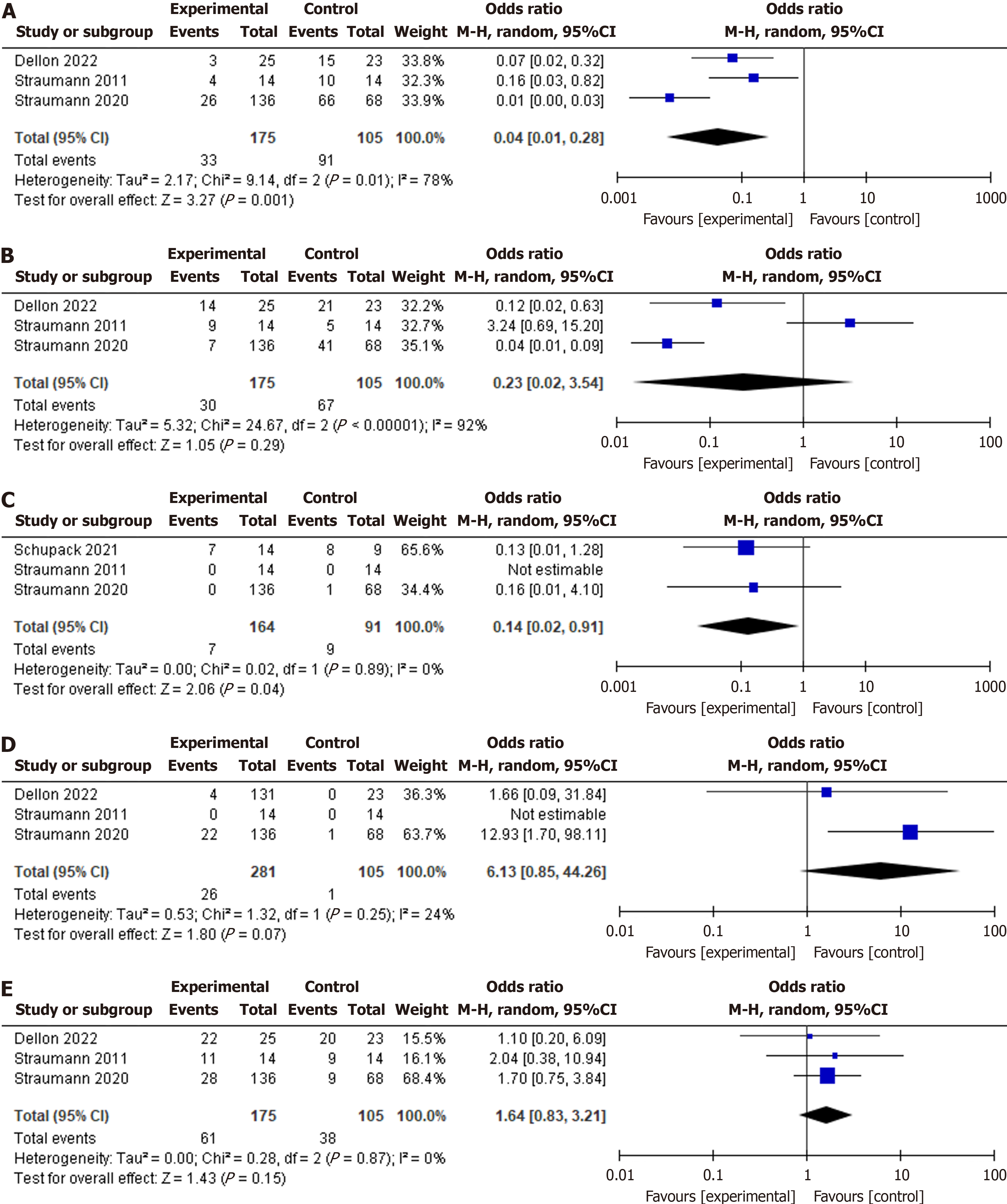
Histological recurrence: Histologic recurrence was significantly lower with STC (OR: 0.04, 95%CI: 0.01-0.28, P < 0.00001, I2 = 78%), see Figure 2A. Sensitivity analysis confirmed the robustness of the results (P < 0.0001).
Symptomatic recurrence: Symptom recurrence was similar between the STC and control groups (OR: 0.23, 95%CI: 0.02-3.54, P = 0.29, I2 = 92%), see Figure 2B. On sensitivity analysis, the older Straumann study was excluded due to the study’s use of an older STC formulation and the STC group was found to have significantly less recurrence of symptoms (OR: 0.05, 95%CI: 0.02-0.17, P = 0.00001, I2 = 39%)[13], Supplementary Figure 1.
Repeat dilation: The odds of repeat dilation were significantly lower in the STC group (OR: 0.14, 95%CI: 0.02-0.91, P = 0.04, I2 = 0%), see Figure 2C.
Candida infection: Candida infection odds were similar between groups (OR: 6.13, 95%CI: 0.85-44.26, P = 0.07, I2 = 24%), see Figure 2D.
Concomitant PPI usage: Proportion of concomitant PPI use was similar between groups (OR: 1.64, 95%CI: 0.83-3.21, P = 0.15, I2 = 0%), see Figure 2E.
Risk of bias analysis: The included RCT studies exhibited a low risk of bias, see Supplementary Figure 2. When evaluated using the RoB-2 tool, the observational Schupack et al[16] study had a high risk of bias.
Publication bias assessment: A publication bias assessment was deferred because fewer than 10 total studies were included in the final analysis.
Guidelines for the long-term management of EoE are evolving. The latest guidance from the American College of Gastroenterology suggests the use of maintenance strategies, although with low quality of evidence[17]. Current evidence has shown disease activity recurrence regardless of treatment type[18,19]. Thus, there is a need to further evaluate how effective STC are compared to placebo and if there are significant drawbacks.
This systematic review and meta-analysis confirmed the efficacy of STC maintenance treatment in sustaining histologic remission over the course of months to a year. We also found that the totality of available RCT data comparing STC to control did not find a difference in symptomatic recurrence. However, upon sensitivity analysis which excluded a study due to its use of an older inhaled STC regimen, we found STC to be efficacious in maintaining symptomatic remission. This suggests that newer formulations of STC such as budesonide oro-dispersible tablets and oral suspensions may have improved outcomes due to more targeted delivery of the medication to affected tissues.
A recent meta-analysis of proportions evaluated the efficacy of available maintenance treatments and found biologics such as dupilumab to potentially have higher efficacy in maintaining deep remission compared to STC[20]. However, meta-analyses of proportions represent an indirect comparison, so head-to-head studies between maintenance therapy regimens will need to be conducted to better inform future guidelines. Cost effectiveness remains an important consideration for patient-centered care as it has been shown that STC incurs lower costs than dupilumab[21].
This study is not without limitations. The small number of available studies limits the power of our analysis, and our study may not have enough power to detect differences in candida infection rates on maintenance STC. Data regarding histologic recurrence, more stringent histologic response thresholds, symptomatic recurrence, candida infection, all infectious outcomes, and need for esophageal dilation were not available from all studies. Our main outcomes had relatively high heterogeneity corresponding to heterogenous STC regimens. Our study also only included studies from North America and Europe, limiting external validity. Our exclusion of non-English language studies may have also introduced bias. Additionally, we did not have data beyond 50 weeks of maintenance therapy.
We acknowledge a key limitation in the lack of differentiation or subgroup analysis in our study for different delivery formulations of topical corticosteroids for the maintenance of EoE remission. There may be important differences between oro-dispersible tablets, inhaled nebulized steroids, and oral suspensions. Budesonide and fluticasone may also have different outcomes. We pooled these treatments together based on the availability head-to-head intervention vs placebo trials studying the maintenance phase of EoE treatment. Most available studies on topical corticosteroid treatments for EoE are limited to the induction phase of treatment, rather than the maintenance phase.
Future studies will be needed to better understand the nuances and differences between steroid delivery methods as well as the type of steroid used. The latest guidelines from the American College of Gastroenterology suggest use of the EoE Endoscopic Reference Score to characterize endoscopic findings[17]. Future studies can correlate EoE clinical and histologic outcome findings with this standardized endoscopic scoring system.
The availability of multiple modalities for maintenance EoE treatment is advancing our ability to treat this increasingly recognized disease. Our study demonstrates that for patients who achieved successful remission of EoE with STC induction therapy, maintenance therapy can effectively sustain histologic remission, while newer regimens may be effective in preventing symptom recurrence compared to placebo. Further studies will be needed to increase the quality of evidence for the positioning of maintenance therapies.
We acknowledge the mentorship and support of Dr. Tapasdip Gajjar and Dr. Erik Rahimi. As program directors of their respective graduate medical education programs, their support for trainee scholarly activity is greatly appreciated.
| 1. | O'Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, Rothenberg ME. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 2. | Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in eosinophilic esophagitis: Systematic review and meta-analysis of placebo-controlled randomized clinical trials. J Gastroenterol Hepatol. 2016;31:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154:319-332.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 564] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 4. | Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015;373:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 5. | Lucendo AJ, Molina‐infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González‐cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez‐sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence‐based statements and recommendations for diagnosis and management in children and adults. UEG Journal. 2017;5:335-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 731] [Article Influence: 91.4] [Reference Citation Analysis (1)] |
| 6. | Straumann A, Spichtin H, Grize L, Bucher KA, Beglinger C, Simon H. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660-1669. [RCA] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 547] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Lucendo AJ, Miehlke S, Schlag C, Vieth M, von Arnim U, Molina-infante J, Hartmann D, Bredenoord AJ, Ciriza de los Rios C, Schubert S, Brückner S, Madisch A, Hayat J, Tack J, Attwood S, Mueller R, Greinwald R, Schoepfer A, Straumann A, Tack J, Vanuytsel T, Louis H, Musala C, Miehlke S, Frederking D, Bajbouj M, Schlag C, Nennstiel S, Brückner S, Schmelz R, Heimerl S, Stephan A, Fibbe C, Liedtke (née Laschinsky) N, Keller J, Rosien U, Haag S, Schneider A, Hartmann D, Schmöcker C, Buchholz H, Lammert F, Casper M, Reichert M, Madisch A, Sommer D, Mönnikes H, Stengel M, Schmidtmann M, Müller M, Eckardt A, Wehrmann T, Schubert S, Armerding P, Hofmann WP, Liceni T, von Arnim U, Kandulski A, Weigt J, Börner N, Lutz-vorderbrügge A, Albert J, Zeuzem S, Blumenstein I, Sprinzl K, Hausmann J, Bredenoord A, Bredenoord A, Warners M, Villarin AL, Arias ÁA, Tejero Bustos MÁ, Carrillo Ramos MJ, Olalla Gallardo JM, Tosina RJ, Molina-infante J, Zamorano J, Vaquero CS, Francés SC, Pérez T, Rodriguez T, Ciriza de los Ríos C, Rodríguez-valcárcel FC, Castel de Lucas I, Juan AP, Barenys M, Pons C, Martinez IP, Lauret ME, García AC, Rubio E, Straumann A, Hruz P, Brunner S, Hayat J, Poullis A. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology. 2019;157:74-86.e15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 8. | Tomás-Pérez M, Domenech-Witek J, Ávila-Castellano MR, Carballas-Vázquez C, Vásquez-Bautista AA, Jover-Cerdá V, González-Mendiola R. Position Paper on the Treatment of Eosinophilic Esophagitis With Dupilumab. J Investig Allergol Clin Immunol. 2024;0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17537] [Article Influence: 1096.1] [Reference Citation Analysis (1)] |
| 10. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6901] [Article Influence: 345.1] [Reference Citation Analysis (0)] |
| 11. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40550] [Article Influence: 1448.2] [Reference Citation Analysis (2)] |
| 12. | DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1795] [Article Influence: 94.5] [Reference Citation Analysis (1)] |
| 13. | Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 14. | Straumann A, Lucendo AJ, Miehlke S, Vieth M, Schlag C, Biedermann L, Vaquero CS, Ciriza de Los Rios C, Schmoecker C, Madisch A, Hruz P, Hayat J, von Arnim U, Bredenoord AJ, Schubert S, Mueller R, Greinwald R, Schoepfer A, Attwood S; International EOS-2 Study Group. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology. 2020;159:1672-1685.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 15. | Dellon ES, Collins MH, Katzka DA, Mukkada VA, Falk GW, Morey R, Goodwin B, Eisner JD, Lan L, Desai NK, Williams J, Hirano I; ORBIT2/SHP621-302 Investigators. Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension. Clin Gastroenterol Hepatol. 2022;20:1488-1498.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Schupack DA, Ravi K, Geno DM, Pierce K, Mara K, Katzka DA, Alexander JA. Effect of Maintenance Therapy for Eosinophilic Esophagitis on Need for Recurrent Dilation. Dig Dis Sci. 2021;66:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Dellon ES, Muir AB, Katzka DA, Shah SC, Sauer BG, Aceves SS, Furuta GT, Gonsalves N, Hirano I. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025;120:31-59. [PubMed] [DOI] [Full Text] |
| 18. | Philpott H, Dellon ES. The role of maintenance therapy in eosinophilic esophagitis: who, why, and how? J Gastroenterol. 2018;53:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Dellon ES, Woosley JT, Arrington A, McGee SJ, Covington J, Moist SE, Gebhart JH, Galanko JA, Baron JA, Shaheen NJ. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020;18:1483-1492.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Barchi A, Massimino L, Mandarino FV, Yacoub MR, Albarello L, Savarino EV, Ungaro F, Danese S, Passaretti S, Bredenoord AJ, Vespa E. Clinical, Histologic, and Safety Outcomes With Long-term Maintenance Therapies for Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Hiramoto B, Muftah M, Flanagan R, Shah ED, Chan WW. Cost-Effectiveness Analysis of Current Treatment Options for Eosinophilic Esophagitis. Am J Gastroenterol. 2025;120:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |